- •
The outcome from the treatment of invasive fungal infections remains poor for many patient groups, despite the availability of new antifungal agents.
- •
Combining agents offers the potential to improve response and reduce mortality.
- •
This review sets out the case for the use of combination therapy and outlines the settings where it has been shown to be of benefit and where further clinical study may well support evidence from in-vitro and animal studies.
- •
The incorporation of this evidence in the current international guidelines for antifungal therapy is also discussed, emphasising the consensus for the use of combined antifungals in defined infections.
Invasive fungal infection continues to exact a high cost in terms of morbidity and mortality, despite considerable progress in diagnosis and therapy in the latter stages of the last century. Disseminated disease may result in respiratory failure, neurological impairment, or loss of vision, to name just a few of the more common complications, while the survival rate from invasive aspergillosis is less than 20% in some patient groups1. It is, therefore, not surprising that attempts have been made, over the years, to improve the outcome of therapy by combining existing antifungal agents.
A PubMed search using the terms “combination antifungal therapy” produces a list of more than 10,000 papers, although the numbers appear to have plateaued over the last few years. Many of these are reports of in-vitro studies of agents unlikely ever to be used for treating human invasive fungal infections; but even searching for clinical reviews on this topic reveals more than 1500 articles, demonstrating the importance of this concept and the high level of interest in it.
This essay will set out the case for the use of combination therapy, and outline the settings where it has been shown to be of benefit and where further clinical study may well support evidence from in-vitro and animal studies. It will not consider the strategy of combining an antifungal drug with an immunomodulator, although a number of small studies have suggested that such therapy may improve outcome over antifungal therapy alone.
Why combine antifungal agents?The main potential benefits of combination therapy are set out in Box 1. The prime goal is to improve response and reduce mortality. This would be the consequence of a positive interaction between the agents (synergy or addition in pharmacodynamic terms), but the addition of another agent might allow a dose reduction in another. This could be a very worthwhile outcome, given the toxicity of some antifungal agents, particularly amphotericin B (AMB). The in-vitro and animal model evidence for these scenarios will be discussed in more detail later.
Elsewhere in infectious diseases management, the most impressive examples of combination antimicrobial therapy producing improvements in outcome have been with anti-tuberculous and anti-retroviral therapy. Here, using agents with different mechanisms of action preserves the efficacy of others by preventing the emergence of resistance during treatment. This is particularly important during the early phase of therapy where high organism numbers make even low resistance mutation rates liable to lead to the selection of resistant organisms. The use of flucytosine (5FC) alone has been shown to result in the rapid emergence of resistance during therapy of both Candida and Cryptococcus infection2 and its value as an anti-yeast drug is preserved by combining it with another suitable anti-yeast agent.
Emergence of resistance to treatment appears to be less of an issue with other antifungal agents, although obtaining isolates during therapy from deep infection sites is uncommon and so it is possible that some clinical failures are due to selection of resistant organisms. Development of azole resistance during long term therapy of chronic pulmonary aspergillosis is well documented3. Combination therapy might, conceivably, prevent this, but there are very few oral options and the strategy has not been tested in the clinical setting.
Is combining antifungal agents plausible?The question is: do the antifungal agents available for invasive infections have appropriate mechanisms of action likely to have a positive benefit if they are combined? In fact, there are a number of potential mechanisms for positive interaction and these have been extensively reviewed by Johnson and colleagues4.
Amphotericin B increases the permeability of fungal cell membranes which might facilitate the penetration of 5FC5. This is likely to have a benefit in the treatment of fungi with known susceptibility to 5FC, such as Candida species or Cryptococcus neoformans, but it is possible that allowing higher intra-cellular levels of 5FC might have a positive therapeutic effect in fungal infections caused by species considered less susceptible to 5FC, such as Aspergillus species.
The inhibition of an additional stage of a common biochemical pathway is likely to result in a further reduction of the end product. Hence the combination of terbinafine and an azole, acting on squalene epoxidase and lanosterol 14-demethylase, respectively, at different stages of the ergosterol synthetic pathway, should result in a positive interaction.
