The incidence of neuroendocrine tumors of the rectum has been increasing in the last decades, partly due to improved investigation. They are mostly well-differentiated small tumors with a rather good overall prognosis. In the last few years, some aspects of neuroendocrine tumors have been evolving. In 2010, the World Health Organization proposed a new classification, indicating that these tumors, as a category, should be considered malignant. Afterwards the European Neuroendocrine Tumor Society published their guidelines for the management of colorectal neoplasms. Treatment algorithm is mainly based on tumor size and grading and, in general, well-differentiated rectal tumors <2cm can be endoscopically resected. Endorectal ultrasound plays a particularly important role by accurately assessing tumor size and depth of invasion prior to resection. There are no specific recommendations on the optimal endoscopic resection method, but data from recent studies suggests that modified endoscopic mucosal resection techniques and endoscopic submucosal dissection have superior complete resection rates.
A incidência dos tumores neuroendócrinos do reto tem vindo a aumentar nas últimas décadas, em parte devido a uma maior investigação. Estes são sobretudo tumores pequenos, bem diferenciados e com um bom prognóstico global. Nos últimos anos, alguns aspetos relacionados com os tumores neuroendócrinos têm vindo a evoluir. Em 2010, a Organização Mundial da Saúde propôs uma nova classificação, sugerindo que estes tumores, enquanto categoria, devem ser considerados malignos. Posteriormente, a Sociedade Europeia de Tumores Neuroendócrinos publicou as suas recomendações para a orientação das neoplasias colorretais. O algoritmo de tratamento baseia-se principalmente no tamanho do tumor e no grau tumoral e, em geral, tumores do reto bem diferenciados, inferiores a 2cm, podem ser submetidos a resseção endoscópica. A ecografia endorretal desempenha um papel particularmente importante ao permitir avaliar com precisão o tamanho do tumor e profundidade de invasão antes da resseção. Não existem recomendações específicas sobre o método de resseção endoscópica ideal, no entanto, dados de estudos recentes sugerem que técnicas modificadas de mucosectomia e disseção da submucosa têm taxas superiores de resseção completa.
Neuroendocrine neoplasms in the digestive system are generically referred as gastroenteropancreatic tumors (GEP-NETs). GEP-NETs constitute a heterogeneous group of tumors arising from neuroendocrine cells of the embryological gut that share a common phenotype with immunoreactivity for the neuroendocrine markers, chromogranin A and synaptophysin1. Some tumors produce a variety of hormones and amines leading to distinct clinical syndromes (functioning tumors).2
These neoplasms used to be called “carcinoids”, the original term used by Oberndorfer, in 1907, to describe tumors that appeared to have a more benign behavior than carcinomas. However this term has been progressively abandoned in favor of neuroendocrine neoplasms.3
2ClassificationPrevious classification of GEP-NETs divided tumors based on embryonic derivation, in those of the foregut (respiratory tract, upper gastrointestinal tract and pancreas), midgut (small bowel and right colon) and hindgut (the remaining two thirds of colon and rectum). However, even within this classification there is marked heterogeneity and differences in behavior of tumors and thus classification is better based on anatomic location and grading of the tumor.3
In 2010, the World Health Organization (WHO) defined a classification for neuroendocrine neoplasms of the digestive system, according to which all tumors are considered malignant with the potential to metastasize. Tumor grade is based on mitotic count and on proliferation (Ki-67 index) and the three levels defined are described in Table 1.
Tumor grade according to mitotic count and proliferation.
| WHO grade | Mitotic count (10 HPFa) | Ki-67 index |
|---|---|---|
| G1 | <2 | ≤2% |
| G2 | 2–20 | 3–20% |
| G3 | >20 | >20% |
When grades assessed by mitotic count and Ki-67 differ, the higher grade is assumed. This new classification recognizes the following categories: neuroendocrine tumor (NET), neuroendocrine carcinoma (NEC) and mixed adenoneuroendocrine carcinoma.
NETs are well-differentiated neuroendocrine neoplasms, with low cellular atypia and proliferative activity and comprises grade G1 or G2 tumors. NECs are a poorly differentiated neuroendocrine neoplasm, showing marked cellular atypia and high proliferative activity. NECs are grade G3 tumors and two categories are recognized: large cell NEC and small cell NEC.4,5
Jernman et al6 demonstrated that this new WHO classification predicted the metastatic potentional of rectal NETs better than the previous 2000 classification, since G1 NETs had an indolent clinical course and G2 NETs often metastasize. Yamaguchi et al7 also concluded that the classification of gastrointestinal NETs into G1 and G2 based on Ki-67 index was appropriated to predict metastases and recurrences; for Salama et al8 Ki-67 appears as a reliable and reproducible marker for the grading of NETs and more superior than mitotic rate.
