The amounts of total and sorbed A1, Ba, Cr, Fe, Mn, Ni, and V present in three sediment cores from the South of the Gulf of Mexico (N1, N2, and N3) were determined. Of the three samples studied herein (N1. N2, and N3), one of them (N2) is associated to a natural “chapopotera”. ICP/MS analyses show that total concentrations of Al, Fe, Mn, Ba, Cr, Ni, Pb, and V for N1 and N3 were found to be ca. 4.2, 2.3, 206.5, 165, 91, 72, 14 and 97mg kg−1, and ca. 5.2 (Al2O3), 4, 401.50, 269, 89, 62, 18, and 118mg kg−1 for N2. As evidenced by the correlation matrices, there is a positive association among metals distribution, except for the case of Mn, regardless of the sediment core. Nevertheless, Cluster and Principal Components Analyses denote variability in metal-spatial distribution, signature variability in the composition of the water column. Magnitude values for the enrichment factor (EF) relative to background values found in the Southern Gulf of Mexico (bulk seawater) for Al, Fe, Mn, Ba, Cr, Ni, Pb, and V were estimated. The outcome of this work show distinctive EF values, ranging from moderate to high EF values, regardless of the core source.
Se determinaron las cantidades total y adsorbidas de Al, Ba, Cr, Fe, Mn, Ni, y V presentes en tres núcleos de sedimentos del Sur del Golfo de México (N1, N2 y N3). De las tres muestras estudiadas en el presente documento, una de ellas (N2) se asocia a las chapopoteras naturales. El análisis en ICP/MS demuestra que las concentraciones totales de Al, Fe, Mn, Ba, Cr, Pb y V para el N1 y N3 son 4.2, 2.3, 206.5, 165, 91, 72, 14 7y 97mg kg−1, y para N2 son 5.2 (Al2O3), 4, 401.50, 269, 89, 62, 18, and 118mg kg−1. Como se evidencia por las matrices de correlación, existe una relación positiva entre la distribución de los metales excepto para el Mn, independientemente del núcleo de sedimentos. Sin embargo, el Cluster y análisis de componentes principales denotan la variabilidad de la distribución espacial del Mn, la cual se confirma en la columna de agua. También se calcularon valores de magnitud para el factor de enriquecimiento (EF) de Al, Fe, Mn, Ba, Cr, Ni, Pb, y V, relativos a los valores del fondo que se encuentran en el sur del Golfo de México (mar grueso). Finalmente, el resultado de este trabajo muestra valores y comportamiento distintivos del EF, que van de moderados a altos, independientes de su fuente principal.
Metals are pivotal to biogeochemical processes, thereby the importance of understanding their partitioning mechanism(s) in marine sediments; particularly so in either mineral surfaces or biological matrices. In the former case, metal sorption in marine sediments will vary depending on pH, redox potential, and the composition of suspended solids (Kitano and Fujiyoshi, 1980; Hakansson et al., 1989; Calmano et al., 1993; Wiechula et al., 2000). In the latter case, metals will interact with organic matter (humic acid), (HS−, S=), and react with other metals, prior to entering to the trophic chain (Ford and Ryan, 1995; Bosecker, 1997; Schippers and Sand, 1999; Gutjahr et al., 2007; Sparrevik et al., 2009; Scholz et al., 2009). Three main rivers, namely, Grijalva, San Pedro, González, Tonalá, and Coatzacoalcos drain to the South of the Gulf of Mexico. These rivers carry high contents of suspended solids. Such high turbidity has been attributed in part to changes in the land use and deforestation (Vázquez et al., 2006; Vela, 2005). In particular, recent reports provide evidence to show water inflows from coastal rivers and lagoons containing suspended solids concentrations as high as ca. 10439 ton d−1 (PEP-UNAM, 2008; Scholz et al., 2009), with chemical components found in the deep zone, ≥ 3,000m. The presence of high amounts of suspended material has been explained by the inflows of the Coatzacoalcos and Grijalba-Usumacinta rivers in light that marine currents are responsible for distributing particulated coastal supplies into the deep zone (Vazquez and Alexander, 2008). Other anthropogenic-borne sources of particulated material prevailing in this geographical region include the presence of the oil industry and imminent natural oil spills commonly referred as “chapopoteras”, marine-transit discharges, or atmospheric precipitation of Pb, Ni, Ba, V, SOx and NOx particles (Prospero, 1999; Prospero et al., 2004). All these input sources ensure the continuous accumulation of metals at the sediment-water pore interface. The purpose of this work was to study the spatial distribution of Al, Ba, Cr, Fe, Mn, Ni, Pb and V, in three distinct sediment environments in from the South of the Gulf of Mexico. The study was conducted to better understand metals partitioning as characterized by Cluster and Principal Component Analyses based on Enrichment Factor (EF) determinations.