Inhibition of efflux transport mechanisms by one agent might increase the intracellular levels of another. Johnson has proposed this mechanism for the added benefit of AMB, blocking the efflux of 5FC when the two are combined4.
Targeting of independent sites or receptors is also likely to have a beneficial effect and this has been proposed as the mechanism for a positive interaction between azoles or AMB (acting on the cell membrane) and the echinocandins (acting on the cell wall).
It is also possible that an agent with a rapid kill rate could be followed by another with fungistatic activity, once the fungal burden had been reduced4. Hence sequential combination therapy might facilitate therapy, even if the two agents were not acting simultaneously.
What is the evidence that combination antifungal therapy might be effective?In-vitro dataThe basis for combination antimicrobial therapy was originally established by in-vitro experiments, such as those demonstrating synergy between aminoglycosides and beta-lactams. In-vitro testing of antifungal agents was hampered for many years by lack of standardisation of susceptibility testing. Nevertheless, a number of drug-drug/organism combinations have consistently been found to yield a positive benefit over the individual drugs alone, and this effect has been confirmed in several studies carried out in the modern era of standardised testing.
Potential increased efficacy is demonstrated in the laboratory or in animal models by synergy (the combination produces an effect greater than the sum of the activities of the individual agents), or addition (the effect is equal to the sum of the activities of the individual agents).Whilst the definitions of these may vary in different studies, the principles remain the same.
Traditionally, in-vitro interaction testing has taken the form of liquid or solid media experiments, examining the impact on growth or via time-kill measurements. However, an understanding of the drug targets and the genetic basis for resistance is opening up the way for alternative approaches to screen for potentially beneficial drug combinations. The study of chemogenomics using gene deletion libraries of different fungi enables compounds conferring growth inhibition or cell death associated with particular genes or gene clusters to be identified. Different compounds which inhibit strains with the same gene deletions may act on the same cellular pathways. Using bioinformatics, compounds can be combined in-silico so that many potential combinations can be investigated without the expensive and time consuming laboratory work previously involved6. Promising paired chemogenetic profiles can then be studied by conventional in-vitro and animal models. This approach has revealed a number of new combinations with confirmed in-vitro synergistic effects, including against strains resistant to one of the pair, as well as confirming known synergistic or additive combinations6. One example is the combination of wortmannin with fluconazole (FLU) against FLU resistant strains of Candida albicans. Despite having extremely high MICs to FLU, the addition of wortmannin resulted in a strong cytotoxic effect6.
Hence, it is conceivable that the in-silico testing of new pairs of compounds may result in greatly enhanced activity, which can then be studied in animal models.
The logical combination of AMB plus 5FC against Cryptococcus neoformans has produced variable results in early studies. However, those performed over the last twenty years have demonstrated synergy or indifference7–9. Schwarz and colleagues used several methods to study interactions against 30 clinical isolates of Cryptococcus neoformans and found synergy against 77% of the isolates using a checker board technique, and consistent synergy by time kill studies at 72 hours10. Indeed, this effect has been shown to hold for some 5FC resistant isolates11.
Flucytosine and azole combinations have been found to be indifferent or synergistic in most studies. The findings are method-related, with studies using checker board techniques demonstrating synergy for more than 60% of isolates, but time kill experiments only showing indifference9.
In-vitro studies using Candida species have found positive interactions most consistently for AMB plus 5FC11. In addition, benefits have been shown for isolates resistant to 5FC12.
Micafungin combined with FLU has demonstrated species dependent synergy by the checkerboard method, with the greatest effect seen with Candida albicans13. A positive effect was seen with the less echinocandin susceptible C. parapsilosis strains, and the authors suggest that such a combination would be useful in infections caused by FLU less susceptible strains of this species. However, a number of other echinocandin/azole combination studies have found variable results. Of interest, sequential studies of an echinocandin (caspofungin or anidulafungin) followed by voriconazole (VOR) or posaconazole have shown increased damage to Candida species biofilms when compared with the individual agents alone14.
