Las distintas especies de Fusarium son principalmente patógenos de plantas que ocasionalmente pueden originar enfermedades en humanos. Varios miembros de este género de hongos han causado una gran destrucción de cultivos alimentarios. Suelen haber especies de Fusarium en el suelo y en la materia vegetal en descomposición, pero también se pueden hallar en el agua. Algunas infecciones localizadas, como queratitis, sinusitis e infecciones en piel y uñas pueden afectar a personas inmunocompetentes. Sin embargo, las sinusitis invasoras y las infecciones pulmonares, de rápida diseminación, son habituales en pacientes muy inmunodeprimidos, especialmente en aquellos que son profundamente neutropénicos. El inicio agudo de lesiones dolorosas en la piel y el crecimiento de moho en cultivos sanguíneos son indicadores de una fusariosis diseminada en pacientes inmunodeprimidos. El organismo crece fácilmente en laboratorio, pero la identificación de la especie es complicada. Las biopsias de tejidos infectados muestran hifas septadas extremadamente ramificadas que a menudo son indistinguibles de las que presentan las especies de Aspergillus. Las lesiones localizadas responden razonablemente bien a agentes antifúngicos y a la cirugía. Las infecciones pulmonares invasoras y extendidas responden de manera deficitaria a la terapia antifúngica, en parte debido a la resistencia que presentan la mayoría de especies a muchos agentes antifúngicos, pero principalmente por la ausencia de una respuesta efectiva del huésped. La mayoría de los pacientes que tienen infección pulmonar invasoras o diseminada son tratados con una combinación de voriconazol y una formulación lipídica de anfotericina B, con la esperanza de que al menos un fármaco actúe contra el patógeno y se alcancen mejores resultados con el uso de dos fármacos. Desgraciadamente, los pacientes que padecen una fusariosis diseminada y aquellos que permanecen neutropénicos, rara vez sobreviven a la infección, independientemente de la terapia utilizada.
Fusarium species are primarily plant pathogens that occasionally cause human disease. Several members of this genus of molds have caused widespread destruction of food crops. Fusarium species are found primarily in soli and on decaying vegetable matter, but also occur in water. Localized infections, such as keratitis, sinusitis, and skin and nail infections, occur in immunocompetent persons. However, invasive sinusitis and pulmonary infection, as well as widespread dissemination, are the rule in markedly immunosuppressed patients, especially those who are profoundly neutropenic. The acute onset of painful skin lesions and the growth of a mold in blood cultures are clues to disseminated fusariosis in immunosuppressed patients. The organism grows readily in the laboratory, but identification to the species level is difficult. Biopsy of infected tissues shows acutely branching septate hyphae that are often indistinguishable from those of Aspergillus species. Localized lesions respond reasonably well to antifungal agents and surgical excision. Invasive pulmonary and disseminated infection respond poorly to antifungal therapy, in part because of the resistance of most species to many antifungal agents, but more importantly, to the absence of an effective host response. Most patients who have disseminated or invasive pulmonary infection are treated with a combination of voriconazole and a lipid formulation of amphotericin B, with the hope that at least one drug may have activity against the organism, and perhaps better killing can be achieved using two drugs. Unfortunately, patients who have disseminated fusariosis and who remain neutropenic rarely survive the infection, regardless of the therapy used.
Fusarium species are known primarily for their role as important plant pathogens that have caused devastating destruction of many fruits and vegetables. Human fusariosis varies in its manifestations from localized infections, such as keratitis, to intoxications that can occur with ingestion of contaminated foods, to devastating disseminated infections that are seen primarily in markedly immunosuppressed patients. The organism exists world wide, with different species occupying different ecological niches. A hallmark of Fusarium species is their marked resistance to currently available antifungal agents. We will review the epidemiology, clinical manifestations, and treatment options for fusariosis.
OrganismThe genus Fusarium includes over a 100 species, very few of which are human pathogens. The most common species that infect humans are F. solani, F. oxysporum, F. moniliforme (F. verticillioides), and F. proliferatum. F. solani causes approximately 50% of human cases of fusariosis. Fusarium species are predominantly plant pathogens. Several of the species of Fusarium have destroyed certain varieties of food producing plants, such as bananas. They grow well in the soil and are found abundantly on decaying organic material. These molds are also present in water and cause infections of the gills, skin, and shells, of sharks, seals, and marine turtles, respectively.