The American Joint Cancer Commission published, in 2010, a tumor–node–metastasis (TNM) classification system for colorectal NETs similar to the one previous proposed by the European Neuroendocrine Tumor Society (Table 2).9
American Joint Cancer Commission 2010 TNM classification.
| T (primary tumor) | |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor invades lamina propria or submucosa and size ≤2cm |
| T1a | Tumor size <1cm in greatest dimension |
| T1b | Tumor size 1–2cm in greatest dimension |
| T2 | Tumor invades muscularis propria or size >2cm with invasion of lamina propria or submucosa |
| T3 | Tumor invades through muscular propria into subserosa or into nonperitonealized pericolic or perirectal tissue |
| T4 | Tumor invades peritoneum or other organs |
| N (Regional Lymph nodes) | |
| Nx | Regional lymph nodes cannot be assessed |
| N1 | No regional lymph node metastases |
| N2 | Regional lymph node metastases |
| M (Distant metastases) | |
| M0 | No distant metastases |
| M1 | Distant metastases |
| Stage | |
| I | T1N0M0 |
| IIA | T2N0M0 |
| IIB | T3N0M0 |
| IIIA | T4N0M0 |
| IIIB | AnyTN1M0 |
| IV | AnyTAnyNM1 |
In order to assess the relevance of the TNM classification system, Jann et al10 conducted a retrospective study with midgut and hindgut NETs that confirmed the prognostic relevance and applicability of the classification.
In 2012, the European Neuroendocrine Tumor Society (ENETS) released guidelines for the management of patients with colorectal neuroendocrine neoplasms, to reflect the new relevant data on this matter, including the WHO classification.5
3EpidemiologyData from the Surveillance, Epidemiology and End Results (SEER) suggests that the incidence of rectal NETs have been increasing over the last decades; rectal NETs represented 29% of all GEP-NETs in the latest report, establishing the rectum as the most common location, slightly above the small intestine.11 In Europe, as indicated by an English12 and an Austrian13 studies, the rectum was the fifth and fourth most common location with a frequency of 8% and 14%, respectively. In Asia, rectal NETs take on special relevance, representing 56% of all GEP-NETs in Ito et al14 study. In Modlin et al15 report, rectal NETs also appear to be over-represented among the Asian populations within the United States. These data suggests that there are likely genetic pre-disposing factors, although some differences may also be attributable to higher colonoscopy screening rates and better reporting of polyps removed at endoscopy.3
Rectal NETs may have a slight male preponderance16,17 and the diagnosis is usually made in the sixth decade of life.16–18
4Clinical characteristics, prognosisMost patients are asymptomatic and diagnosis is made upon routine lower endoscopy or for investigation of unrelated symptoms. In symptomatic patients, the most frequent symptoms are rectal bleeding, pain, constipation and tenesmus.3,16 Carcinoid syndrome is very rare as the tumors themselves rarely produce serotonin.5
The majority of rectal NETs are small size lesions; in a recent systematic review 79% of tumors were less than 1cm and only 5% were greater than 2cm.19 Kasuga et al20 reported a mean tumor size of 7.1mm in their series. Most lesions are found in the midrectum as suggested by Kim et al21 study, in which 74.8% of tumors were found between 5 and 9.9cm of the anal verge.
Most rectal NETs (89%) are limited to the submucosa layer20 and are low grade tumors; Weinstock et al22 reported that G1 tumors accounted for 88.1, G2 for 8.2 and G3 for 3.5% of rectal neoplasms in their series.
Lymph node and distant metastasis are found in about 8% and 4% of patients, respectively.20
Rectal NETs have a good overall prognosis with a 5-year survival rate of 75.2–88.3%.15
Disease stage is the main prognostic factor of rectal NETs. The 5-year overall survival rates reported are 94–100, 54–74 and 15–37% for patients with localized, nodal positive and metastatic rectal NETs, respectively.23
Risk factors for metastatic disease include tumor size, muscularis propria invasion, proliferation index, lymphovascular and perineural invasion.5,20,23 Yoon et al.16 described distant metastasis rates for tumors ≤1cm, >1 to ≤2cm, and >2cm of 1.7, 15 and 50%, respectively. Weinstock et al22 reported 5-years survival rates of 87.7, 47.6 and 33.3% for patients with G1, G2 e G3 tumors, respectively.