With MICs generally higher for most agents versus Aspergillus species than for Candida, there is considerable interest in demonstrating a positive effect for antifungal combinations. AMB plus 5FC has shown synergy in earlier studies, but indifference has been found in more recent ones. AMB plus echinocandins have generally shown positive interactions, but are isolate and species dependent to some extent. Extended spectrum azoles have also generally shown a positive effect in combination with echinocandins. Perea and colleagues15 found that a combination of caspofungin plus VOR was synergistic in 86% of 48 clinical isolates of Aspergillus species (24 were Aspergillus fumigatus). Likewise, caspofungin plus itraconazole was synergistic against 30 of 31 clinical Aspergillus isolates using MIC endpoints16. Importantly, no evidence of antagonism was shown in these studies.
Of perhaps greater relevance is the fact that synergy with such combinations has been demonstrated against isolates with characterised resistance mechanisms. Hence, anidulafungin plus VOR was shown to be synergistic against 8 of 10 G448S cyp51A VOR resistant mutants of Aspergillus fumigatus17. This suggests a role for combination therapy when samples are culture negative, but when the possibility of azole resistance exists and also a means of preventing the emergence of resistance on treatment – the exact situation in which combination therapy has advanced the treatment of tuberculosis and HIV infection.
In-vivo dataAnimal models have many caveats, but they do provide the means for examining the efficacy of antifungal agents in eradicating an infection and allowing survival of the host, incorporating the many issues of pharmacokinetics versus pharmacodynamics which complicate the simple mechanism of inhibiting or killing a fungus. The demonstration of positive interactions in-vivo is more likely to be relevant to human infection than laboratory data from experiments using non-physiological conditions.
Amphotericin B plus 5FC have been shown to prolong survival over the individual agents alone in a number of different animal models of cryptococcosis. Schwarz and colleagues demonstrated enhanced survival, as well as reduced fungal colony counts in the brain, lung and spleen, in a systemic mouse model18. A number of studies in mice have also shown a survival benefit and reduction in organ colony counts for combined 5FC and azoles. Thus, Allendoerfer found a reduction in brain colony counts with FLU plus 5FC, as well as a prolongation of survival19. However, the evidence for a positive effect with the combination of AMB plus an azole is mixed11.
In earlier studies, 5FC plus AMB have been shown to improve survival or reduce tissue counts of Candida species. Polak20,21 showed a benefit for the combination in mouse models and Thaler22 did likewise in neutropenic rabbits. More recently, in an immunosuppressed systemic Candida albicans mouse model, Hope and colleagues demonstrated enhanced reduction of fungal burden in the kidneys with this combination23.
However, combining 5FC with azoles has produced less clear-cut outcomes, with high dose FLU in combination producing strain dependent effects on survival in C. albicans infected rabbits24.
Animal model studies of AMB plus FLU have again produced inconsistent results, with a rabbit endocarditis model showing that the combination was not superior to AMB alone, despite high dose FLU being used24. In mice, the combination was worse than AMB alone in terms of survival and kidney fungal burden25. However, in a more recent immunocompromised mouse model, the combination was more effective than FLU alone, and at least as effective as the AMB component26. Interestingly, the same group subsequently showed no benefit for the combination of AMB and itraconazole in mice27.
Combinations of echinocandins with AMB have been studied using several different Candida species, including C. albicans28 and C. parapsilosis29, and all have shown a benefit, although the enhanced effect against C. parapsilosis was lost at higher doses of caspofungin29. Combining echinocandins with azoles in mice does not seem to add to the activity of the echinocandin alone30.
The combination of AMB with an azole has produced mixed results in different models of invasive aspergillosis, but, overall the effect has been one of indifference31. However, echinocandin containing combinations have more often produced a positive effect than indifference and, importantly, they are not antagonistic. AMB plus caspofungin showed a reduction in kidney fungal burden over the individual drugs28, although others have shown no benefit in a central nervous system aspergillosis model in mice32, a setting in which neither drug penetrates well to the site of infection.