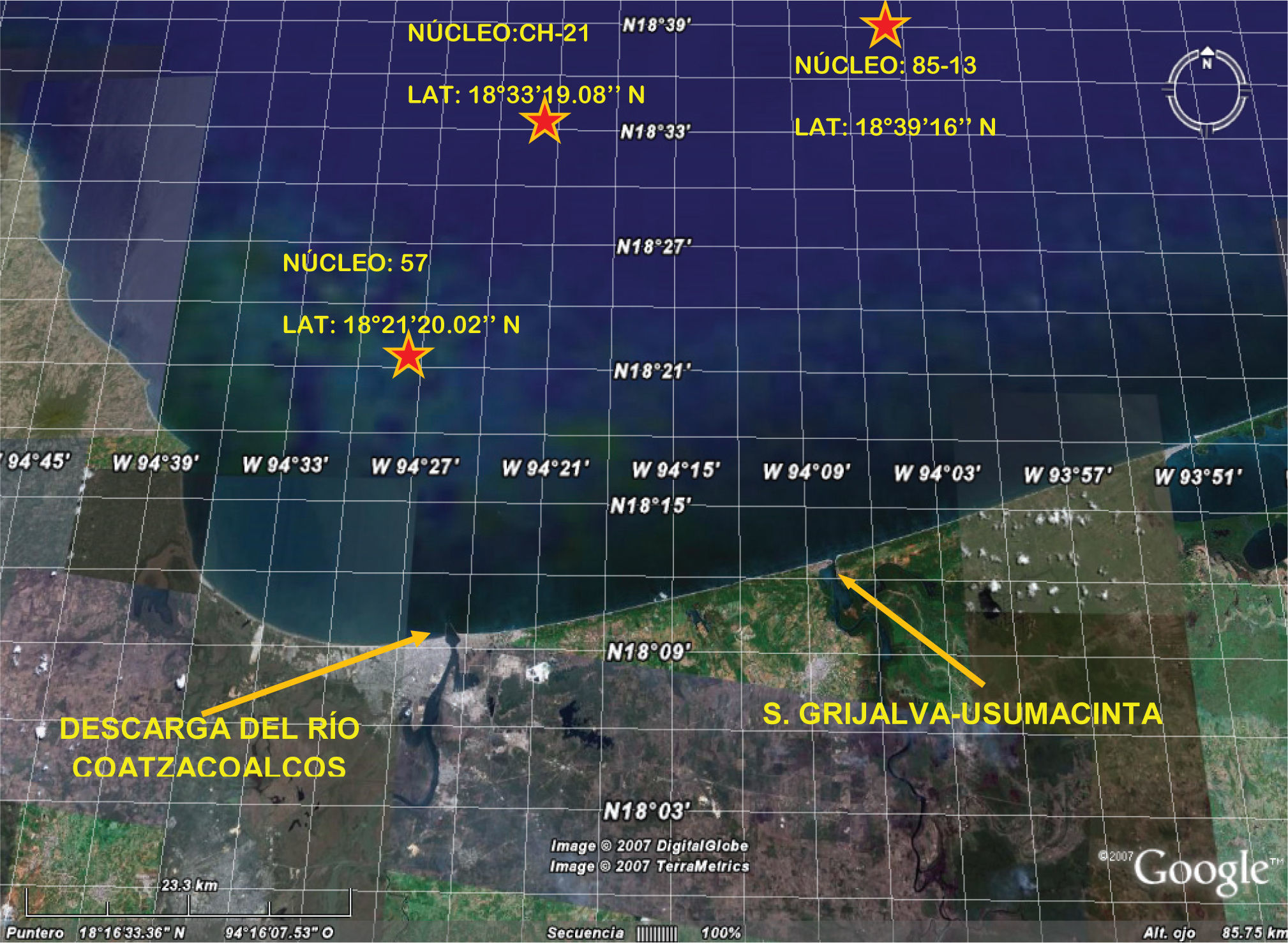
Material and methodsThree sediment cores were collected on board of the B/O (Oceanographic Vessel) Justo Sierra which belongs to the Universidad Nacional Autonoma de Mexico (UNAM). The collection sites for the sediment cores were located at the forefront of the Coatzacoalcos and Grijalva-Usumacinta. The site was affected by a natural oil spill (Figure 1).