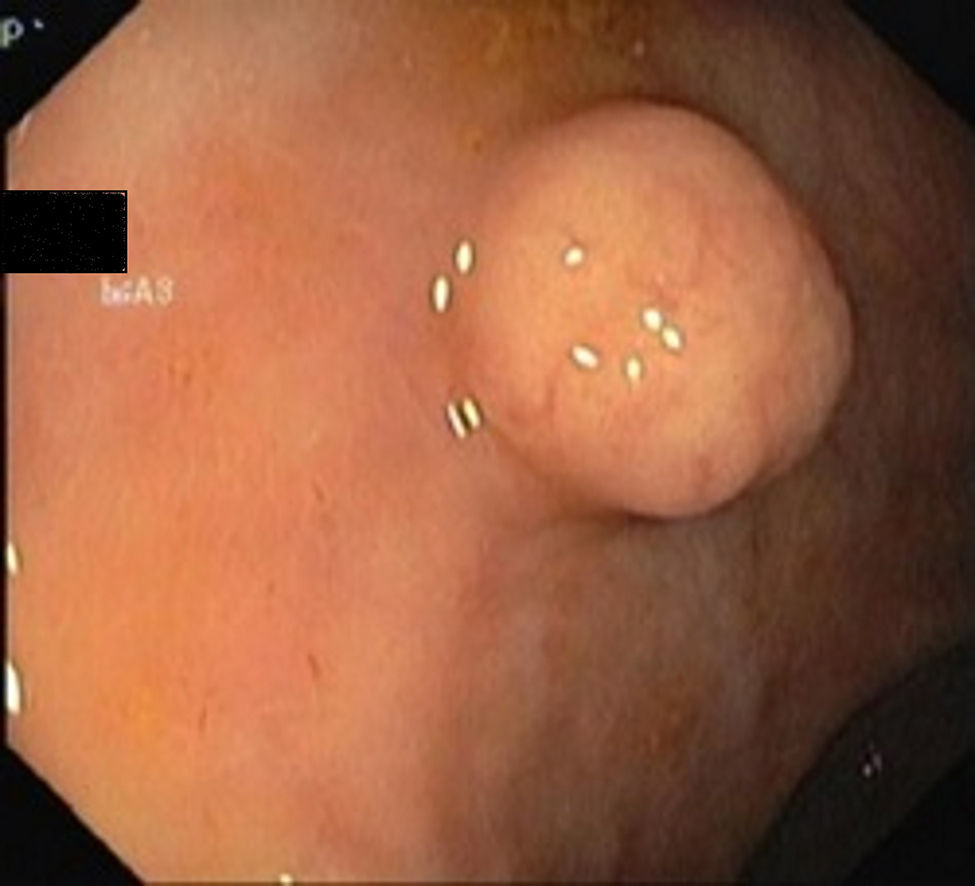
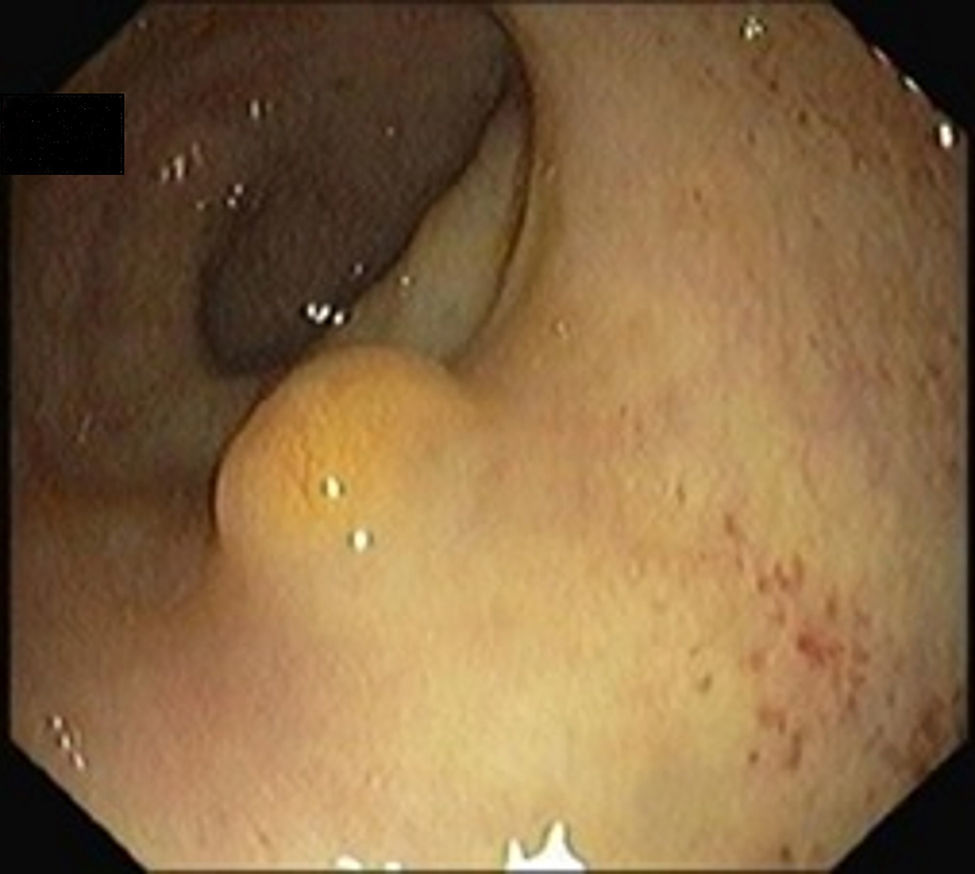
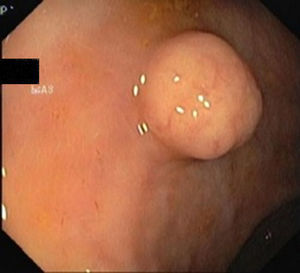
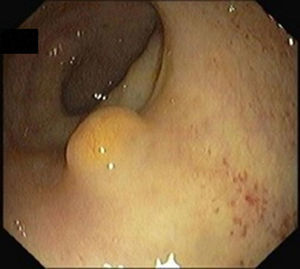
5Diagnosis and staging5.1ColonoscopyRectal NETs appear usually as small, sessile, submucosal tumors covered with yellow discolored mucosa.19 Atypical endoscopic features such as unusual tumor shape (semipedunculated and ulcerofungating), color (hyperemia) and surface change (depression, erosion, and ulceration) can be associated with metastasis.21
A full colonoscopy is recommended to exclude concomitant colonic disease and the possibility of synchronous carcinoma5 (Figs. 1 and 2).
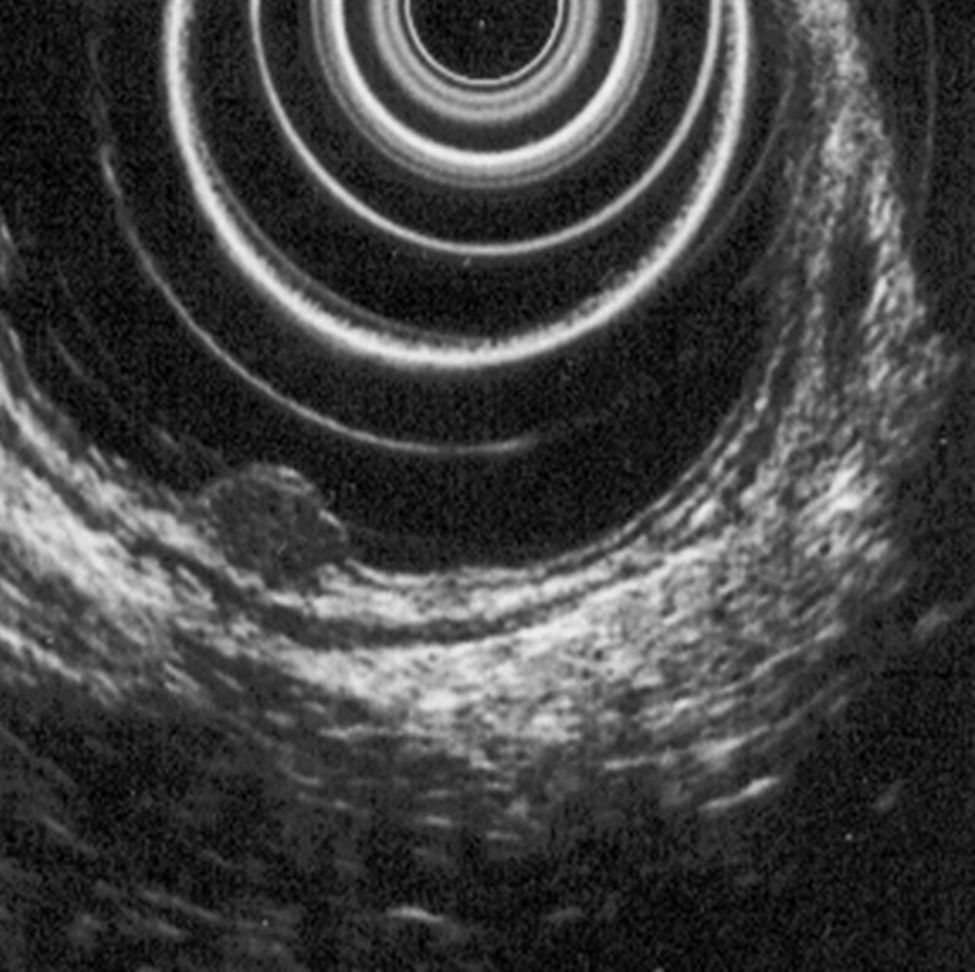
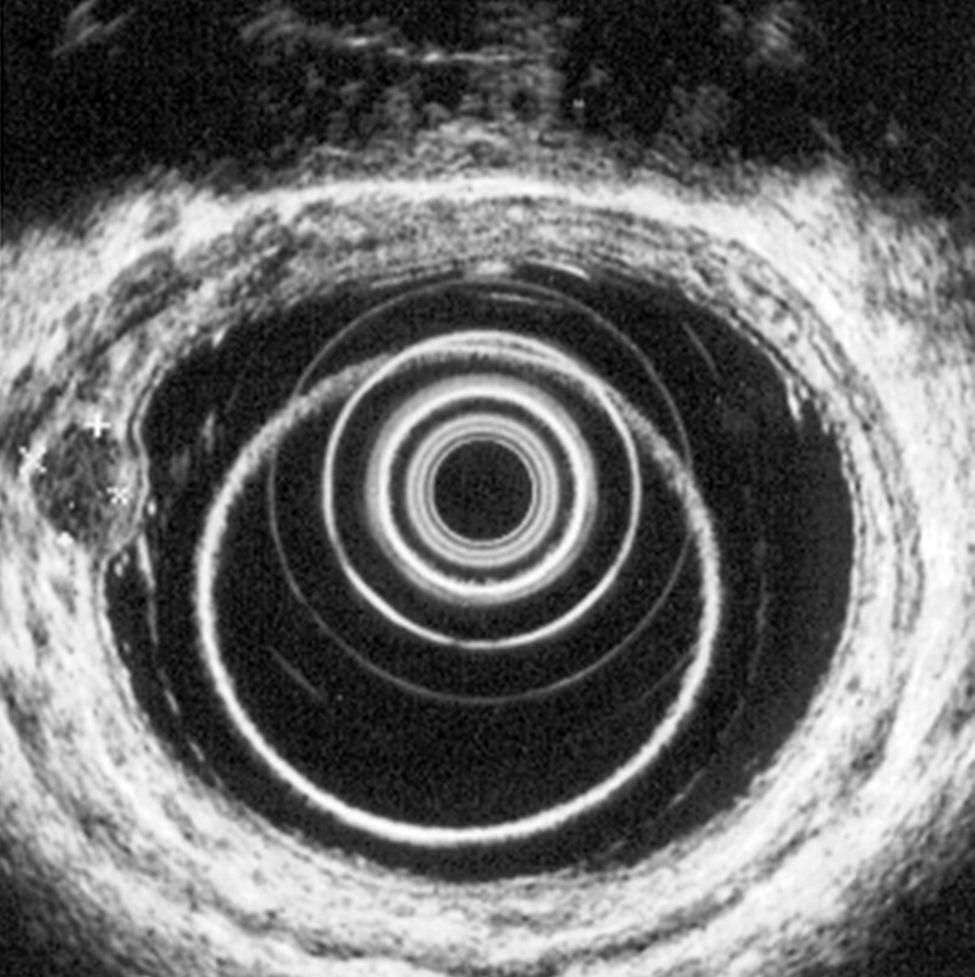
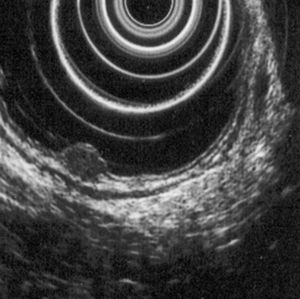
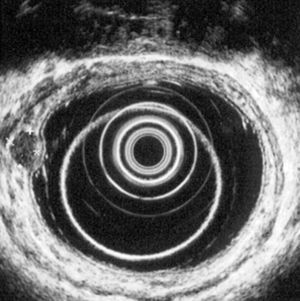
5.2Rectal ultrasoundRectal ultrasound (RUS) can accurately assess tumor size, depth of invasion and presence of pararectal lymph node metastases, which is particularly important to determine the adequate treatment modality.5 Kobayashi et al performed ultrasonographic evaluation with echocolonoscopes in 21 lesions and ultrasonic probes in 32 lesions. Rectal NETs appeared as well-demarcated, homogenous, isoechoic or hypoechoic lesions and the depth of invasion was correctly identified in all lesions (submucosal in 49/52 lesions). RUS could accurately diagnose the invasion depth of lesions as small as 2mm in diameter.24 High frequency ultrasound probes proved to be especially useful in assessing invasion of small lesions limited to the mucosa or submucosa25 and therefore can be particularly adequate for evaluating the depth of invasion in neuroendocrine rectal tumors. Another two studies found an accuracy of 91 and 100% for determination of tumor invasion with RUS.26,27
According to ENETS recommendation RUS should be performed prior to treatment to determine tumor invasion5 (Figs. 3 and 4).
5.3Computed tomography and magnetic resonanceIn rectal NETs, the role of computed tomography (CT) is not to detect the primary tumor nor to appreciate its invasion of the rectal wall, but to detect regional and distant metastases. CT has a reported mean detection rate for liver metastasis in neuroendocrine tumors of 81%. The CT appearance of NET lymph node metastases is similar to those from other malignant tumors, although a marked contrast enhancement is frequent.2