Azoles in combination with echinocandins have generally produced a positive effect, although the magnitude of this effect has varied in different studies. Kirkpatrick and colleagues found reduced tissue counts with the combination of VOR plus caspofungin, but no prolongation of survival in neutropenic guinea pigs33, whereas the addition of micafungin to ravuconazole in a neutropenic rabbit model reduced tissue fungal burden and increased survival over either drug alone34. Calvo and co-workers have recently shown that the addition of VOR to anidulafungin prolongs survival in mice infected with three strains of Aspergillus flavus, although there was only a significant benefit for one strain with the combination in comparison with VOR alone35. In addition, the combination had a significant impact on tissue colony counts in comparison with anidulafungin, and was superior to VOR for one strain. It was noted that the combination was comparable with VOR in reducing galactomannan levels.
I have so far concentrated on the common invasive mycoses but there are infections caused by fungi which have high MICs to all the available systemic antifungals. Combination therapy offers the possibility of successful treatment, but is there evidence to support this approach?
One such infection is that caused by Scedosporium prolificans which is becoming an increasing issue in Australia, the US and Southern Europe. In-vitro studies have shown positive interactions between VOR and terbinafine against this fungus36, and with echinocandins, azoles and AMB37. These interactions have been supported by in-vivo studies, including one of a recent murine model of disseminated infection caused by a clinical isolate of S. prolificans. The authors examined the double and triple interactions of AMB, VOR and micafungin and found that micafungin combined with AMB or VOR significantly increased survival and reduced tissue counts in the brain and kidney. However, the triple combination gave no additional benefit.
It is unlikely that randomised comparative controlled trials of combination therapy for this uncommon infection will ever be conducted, but these studies point the way towards appropriate treatment, and the consensus for these novel therapies has now been established for this condition.
Are there consistent effects with particular organism-drug combination interactions?One explanation for a lack of overall consistency in different studies is the likelihood of concentration dependent effects. Hence, delivering the right concentration of each drug to the site of the infection may be crucial in achieving a beneficial effect. Using a response surface model, Meletiadis and co-workers have shown that the interaction of AMB, caspofungin and VOR is extremely complex and antagonism, as well as synergy, may be seen according to the relative concentrations of the different agents38.
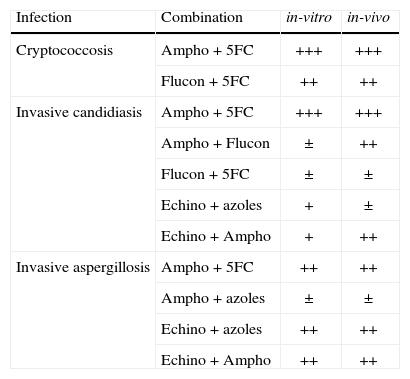
However, a few antifungal combinations have consistently shown positive interactions in both in-vitro and in-vivo studies and these are summarised in Table 2. These have been the ones chosen for clinical trials, but the numbers of trials have been remarkably few. Most studies designed to demonstrate a benefit require the enrolment of hundreds of patients with proven or probable infection. This is a great challenge in the case of invasive aspergillosis (and much more so for the less common conditions such as Scedosporium prolificans infection, previously discussed), where even conducting trials of new single agents for licensing purposes has been extremely difficult.
Relative benefits of different antifungal combinations demonstrated by in-vitro and in-vivo studies.
| Infection | Combination | in-vitro | in-vivo |
| Cryptococcosis | Ampho + 5FC | +++ | +++ |
| Flucon + 5FC | ++ | ++ | |
| Invasive candidiasis | Ampho + 5FC | +++ | +++ |
| Ampho + Flucon | ± | ++ | |
| Flucon + 5FC | ± | ± | |
| Echino + azoles | + | ± | |
| Echino + Ampho | + | ++ | |
| Invasive aspergillosis | Ampho + 5FC | ++ | ++ |
| Ampho + azoles | ± | ± | |
| Echino + azoles | ++ | ++ | |
| Echino + Ampho | ++ | ++ |
One of the earliest combination antifungal studies established the role of AMB plus 5FC in the treatment of cryptococcosis in the pre-AIDS era39. This fulfilled a number of the objectives of combination therapy – increased response rate (although no survival benefit was shown), more rapid sterilisation of the cerebrospinal fluid and less toxicity – despite only enrolling 50 patients. Subsequent studies on HIV positive patients essentially confirmed this benefit. However, perhaps the most informative of these have studied the antifungal activity at the site of infection and demonstrated convincing advantages for the AMB/5FC combination. A multi-national group based in Thailand used the concept of early fungicidal activity (the mean rate of fall in cerebrospinal fluid log CFU counts) in patients treated in a randomised trial with different combinations of AMB, 5FC and FLU. The AMB plus 5FC combination was again superior to the others, and superior to the combination of all three40.