Fusarium species have small microconidia and large fusiform or banana-shaped macroconidia, explaining the genus name1. The macroconidia are distinctive and allow clinical laboratories to define the genus as Fusarium, but further identification to the species level is difficult and is often not routinely performed. Species identification is important for surveillance studies and for defining species-dependent antifungal susceptibility patterns. Although antifungal susceptibility testing gives broad guidance to the choice of drugs, it is not standardized for this genus2. For patients with disseminated infection, correlation of in vitro susceptibility results with clinical outcomes is poor, most likely reflecting the poor immune status of patients with disseminated fusariosis.
EpidemiologyFusarium species occur world wide but, at least for disseminated infection, most cases are reported from large cancer centers in warmer climates in the U.S. and Europe. In the last several decades, increasing reports of disseminated fusariosis have emerged among highly immunosuppressed patients, among whom the highest rates are in those who have a hematological malignancy and are neutropenic or have received an allogeneic hematopoietic cell transplant3-7. Reports from the U.S. have noted the highest concentrations of Fusarium species in the outside air during hotter, more humid months2. Although most cases of disseminated fusariosis are acquired from the outside environment, nosocomial acquisition of this organism related to construction has been documented8. Plants also have been implicated as a source for hospital-acquired infection, and for this reason, live plants are restricted on units in which immunosuppressed patients are cared for. Additionally, there have been reports of hospital water sources, especially showers, yielding Fusarium species on culture9. Although firm proof that showers have been the source of the organism in specific patients is lacking, many transplant units do not allow patients to take showers.
Localized disease is most often related to direct inoculation. This occurs in outdoor workers who experience inoculation of soil containing the organism into skin and subcutaneous tissues. Fusarial onychomycoses are presumably also initiated by local inoculation of organisms from either water or soil. One of the most devastating localized infections results from corneal inoculation by contaminated contact lenses, resulting in severe keratitis.
An outbreak of Fusarium keratitis among soft contact lens wearers in the U.S., Singapore, and Hong Kong from 2004 to 2006 was traced back to a specific contact lens solution (ReNu with MoistureLoc) that was subsequently removed from the market10. The contact lens solution was not intrinsically contaminated, but had lost its usual antimicrobial properties and allowed the growth of several different species of Fusarium in the contact lens cases11. The organisms were introduced by the users themselves, presumably from their hands, and then persisted, most likely in a biofilm in the case and on the contact lens. Introduction of the contaminated lens onto the eye led to destructive keratitis, anterior chamber infection, extension into the vitreous in many cases, and loss of vision.
PathogenesisFusarium species are angioinvasive, a property shared with Aspergillus species and the Mucorales. With invasion through blood vessels, hemorrhagic infarction and necrosis occur. When the conidia are inhaled from the environment, the lungs and the sinuses are primarily affected. This occurs almost entirely in markedly immunosuppressed patients who usually are neutropenic. The depth and length of the neutropenia are important predictors for the development of disseminated infection5,6. Neutrophils are essential for inhibiting hyphal growth, and macrophages can both phagocytize conidia and also attack hyphae. The use of corticosteroids is also a risk factor for development of disseminated infection.
Toxin-induced disease is now rare, but outbreaks have been described in the past in which persons ingesting grain contaminated with toxin-producing Fusarium species developed bone marrow suppression and death, so-called alimentary toxic aleukia. There is no evidence that the mycotoxins produced by Fusarium species contaminating grains play any role in invasive fusariosis.
Clinical manifestationsLocalized InfectionsSkin and Skin Structure InfectionsLocalized infection can follow after local inoculation of Fusarium species into skin or subcutaneous tissues12. In the tropics, eumycetoma has been reported due to F. solani or F. moniliforme, but overall, Fusarium species are not among the most common causes of eumycetoma. In immunocompetent persons who have experienced trauma or who have burn wounds, cellulitis, ulcerative lesions, and abscesses due to fusariosis can occur. Solid organ transplant recipients tend to develop localized nodular or ulcerative lesions of the extremities, likely related to trauma, and not accompanied by dissemination4. There are no specific features in these skin and soft tissue infections that are distinctive for fusariosis.
Although Fusarium species are a less common cause of onychomycosis than dermatophytes, they are an increasingly important cause of infection in immunosuppressed patients, especially those who are neutropenic13,14. In this population, onychomycosis may be the prelude to a painful paronychial infection and resultant cellulitis. Disseminated fusariosis has been reported secondary to Fusarium paronychia in neutropenic patients12.