Magnetic resonance imaging (MRI) is superior for determining liver metastases and can be used where there is uncertainty over the nature of lesions identified on CT.3,5
The ENETS consensus recommend CT/MRI for patients with tumors >10mm in size and when residual and metastatic disease is suspected.5 In the NANETS guidelines it is stated that for tumors smaller than 2cm and confined to the mucosa or submucosa, these studies are not routinely recommended.9
5.4Scintigraphic scanning (Octreoscan)Radiolabelled somatostatin analogs are used to detect somatostatin receptor positive tissue. The detection of primary tumor in the rectum NETs can be difficult because of greater background activity. In addition, some tumors may not express somatostatin receptors, particularly higher grade NETS, and so the presence of metastatic disease is better evaluated by other methods such as CT. In the presence of metastatic disease, this method can be useful to determine somatostatin receptor expression which may have impact in selecting some therapies.3,5
5.5Positive emitron tomography (PET)Nowadays, Octreoscan can be replaced by Gallium-68 DOTA octreotide PET, which has higher sensitivity and specificity.1 However this method is less available.
FDG (18F 6-fluordopamine) PET is helpful for staging high grade/poorly differentiated tumors that do not express somatostatin receptors.3 Octeoscan and PET should be used for staging if residual or metastatic disease is suspected.5
5.6Biochemical markersSerum Chromagranin A is a useful marker in many neuroendocrine tumors but of limited use in non-metastatic rectal NETS.3 It can be useful for monitoring patients with metastatic disease or for surveillance in patients with resected stage II and III tumors.9 Urinary 5-hydroxyindoleacetic acid is of little use as few tumors produce serotonin.3
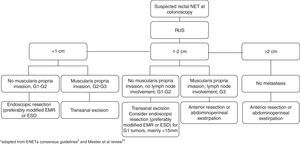