A systematic review of antifungal therapy for invasive candida infection has found no significant difference between the different agents in terms of all-cause mortality41. However, only two randomised trials of combination therapy were included: AMB plus FLU versus FLU42 or VOR43. In the former, standard dose AMB) (0.7mg/kg/day) plus FLU (800mg qd) produced a greater success rate by day 30 (the primary end point), although this was not significant (P=.08). However, the overall success rate was significantly greater in the combination arm (P=.043), and there were significantly fewer episodes with persistently positive blood cultures (6% vs 17%; P=.02), demonstrating the advantage of this approach in the more difficult to treat cases42.
The second study was a sequential, rather than a combination one, but the persistence of the initial AMB is likely to have interacted with the subsequent FLU. However, at the 12 week primary end point there was no significant difference in the clinical and microbiological outcomes or in the percentage of patients with persistent blood cultures.
Hence there is inconsistent and rather sparse evidence to support the use of AMB plus FLU for invasive candida infection – rather like the in-vitro and animal data.
Unfortunately, the more promising AMB plus 5FC combination has not been studied in large trials. A small prospective randomised trial enrolled 72 patients to receive AMB (1.0-1.5mg/kg/day) plus 5FC (2.5g tds) or FLU (400mg qd followed by 200mg qd)44. While there was no significant difference in overall outcome, there was a significant advantage for the combination in the treatment of peritonitis (55% vs 25% success), and in eradication of the pathogen (86% vs 50%). While the authors could be criticised for using lower FLU doses than currently used plus higher doses of AMB, more likely to cause nephrotoxicity, the study does provide some support for the use of combination therapy for the more difficult to treat cases of invasive candida infection.
As already discussed, there have been few studies of combination therapy for invasive aspergillosis, and most of these have been retrospective. A review of more than 6,000 cases of invasive aspergillosis included 249 treated with combination therapy45. The regimens were mostly an AMB product plus either 5FC, itraconazole or rifampicin, accounting for 91% of the total. The response rate was 64% and the mortality 34%, both high in comparison with contemporary and current data for monotherapy.
As noted in the previous sections, echinocandin-containing combinations have shown considerable promise and a number of retrospective studies with historical controls have been published. A small study of 31 patients with haematological malignancies, with proven or probable invasive aspergillosis, and given initial therapy with AMB, investigated salvage therapy with VOR plus caspofungin46. There was a survival benefit (hazard ratio, 0.27; 95%CI, 0.09–0.78; P=.008) in comparison with an historical control group of 16 patients given VOR only.
Several retrospective studies of caspofungin plus liposomal AMB have shown good response rates (up to 75%) suggesting that echinocandin /AMB product combinations should be investigated further in prospective randomised trials47,48. The Combistrat trial has attempted to do just that in a small pilot study49. Thirty patients with proven or probable infection were randomised to receive AmBisome plus caspofungin or high dose (10mg/kg/day) Ambisome alone, with 67% vs 27% (p=.028) responding – similar to the response rates in the retrospective studies.
A much larger RCT of anidulafungin plus VOR versus VOR alone has recently completed50. On the face of it, this study appears to show no significant difference in outcome between the two arms. The mortality at week 6 (the primary end point) in the combination arm was 19.3% compared with 27.5% in the monotherapy arm – a relative risk reduction of 30%, but non-significant (p=.089). Unfortunately, out of the 454 patients enrolled, only 277 were able to be included in the modified intention to treat population for analysis, and this may have rendered the study numbers insufficient to demonstrate a true benefit. Of the 40% that were considered “not evaluable” by the Data Review Committee, 39 died in the monotherapy arm and 26 in the combination arm. This might well have biased the outcome of the study in favour of a non-significant result.