KeratitisCorneal infection following trauma with introduction of plant material or soil or contamination of contact lenses, presents with pain, photophobia, tearing, and decreased visual acuity. The cornea becomes cloudy and may perforate. The infection often spreads into the anterior chamber, and a hypopyon may be seen. In some cases, extension through the globe into the posterior chamber occurs, resulting in vitritis and retinal damage. Early diagnosis allowing aggressive treatment is imperative to retain vision.
SinusitisFusarium species can cause chronic invasive sinusitis. This uncommon infection occurs in patients who are not immunosuppressed but often are older and have underlying illnesses, such as diabetes15. This form of sinusitis slowly progresses over weeks to months, causing increasing pain and discharge from the nares, but it is not life threatening. Fungus ball formation, which almost always occurs in the maxillary sinus, is uncommonly due to Fusarium15.
In patients who are immunosuppressed, sinusitis due to Fusarium species is an aggressive infection with invasion into bone and adjacent structures of the face. Clinically, the patients present with the acute onset of fever, face pain, and discharge from the nares; necrosis of the palate or nasal turbinates is common because of the angioinvasive nature of the mold. It is indistinguishable from infection with the Mucorales, such as Rhizopus and Mucor species, and Aspergillus species. Dissemination can occur secondary to an initial focus in the sinuses.
Other localized infectionsOther manifestations of fusariosis include infections associated with chronic peritoneal dialysis catheters, osteomyelitis, septic arthritis, and a variety of different visceral abscesses2.
Pulmonary InfectionThe lungs are frequently the primary site of infection with Fusarium species, which is not surprising given that inhalation of aerosolized conidia from the environment is a prime means of spread of these molds. Pneumonia is unusual in immunocompetent hosts, but must always be thought of in immunosuppressed hosts, especially those who have a hematological malignancy and who are neutropenic3,5. Symptoms and signs are similar to those seen with aspergillosis, which is more common. Symptoms include fever, cough, malaise, and dyspnea; pleuritic chest pain and hemoptysis appear as the infection progresses.
Any symptoms suggesting pulmonary infection in an immunosuppressed host should trigger an immediate chest radiograph and computed tomography scan of the thorax. Classic findings on computed tomography scan include those seen with other angioinvasive molds and most commonly include multiple nodules of varying sizes, some of which may be surrounded by ground glass opacification signifying hemorrhage (so-called halo sign), and some of which may show cavitation3,16. Disease may remain localized to the lungs, but more commonly, the pulmonary findings are just one manifestation of disseminated fusariosis.
Disseminated InfectionDissemination is the rule when Fusarium infection occurs in those who are immunosuppressed and especially in those who are neutropenic3,5. Suspicion for disseminated infection is raised when a patient who is febrile and appears severely ill develops painful skin lesions (Fig. 1). The lesions have been characterized by Nucci and Anaissie in their literature review of 232 immunocompromised patients who had fusariosis, 72% of whom developed cutaneous manifestations12. The lesions vary from papules to nodules, with or without central necrosis. They can appear as target lesions or classic ecthyma gangrenosum. Uncommonly, bullae and vesicles appear. The lesions are usually on the extremities, almost always multiple, and can become purpuric or hemorrhagic as they evolve. The crucial clue for the clinician is that these lesions are painful, in contrast to the non-painful lesions of candidiasis and aspergillosis.
DiagnosisThe diagnosis of fusariosis is established by growing the organism from a sterile body site. Frequently, a non-sterile site, such as respiratory secretions or skin, is the source for culture, and for these patients, there must be a compatible clinical picture and/or radiological evidence typical for an invasive fungal infection. Fusarium is one of the few genera of molds that are able to sporulate in vivo. Because of this, they are capable of growing in blood culture bottles, and this may be the first hint that a patient has disseminated fusariosis6. This is a valuable clue for clinicians who usually think that a patient who has an invasive fungal pneumonia has aspergillosis. Aspergillosis is clearly much more common than fusariosis, but Aspergillus species only very rarely can be isolated from blood culture bottles. Growing Fusarium from respiratory secretions, including BAL fluid, in a patient who is immunosuppressed and has computed tomography findings suggestive of invasive mold infection is adequate to make a diagnosis of probable fusariosis and begin treatment immediately.
