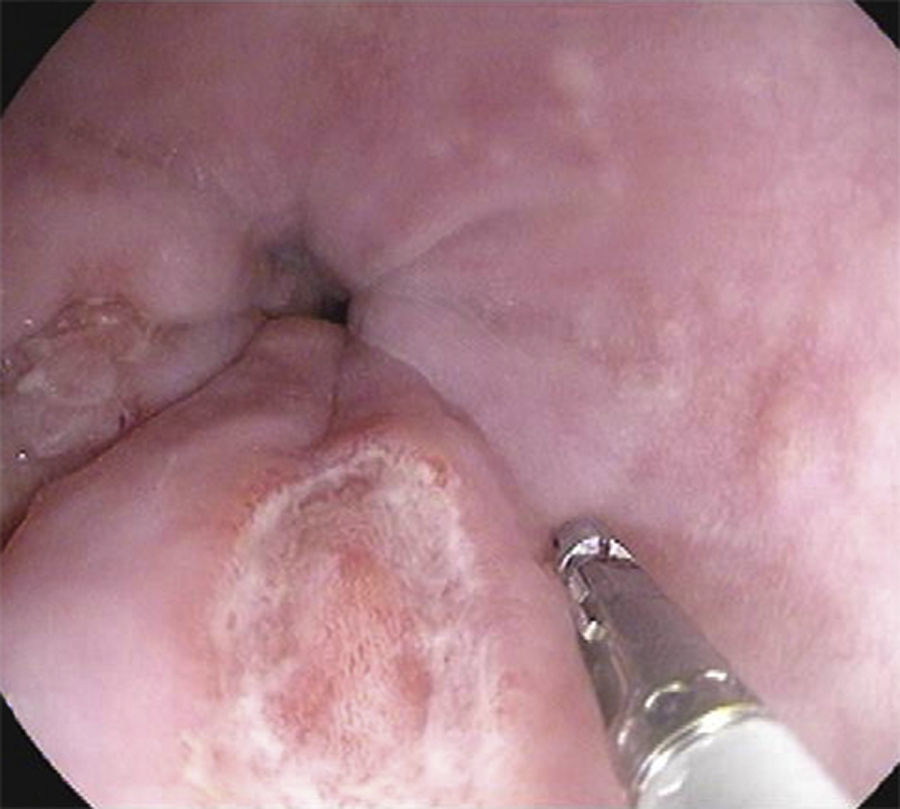
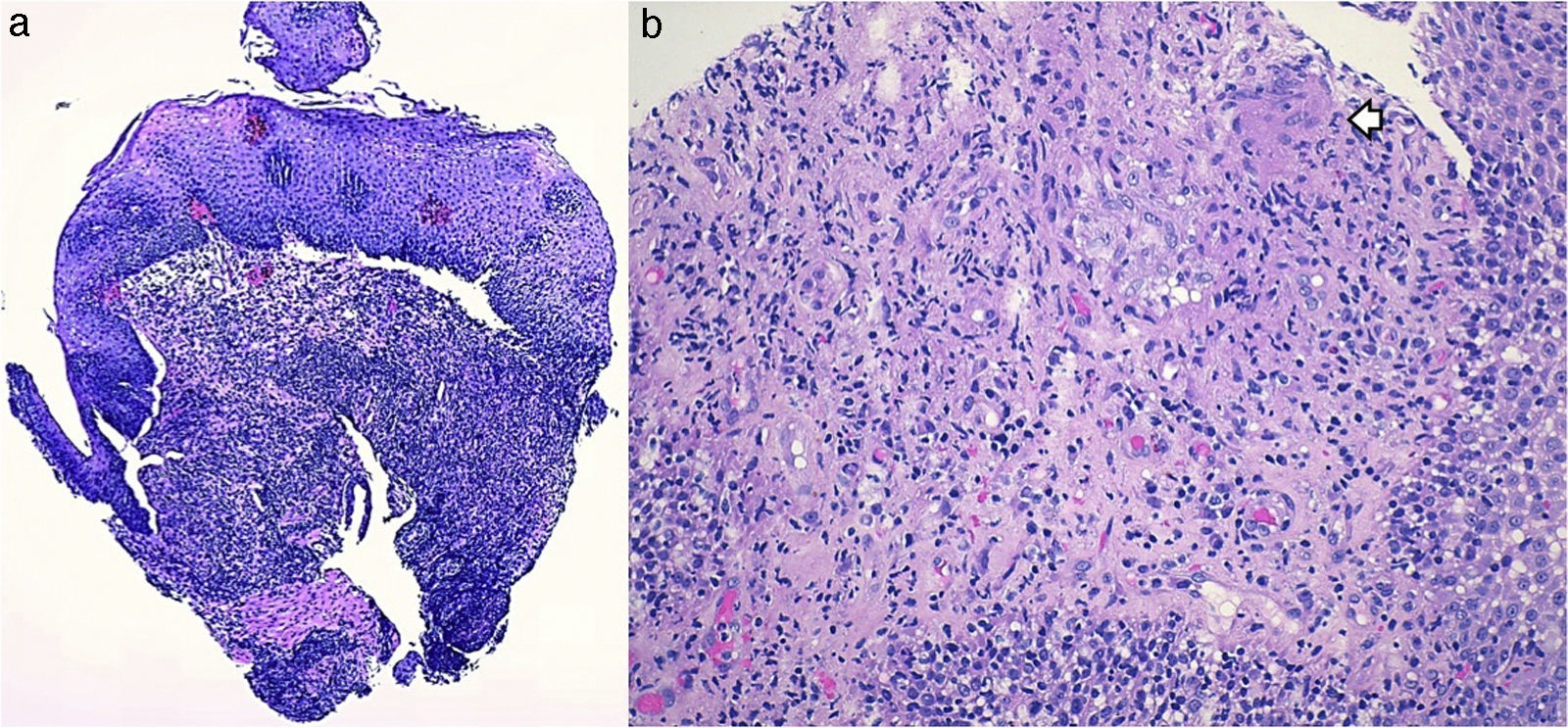
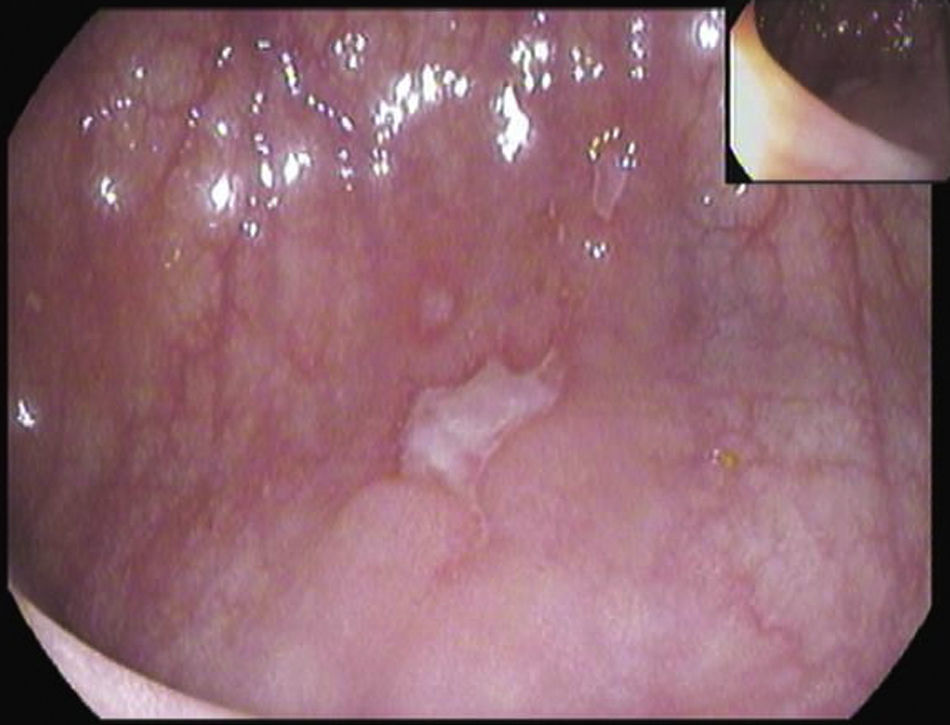
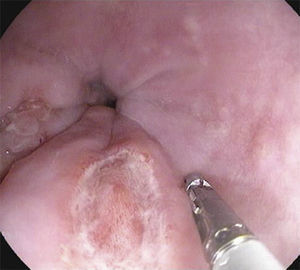
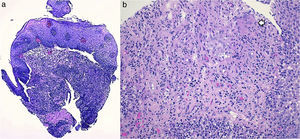
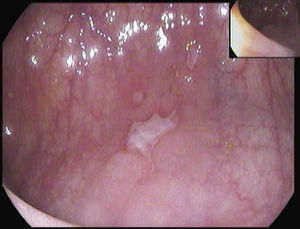
A 26-year-old overweight female patient presented to the emergency department with severe odynophagia for six days and retrosternal pain in the past two weeks. She had a history of appendectomy and a recent diagnosis of irritable bowel syndrome (IBS). The patient denied regular medication or recent use of any drugs, namely antibiotics or non-steroidal anti-inflammatory drugs. Laboratory studies showed an increased C-reactive protein and a negative HIV serology. An esophagogastroduodenoscopy (EGD) revealed several ulcers in the lower third of the esophagus, the largest with 15mm and raised borders (Fig. 1). Biopsies were taken from the edges and bottom of the ulcer. The patient was admitted and empirically started on proton pump inhibitor (PPI) and acyclovir. Serologies ruled out HSV 1 and 2, CMV, EBV and VZV recent infections and syphilis. Histological examination showed an intense chronic inflammatory infiltrate involving the mucosal, submucosal and muscular layers (Fig. 2a) and an epithelioid granuloma with a giant cell (Fig. 2b). There were no viral cytopathic effects or acid-fast bacilli. Hence, our patient had