5.7TreatmentTumor size is the most important predictor of tumor behavior, and although other characteristics are taken into account, this is the main determinant in selecting the treatment option.5,9 Lesions <1cm have a low risk of lymph node and distant metastasis. The outcome of intermediate-sized (between 1 and 2cm) lesions is less clear but they have a higher risk of metastasis and a poorer prognosis compared to those <1cm.5,23 Park et al26 found that the tumor size that constituted a risk factor for metastasis was>1.4cm. Nevertheless, the ENETs recommendations suggest that rectal NETs up to 2cm with low mitotic rate and no muscularis propria invasion or lymph node involvement can mostly be endoscopically ressected.5 For Mestier et al, only tumors <15mm should be treated endoscopically.23 Among tumors <1cm, G2 grade cannot always be considered as a high risk factor taking into account the broad heterogeneity (mitotic count 2–20 and Ki-67 index 3–20%). The “low” G2 tumors, may behave like G1 tumors and could theoretically be treated in the same manner.23
Based on endoscopic and ultrasonographic characteristics, local excision is often performed without biopsy and the subsequent management will depend on margin status and risk factors for local and distant recurrence.5,23 A study found that performing biopsy before endoscopic excision was associated with incomplete endoscopic resection. The authors pointed out that the lesion may be flattened and margins blurred after biopsy and snaring and targeting the lesion afterwards may be more difficult and also tissue fibrosis after biopsy may also disturb ressection.28
Various endoscopic resection techniques have been applied to rectal NETs, including conventional polypectomy, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). Other techniques, derived from conventional EMR have been used and include EMR with cap aspiration (EMR-C), endoscopic submucosal resection using a ligation device (ESMR-L) and EMR using a dual-channel endoscope (EMR-D). There are no specific recommendations for selecting the optimal endoscopic treatment, however it is important to attain a complete tumor resection. Unsuccessfully resected lesions will need follow-up or additional treatment.23
With conventional polypectomy, it is difficult to achieve a tumor-free resection margin, as NETs generally penetrate the muscularis mucosa into the submucosal layer. Son et al. reported a pathologically complete resection rate of only 18.5% after polipectomy.29 Data regarding complete resection rates with conventional EMR are highly variable; in three different studies these rates were 42.9, 64.3 and 77.4%.29–31