The cores (N1, N2, and N3) were collected in 0.01m3 boxes. Each core was 12cm in diameter. The cores thickness varied from 12 to 31cm. The cores were collected using hydraulic PVC tubes. The tubes were washed with an acid solution and water Type III (millipore, Milli-Q) prior to use. The sediment cores were stored in plastic bags right after collection. Plastic bags were opened right before use to prevent oxygen diffusion and a subsequent plausible metal-oxidation. In all samples, the supernatant water from the core top was removed. The cores were then sealed and cap-closed using Teflon cylinder(s). All cores collected were kept at 4°C prior to analyses (Figure 1).
The sediment samples were obtained by sectioning the cores at 0.5-cm regular intervals for N1 and N2, and 1.0-cm regular intervals for N3. Twenty six samples for N1, 64 for N2, and 30 for N3 were obtained. The depths selected for this study were 2100 (N2), 250 (N1), and 15m (N3). Sections of dry sediment were homogenized with an agate mortar and stored in high-density plastic jars. For determining total-metals contents, dry-sediment samples were digested in a 1:1 HCl: HNO3 mixture inside a CEM MARS 5 microwave, according to a method reported by Tessier et al. (1979); De Lorenz (2004); Vázquez et al. (2004), and (2006); PEP-UNAM, (2008). Metal quantification was conducted using an ICP/MS and Atomic Absorbance equipped with an impact cell and an automatic sampler. All experiments were analyzed randomly and by duplicates. The analytical recoveries relative to standard solutions (High-purity standards; Lot number 830702, Cat. Number CRM-MS-S) for total-metal determinations were 91.5% for Al; 94.5% for Ba; 98.4% for Cr; 99.5% for Fe; 95.4% for Mn; 95% for Ni; and 97.5% for V. For sorbed metals determinations, the analytical recoveries corresponded to ca. 96% for Al; 93% for Ba; 87% for Cr; 98% for Fe; 85% for Mn; 91% for Ni; and 95 % for V.
Results and discussionOur results show atypical values for all metals studied. The magnitude values for the concentrations of total metals revealed a broad distribution for FeT (total Fe), MnT, BaT, and AlT. Mean values obtained indicate a higher data dispersion for the 50 and 75% quartiles, along with a concomitant negative data slanting. A comparison of the degree of data dispersion for the contents of sorbed and total metals for N1 shows a greater magnitude value for the contents of sorbed metals. A comparison between data trends obtained for N1 and N2 shows a difference in the distribution of total metals perhaps because of its proximity with natural “chapopoteras” (Scholz et al., 2009). The concentrations of MnT are higher in magnitude for N2. Also, the distribution of sorbed metals was also found to vary from N1 to N2. The distribution of the data for sorbed metals in N2 was found to present a greater dispersion. This is particularly the case for either sorbed Ni (Nia) or V (Va), arguably due to the presence of “chapopoteras”. Also, sorbed Fe (Fea), Mn (Mna), and Ba (Baa) were found to present lower magnitude values in N1 compared to N2 (S1, Supplementary Information Section).