a non-caseous esophageal granulatomatosis. We excluded tuberculosis, sarcoidosis and granulomatosis with polyangiits (Wegener's granulomatosis) based on a negative Mantoux and IGRA tests and normal chest X-ray, angiotensin conversion enzyme levels, serum electrophoresis and renal function. At this point, we considered the hypothesis of Crohn's disease and given the patient's complaints of intermittent diarrhea and abdominal discomfort, labeled as IBS, an ileocolonoscopy was performed. Several areas of erythema with aphthous erosions, and ulcers, stellar and circular, the largest with 10mm, were found in the colon and rectum (Fig. 3); and a posterior commissure anal fissure was also identified. Biopsies showed architectural gland distortion, goblet cell depletion, cryptitis and submucosal lymphoplasmacytic infiltrate, findings consistent with Crohn's disease. The PPI was maintained and the patient was started on swallowed fluticasone (500mcg two times a day) with significant improvement of the proximal symptoms. In turn, a decision was made to start azathioprine and infliximab, due to the patient's young age, upper gastrointestinal and colonic involvement and perianal disease. An EGD repeated six months after presentation showed complete esophageal mucosal healing.
Foregut Crohn's disease has an estimated incidence of 1–13% in patients with ileocolonic disease. Moreover, esophageal Crohn's disease lesions are reported in 15% of adults and 44% of pediatric patients with Crohn's disease, when an EGD is systematically performed, suggesting that this entity is probably underdiagnosed.1 In contrast, isolated esophageal Crohn's is extremely rare with very few reported cases.2 The diagnosis implies ruling out other causes of esophagitis, namely, reflux disease, medications, viral, fungal and mycobacterial infections, sarcoidosis, vasculitis and carcinoma.2 Endoscopic features are not specific and include erythema, erosions and ulcers, aphthous and superficial or deep punched-out, strictures and fistulas.2–4 As for other Crohn's disease locations, non-necrotizing granulomas are uncommonly seen (7–9% in esophageal biopsies)2 and a high index of suspicion is needed to consider this diagnosis in the absence of known extra-esophageal Crohn's disease. Therapeutic options include systemic corticosteroids, immunosupressants and anti-TNFα, the latter reserved for severe and refractory disease.2–5 PPIs have no proved efficacy in mucosal healing and are used for symptom relief. Finally, topical swallowed aerosolized corticosteroids were shown to induce esophageal healing in a recent case-report and the authors propose topical steroids as an effective adjuvant therapy.6
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.