EMR-C and ESMR-L allow the creation of a pseudopedicle prior to resection generally obtaining a deeper excision comparing with EMR and polypectomy.32 In EMR-D the lesion can be pulled into the snare using a grasping forceps before closing the snare and proceed with the resection.33 Two studies reported a complete resection of 100 and 93.9% with ESMR-L compared to 77.4 and 65.5% with conventional EMR, respectively.30,34 Son et al29 reported tumor-free margins in 71.7% of 53 patients treated with EMR-C compared to 42.9% treated with EMR. In Jeon et al35 study EMR-C was performed as a salvage treatment in 31 patients that failed en bloc excision after primary EMR or polypectomy, achieving clear resection margins in all cases. Two studies reported complete resection rates of 74.1% and 84.6% for EMR-D.34,36 In the first, the resection rate of this method was similar to conventional EMR. A concern with this technique is that the mucosa can be torn before the tumor is adequately elevated with the grasping forceps.37
ESD is a good therapeutic option, but at the same time is a more challenging procedure, because of its technical difficulty, the need of special devices and of an experienced endoscopist. Lee et al31 found a pathological complete resection rate of 82.6% for ESD compared with 64.3% for EMR group. Another two studies reported a complete resection rate of 97.7 and 80.6% for ESD, and on both these rates were similar to those achieved with ESMR-L.30,38 Choi et al38 suggests that ESMR-L may be considered the treatment of choice for rectal NETs based on comparable histologically complete resection rates and given the advantages of easier and shorter procedure time. Kim et al30 did not find an inferior procedure time for ESMR-L compared to ESD but several factors could explain these finding: the definition of procedure time did not include the time during post-procedure bleeding control which can be considerable during ESD; ESD in the rectum may be easier than other parts of the colon and finally ESD was performed by a highly trained endoscopist.