Overall data trends showed higher dispersion values for N1. Finally, the amounts of Fe, either in dissolved (Fes) or sorbed (Fea) form, were found to surpass in magnitude those registered for any other metal. This was found to be the case in either N1 and N2. Total Ba (BaT), Cr (CrT), Pb (PbT), and Mn (MnT) magnitude values were found to be similar in N2 and N3. By contrast, total Fe (FeT), Ni (NiT), and V (VT) magnitude values in N3 were found to be higher relative to those found for N1 or N2. Total Fe (FeT), Mn (MnT), Ba (BaT), and V(VT) mean ± S.E. values indicated a greater data dispersion between the 50 and 75% quartiles, along with a concomitant negative slanting of the data (S2 and S3, Supplementary Information Section).
The partitioning behavior of metals in the environment depends on the enlargement on their intrinsic reactivity, as well as biogeochemical conditions. In particular for Al, a conservative element, the environmental fate is constrained by diffusion and precipitation-dissolution reactions processes (Caschetto and Wollast, 1979; Carroll et al., 2002). For these reasons, various researchers have suggested the usage of Al as an indicator of anthropogenic activity (Rubio et al., 2000; Rubio et al., 1998; Covelli and Fontolan, 1997; Balls et al., 1997; Marcet et al., 1997; Ryan and Windom, 1988). Pertinent herein is the fact that Al is found in abundance in native clay minerals from the South of the Gulf of Mexico (Scholz et al., 2009; PEP-UNAM, 2008; Vázquez et al., 2000). The variability observed for the Cr distribution among sediment cores can be attributed in part to the presence of either organic matter (Salomons and Förstner, 1984), iron and manganese oxides (Williams et al., 1974; Vázquez et al., 2000, 2004 and 2006), or iron-calcareous residues (Jickells and Knap, 1984). In deep zones, Cr becomes immobilized when reacting with aqueous Fe (II) (Lan et al., 2006). Iron reacts to form insoluble sulfides (Hurtgen et al., 1990; Carol et al., 2002). Data showing high concentrations of soluble iron, however, is explained by the sorption-desorption processes coupled with metal complexation by bioorganic compounds. The variability observed for the amounts of soluble Mn is explained in part by the formation of manganese oxides or manganese organic-matter complexes. Given that the sampling sites are biologically active (Anschute et al., 2005), we do not discard the notion that Mn (III) becomes the predominant species. The variability observed among the contents of Ni can be explained by the interaction(s) with sulphide species or organic matter (Ni-OM); bioturbation; desorption; migration of Ni from the deep zone to the surface; bio-transformations; or the ionization of NiCO3 species at the core deep zone (Williamson and Wilcock, 1994). Variations in Pb accumulation observed in the core deep zones can be explained in part by the formation of insoluble Pb sulfides, Pb-organic matter complexes (Huerta and Morse, 1992; Vázquez et al., 2004), or PbCO3. Nevertheless, the high amounts of source Pb observed throughout the core are an indication of undersaturation with respect to pyrite or other insoluble sulfides (Huerta and Morse, 1992). The variability in the amounts of soluble V registered can be explained by the complexation with organic compounds originated the nearby “chapopoteras” (Scholz et al., 2009). Vanadium is found in the environment primarily as V(V). Vanadium (V) interacts with a variety of organic compounds including humic and fulvic acids, and minerals, most importantly with iron oxides (Van and Jawoski, 1980).