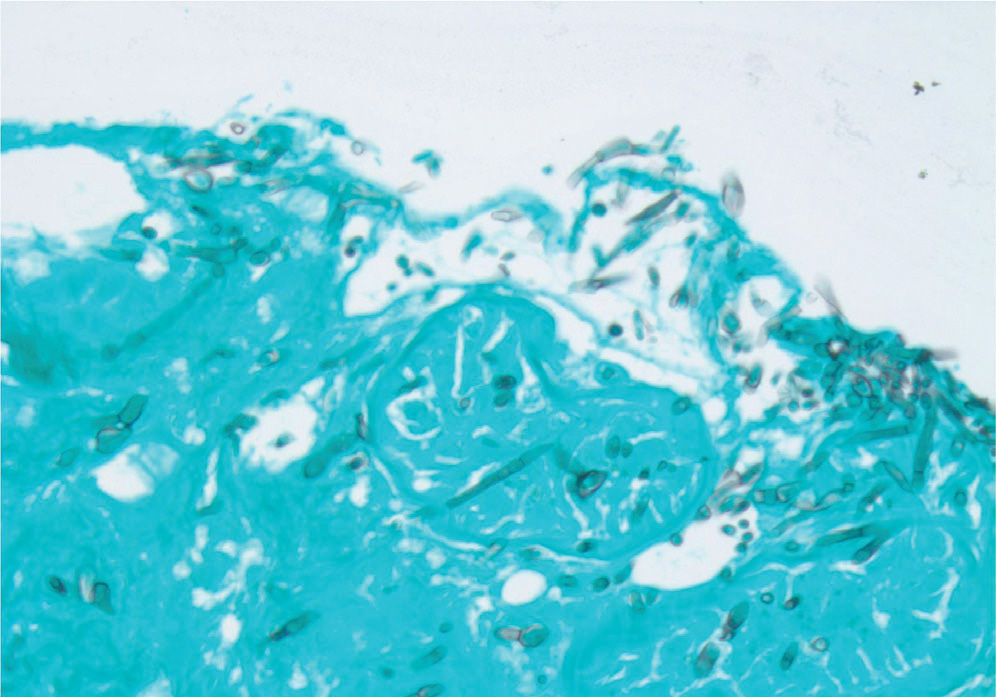
Histopathological examination of biopsy material from skin lesions or pulmonary infiltrates shows acutely branching septate hyphae that are indistinguishable from those of Aspergillus and Scedosporium species (Fig. 2). Cytological preparations from BAL fluid and scrapings from keratitis lesions show similar septate hyphae. Histopathology defines the extent of tissue invasion, but culture is needed to differentiate the specific hyaline mold that is causing disease.
Methenamine silver stain of the biopsy of one of the skin lesions from the patient shown in Figure 1. Acutely branching septate hyphae are scattered throughout the tissue.
There are no antibody or antigen detection tests available to aid in diagnosis. PCR has been used in some cases using universal primers or genus-specific probes for Fusarium in BAL fluid, skin biopsy specimens, and ocular samples6. In situ hybridization has been reported to be useful to differentiate Fusarium from other hyaline molds in a small number of patients17.
TreatmentLocalized InfectionsTreatment of localized Fusarium infections of the skin and subcutaneous tissues is usually successful. If the infection progresses to become a mycetoma, treatment is more difficult, and surgery along with antifungal therapy is often required. Fusarium onychomycosis should be treated with systemic azole therapy. In immunosuppressed patients, especially those who have hematological malignancies and are neutropenic, onychomycosis should be treated as soon as possible by removing the nail and giving systemic antifungal therapy, usually voriconazole (VOR).
The most difficult local infection to treat is keratitis; although the organism is eradicated, visual acuity often remains poor. The standard treatment for fungal keratitis has been topical natamycin, but this has not been effective in many cases of Fusarium infection. With extension into the chambers of the eye, systemic antifungal agents should be used, and most cases require a keratoplasty to remove the infected lens. Increasingly, VOR is used for fusariosis as well as other ocular fungal infections18. Topical VOR as well as oral VOR, at the doses noted below, are used. With extension into the vitreous body, intravitreal VOR is given in addition to systemic therapy.
Pulmonary and Disseminated InfectionsInvasive Fusarium infections are exceedingly difficult to treat because most species are resistant to many antifungal agents2. The most common species that infects humans, F. solani, is the most resistant. The most active drugs appear to be amphotericin B (AMB), VOR, and posaconazole (POS), but the minimum inhibitory concentration required to inhibit growth is higher than that noted for other molds, such as Aspergillus fumigatus. Fluconazole and itraconazole are not active against most Fusarium species, and echinocandins have no activity against Fusarium species. In vitro, terbinafine has activity against some species and based on these data, has been used as an adjunct along with other agents, but it never should be used as monotherapy. In vitro susceptibility studies do not correlate well with outcomes of treatment, and the range of minimum inhibitory concentration values varies for different species and among different laboratories2.