A recent meta-analysis reported a higher rate of pathological complete resection among patients treated with the modified EMR techniques (EMR-M) or ESD than among those treated with conventional EMR (OR 0.42), while there was no significant difference between the first two groups (OR 1.19). The procedure time for ESD was longer than those for EMR or EMR-M groups and it was insignificant between EMR and EMR-M. There were not detected significant differences in complications or recurrence between the three groups.39
After excision, the resection area must be tattooed in order to facilitate the lesion site location in case the positive margins are identified and further resection or vigilance is required.9
Although a negative resection margin is desirable, its positivity is not a completely satisfactory predictor of remnant tumor, relapse or metastases. By cauterization during endoscopic resection, the destruction of adjacent tumor cells can sterilize the resection site.23 In a study with 107 rectal NETs ≤10mm treated by conventional EMR and polypectomy, 15.9 and 34.6% had positive or indeterminate margins, respectively. None of the patients experienced local or distant recurrence during follow-up.40 In another study, 4 patients with positive resection margins underwent transanal endoscopic microsurgery (TEM) as a salvage operation. Posterior pathologic assessment only showed changes due to scarring without a remnant tumor.30
Recommendations for incomplete resection with endoscopic methods consist in annual follow up for tumors <1cm and G1 grading and transanal excision for tumors between 1–2cm and <1cm with G2 and G3 grading.5
In addition to salvage therapy in case of incomplete resection with endoscopic therapy, transanal excision is commonly performed as a primary therapy for intermediate-sized rectal NETs confined to the submucosa or for patients with smaller tumors invading the muscularis propria in whom lymph node metastasis has been excluded.5,9 TEM also allows full thickness excisions and several advantages over conventional transanal excision by providing an improved visualization, exposure and access to higher lesions in the rectum. Kinoshita et al.41 performed 27 TEM procedures (14 as a primary excision and 13 as completion surgery after incomplete endoscopic resection) attaining clear resection margins in all patients.