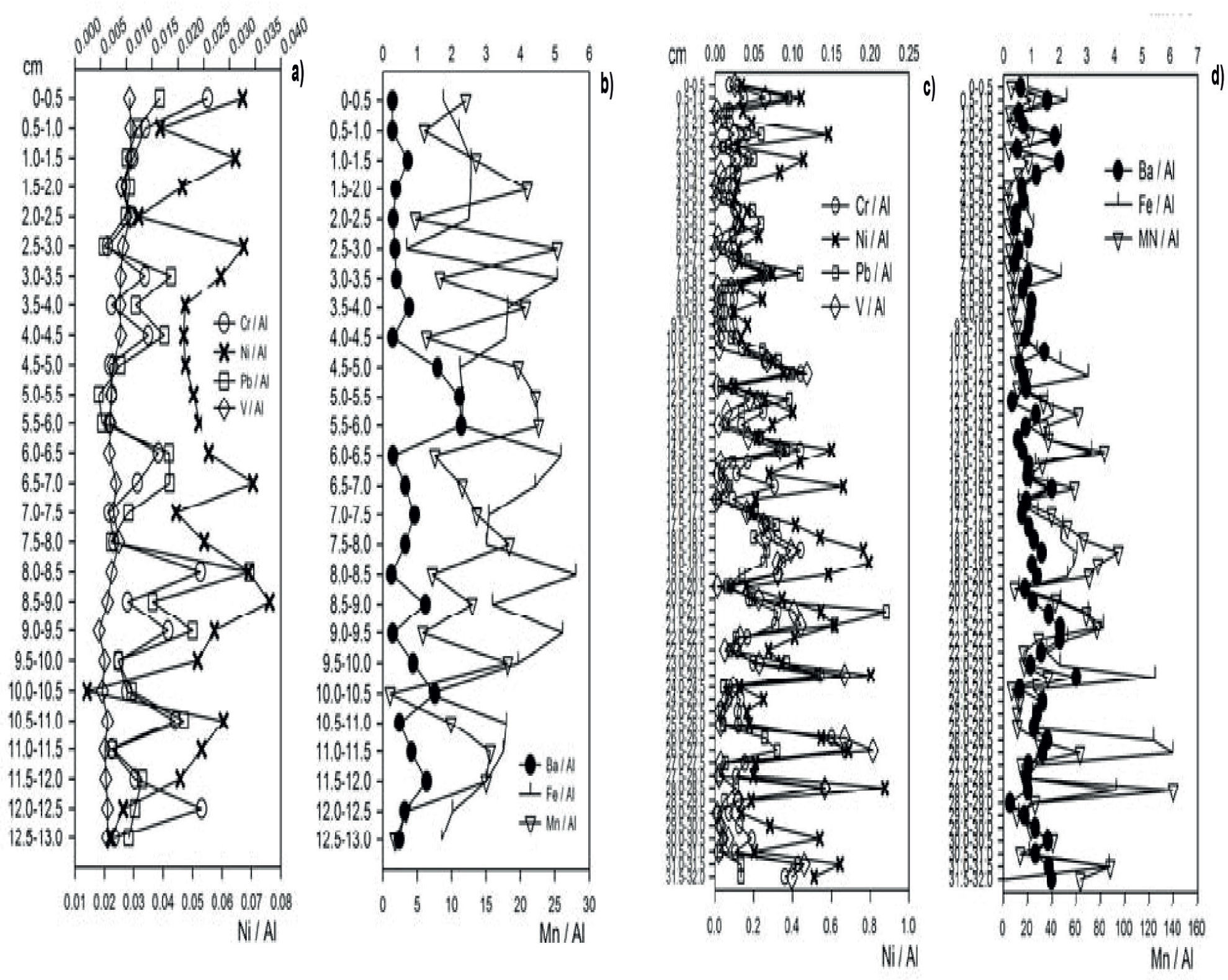
Metal Distribution and Principal Component AnalysesCore N1. The normalization of total metals data trends for Cr (CrT), Ni (NiT) and V (VT) in N1 did not show significant changes because anthropogenic activities provided the lack of variation with depth (Figure 2). On the other hand, normalized BaT values present changes throughout the core perhaps due to the supplies of barite (BaCO3; PEP-UNAM, 2008). Normalized FeT and MnT values show changes along the core. Variations in the concentration of these redox-active metals (Stumm and Morgan, 1996) is related to diagenetic processes, which are strongly dependent on the concentration of dissolved O2, pH, and EH values (Finney and Huh, 1989). At the same time, the high FeT and MnT values reported (Figure 2) in the core-upper layer(s) is consistent with inputs from the Coatzacoalcos River.
Normalized FeT and MnT values show changes along the core. Variations in the concentration of these redox-active metals (Stumm and Morgan, 1996) are related to diagenetic processes, which are strongly dependent on the concentration of dissolved O2, pH, and EH values (Finney and Huh, 1989). At the same time, the high FeT and MnT values reported (Figure 2) in the core-upper layer(s) are consistent with inputs from the Coatzacoalcos River.