In general, a lipid formulation of AMB, VOR, or POS is used for most patients who have disseminated infection or serious localized infection5,6,19-25. Many clinicians use dual therapy with a lipid formulation of AMB and VOR for patients who have disseminated infection22,24,25.
Amphotericin B is almost always given as a lipid formulation (liposomal AMB or AMB lipid complex) so as to decrease the risk of nephrotoxicity and also to allow an increase in the daily dose. The dosages recommended are 3-5mg/kg daily, but in ill patients, doses as high as 10mg/kg daily have been used. One study noted a partial or complete response in 12 of 26 (46%) patients who received high dose AMB lipid complex23.
A retrospective review of 73 cases of invasive fusariosis treated with VOR found an overall partial or complete response in 34 (47%), most of whom had disseminated infection, and most of whom had received prior antifungal therapy21. The best response rate was seen in patients who were least immunosuppressed, which is not surprising; all patients who remained neutropenic throughout the course of therapy died.
Salvage therapy with POS has been shown effective for some patients. In one series, 10 of 21 (48%) patients achieved a complete or partial response with POS therapy after they had failed or developed intolerance to prior therapy. However, only 20% of leukemic patients with persistent neutropenia responded compared with 67% of leukemic patients whose neutropenia resolved20.
Case reports describing the efficacy of combination antifungal therapy are increasingly published. A review of case reports of patients treated with different combinations of antifungal drugs noted a 70% response to combination therapy24. However, in most of these reports, neutropenia was not taken into account, and it is likely that resolution of neutropenia was the primary determinant of outcome. The largest series reported retrospectively on 37 patients who received combination antifungal therapy. For 27 of these patients, therapy consisted of a lipid formulation of AMB and a triazole. After 90 days of dual therapy, 41% were noted to have achieved a complete or partial response25, which is similar to the response noted with monotherapy in prior studies20,21. Thus, it is not clear that combination therapy is any more effective than monotherapy. However, if one is unsure of the susceptibilities of the infecting organism, which is the usual scenario, it seems reasonable to treat with a combination of drugs in the hopes of having at least one active drug in the treatment regimen.
We generally initiate therapy with intravenous VOR, at a dosage of 6mg/kg twice on the first day, and then 4mg/kg twice daily thereafter, combined with intravenous liposomal AMB, at a dosage of 5mg/kg daily, which can be increased to 10mg/kg daily, if needed. After the patient improves, we step down to oral VOR, 200-300mg twice daily on an empty stomach. Voriconazole levels should be obtained to ensure appropriate serum concentrations are attained. Posaconazole can also be used as step-down therapy (but not initial therapy) at a dosage of 400mg twice daily with food, and levels also should be obtained to ensure adequate absorption, which is often problematic with this drug.
Adjunctive therapy with granulocyte colony stimulating factor has been used in some centers in an attempt to improve the outcome of patients who remain neutropenic2,25. Others have infused granulocytes that were obtained from donors who had received granulocyte colony stimulating factor to stimulate higher numbers of more active granulocytes2.
OutcomesRather than the specific antifungal agent used, the most important factor predicting the outcome of invasive fusariosis is the recovery of the patient's immune function, especially recovery from neutropenia. In several multicenter and single center reports of patients who had hematological malignancies or who were hematopoietic cell transplant recipients, persistent neutropenia and corticosteroid therapy predicted poor outcomes3,5,6,19. Mortality among this type of patient with disseminated fusariosis has been reported to be 81% to 87%2. It is almost always the case that if neutropenia fails to resolve, a patient with disseminated fusariosis will die of this infection.
Patients who are less immunosuppressed and not neutropenic, such as solid organ transplant recipients, have a better outcome than hematopoietic cell transplant recipients. Solid organ transplant recipients tend to have localized skin and subcutaneous Fusarium infections rather than disseminated infection, and they generally recover from their infection4. Immunocompetent patients who have localized inoculation disease have a good prognosis. The worst prognosis for localized infection is noted with keratitis due to Fusarium species; even with a corneal transplant, visual acuity may remain poor.
Conficts of interestThe author declared no conflicts of interest