Rectal tumors >2cm, between 1 and 2cm with muscularis invasion, T3 or T4 stage and G3 grading and tumors with lymph node involvement should be treated similarly to adenocarcinoma, with anterior resection and total mesorectal excision or abdominoperineal exstirpation depending on distance to the anal verge.5
The general management of rectal NETs is summarized in Fig. 5.
5.8Therapy for advanced diseaseRectal NETs are mostly localized and non-functioning tumors. There is few data regarding the specific treatment of metastatic rectal NETs and further studies are necessary. Therapeutic approaches described for the management of metastatic disease associated with neuroendocrine neoplasms include surgical, medical, radiological and nuclear medicine strategies.42
Somatostatin analogs (SA) are the standard therapy in functioning NET of any site, as they control symptoms by inhibiting serotonin and other vasoactive substances secretion. In addition, there is some data suggesting that SA may also have an antiproliferative effect. Therefore, SA may be used in functioning and non-functioning tumors, particularly in cases where there is uptake in Octreoscan or Gallium-68 DOTA octreotide PET. Interferon alfa also seems to have an anti-secretory and antiproliferative effect and is equally effective in functioning and non-functioning tumors.9,42
Systemic chemotherapy is recommended for G3 NEC and is rarely used in G1 and G2 NETs given the poor results in these patients.5,42
In peptide receptor radiotarget therapy, a SA is linked to a radioisotope allowing high dose radiotherapy to be delivered to the tumor cells. These therapy can be used for inoperable metastatic neuroendocrine neoplasms positive for somatostatin receptors.3,5
Surgical resection of liver metastasis has been used either in a curative intent or to reduce liver tumor burden with debulking resections.42
Various ablative and locoregional procedures have also been described and include radiofrequency ablation, laser-induced thermotherapy, selective hepatic transcatheter arterial embolization or chemoembolization and selective internal radiotherapy. The option depends on the local expertise and extension and location of liver involvement. These methods can be used as sole therapies or in combination with surgery or medical treatment.42
6Follow-upNeuroendocrine tumors can recur even many years after resection, but there is little consensus about the best surveillance strategy. The ENETs recommend3,9 follow-up for 10 years5 and the NANETs for 7 years.9
ENETs recommendations suggests that tumors <1cm, G1–G2 grading, with no muscularis propria and lymph node involvement completely resected do not require regular follow-up. In tumors <1cm with G3 grading and in tumors between 1 and 2cm, follow-up may be performed on annual basis. Tumors >2cm always require follow up on an annual basis for G1–G2 tumors and on every 4–6 months in the first year and finally at least annualy, for G3 tumors. Although there is not a specific protocol for surveillance, these consensus recommend colonoscopy, RUS, MRI for rectal evaluation, TC or MRI for liver metastasis and cromogranin A.5
The NANETs do not recommend routine surveillance for stage I tumors; for stage II and III tumors follow-up may be performed in annually.9
7ConclusionsAlthough neuroendocrine rectal tumors are rare they are increasing in incidence and recent updates have been made regarding the classification, diagnostic and therapeutic approach. Despite a relatively indolent behavior, they are malignant and can metastasize. Most reported risk factors for metastatic disease are tumor size >1cm, muscularis propria invasion, high proliferation index and lymphovascular invasion. In general, low risk tumors can be treated by endoscopic resection and high risk tumors need surgical excision. Conventional polypectomy and EMR have lower complete resection rates compared to modified EMR techniques, such as EMR-C, ESMR-L and ESD. The prognosis of patients with metastatic disease is poor and data on the specific treatment of these patients is scarce.
Conflicts of interestThe authors have no conflicts of interest to declare.