In fact, a subgroup analysis of those patients with positive galactomannan antigen assay results found that 15.7% of those treated with the combined regimen died by week 6, versus 27.3% in the monotherapy arm, a significant reduction (p<.05). This suggests that those diagnosed by other means, who are, therefore, likely to have more advanced disease, are less likely to benefit from the combination than those with a moderately severe infection. This is commensurate with the findings from the Rex candidaemia study42 where the benefit of the AMB plus FLU combination was greatest for those with an intermediate APACHE II score, in comparison with those with mild or severe severity of illness.
There was no difference in mortality between the different treatment groups in patients who had received an allogeneic stem cell transplant and those receiving other therapy. Importantly, this study showed no excess of treatment-related adverse events with the combination regimen. The majority of adverse events were considered to be related to VOR in both arms50.
Hence, overall, this trial showed that an echinocandin/triazole combination is a safe treatment regimen, likely to improve outcome in moderately severe invasive aspergillosis in patients with haematological malignancies, the group with the highest mortality1.
What do the guidelines say?The current guideline from the Infectious Diseases Society of America (IDSA) for the management of cryptococcosis recommends the combination of AMB plus 5FC for initial therapy of meningoencephalitis and for disseminated or severe infection at other sites51. For HIV-positive patients, the World Health Organisation guidelines are very similar, again emphasising the benefit of combination therapy in this setting52.
The principle international guidelines for the management of invasive candida infection are those of the IDSA53, the European Conference on Infection in Leukaemia (for patients with haematological malignancies)54, and the recent European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group Diagnostic & Management Guideline for Candida Diseases 201155. There are, of course, a number of national guidelines providing recommendations on candida infection therapy.
The IDSA guidelines recommend monotherapy for almost all indications, but consider the combination of 5FC with an AMB product as primary therapy for central nervous system infections, candida endophthalmitis and endocarditis, although all of these are based on evidence rated as level III: “Evidence from opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees”53.
The European Conference on Infection in Leukaemia guidelines have made no recommendations on combination therapy for neutropenic patients, although it is noted that there is a paucity of data on candidaemia therapy in these patients54.
The Fungal Infection Study Group guidelines, again recommend monotherapy for the majority of indications, but, like the IDSA, consider combinations including 5FC as appropriate for central nervous system infections, endocarditis and endophthalmitis, with a moderate strength of recommendation and level of evidence55.
All the international guidelines dealing with the management of invasive aspergillosis, of course, pre-date the recently presented VOR/anidulafungin trial and so are based on relatively little evidence. The IDSA do not recommend combination therapy for routine primary therapy, but make a BII recommendation for unspecified combination therapy in the salvage setting56. The European Conference on Infection in Leukaemia guidelines likewise recommend against combination therapy for first line treatment, but rate as CII a recommendation of caspofungin plus either VOR or a lipid–associated AMB product for salvage treatment. It will be interesting to see how the Marr study will impact on these recommendations.
ConclusionsThis has been an article supporting the concept of combination therapy in certain clinical settings and it has, therefore, been selective, to some extent, in citing data showing a positive benefit. The con debate will almost certainly do the opposite (perhaps even from within the same studies) and, of course, the truth lies somewhere in between these two extremes.
There is clearly variable evidence for improved efficacy. We need to choose the settings where combination therapy is likely (and needed) to make a difference. These should be when there are infections with difficult to treat organisms and/or in certain anatomical sites, where evidence suggests that combination will provide an improved outcome. We should not expect combination therapy to be a universal principle. There are relatively few indications for combining agents when treating bacterial and viral infections, and we must accept that the same goes for invasive fungal infections.
Differences in outcome with different animal models and with different strains of the same species suggest that PK/PD issues are extremely important for some combination/organism interactions. Optimisation of outcome may require more individualisation of therapy which could be delivered by means of pharmacogenomics and fastidious TDM management in the future. In the meantime, there is an international consensus for the use of combination antifungal therapy for the major opportunistic infections in the specific settings outlined in this article.
Conflict of interestsThe author has received honoraria and/or research grants from Astellas, Gilead, MSD, and Pfizer.
I would like to thank JCT Kibbler for help with earlier drafts of the manuscript.