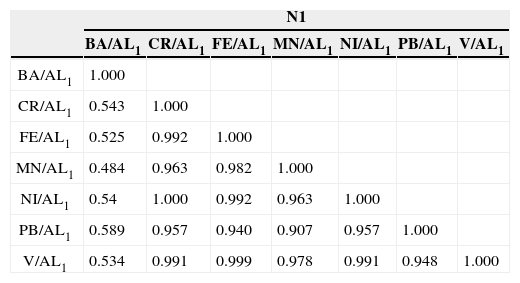
A significant statistical correlation (p<0.05, n=26) (Table 1) between normalized total metals at 0–13cm depth becomes evident. These results further confirm the idea of the existence of a significant supply of suspended solids from the Coatzacoalcos River and/or atmospheric particles (PEP-UNAM, 2008; Vázquez et al., 2000). Cluster and Principal Components Analyses for total metals (S4, Supplementary Information Section) further suggest the prevalence of similar geochemical processes occurring at different core depths. Likely, a non-homogeneous distribution of organic matter of showed about microenvironments with varying “redox” potential values.
Multiple correlation for total and sorbed metals
| N1 | |||||||
|---|---|---|---|---|---|---|---|
| BA/AL1 | CR/AL1 | FE/AL1 | MN/AL1 | NI/AL1 | PB/AL1 | V/AL1 | |
| BA/AL1 | 1.000 | ||||||
| CR/AL1 | 0.543 | 1.000 | |||||
| FE/AL1 | 0.525 | 0.992 | 1.000 | ||||
| MN/AL1 | 0.484 | 0.963 | 0.982 | 1.000 | |||
| NI/AL1 | 0.54 | 1.000 | 0.992 | 0.963 | 1.000 | ||
| PB/AL1 | 0.589 | 0.957 | 0.940 | 0.907 | 0.957 | 1.000 | |
| V/AL1 | 0.534 | 0.991 | 0.999 | 0.978 | 0.991 | 0.948 | 1.000 |
| N2 | |||||||
|---|---|---|---|---|---|---|---|
| BA/AL2 | CR/AL2 | FE/AL2 | MN/AL2 | NI/AL2 | PB/AL2 | V/AL2 | |
| BA/AL2 | 1.000 | ||||||
| CR/AL2 | 0.873 | 1.000 | |||||
| FE/AL2 | 0.911 | 0.982 | 1.000 | ||||
| MN/AL2 | 0.166 | 0.237 | 0.229 | 1.000 | |||
| NI/AL2 | 0.856 | 0.968 | 0.961 | 0.380 | 1.000 | ||
| PB/AL2 | 0.888 | 0.942 | 0.935 | 0.172 | 0.892 | 1.000 | |
| V/AL2 | 0.872 | 0.984 | 0.987 | 0.273 | 0.972 | 0.914 | 1.000 |
| N3 | |||||||
|---|---|---|---|---|---|---|---|
| BA/AL3 | CR/AL3 | FE/AL3 | MN/AL3 | NI/AL3 | PB/AL3 | V/AL3 | |
| BA/AL3 | 1.000 | ||||||
| CR/AL3 | 0.807 | 1.000 | |||||
| FE/AL3 | 0.878 | 0.982 | 1.000 | ||||
| MN/AL3 | 0.177 | 0.262 | 0.299 | 1.000 | |||
| NI/AL3 | 0.769 | 0.971 | 0.972 | 0.419 | 1.000 | ||
| PB/AL3 | 0.800 | 0.958 | 0.928 | 0.119 | 0.875 | 1.000 | |
| V/AL3 | 0.813 | 0.992 | 0.989 | 0.321 | 0.990 | 0.924 | 1.000 |
Our results for absorbed metals provide an indication that the accumulation of Cr (Cra) and Ni (Nia) is best explained by anthropogenic activities (Figure 3). With respect to Baa, Fea, Mna, Pba and Va, visible changes are present along the core. Correlations with statistical significance (p<0.05, n=64) (Table 1), except for Ba (Baa) become evident, in consistency with the anthropogenic activities from the petroleum industry.
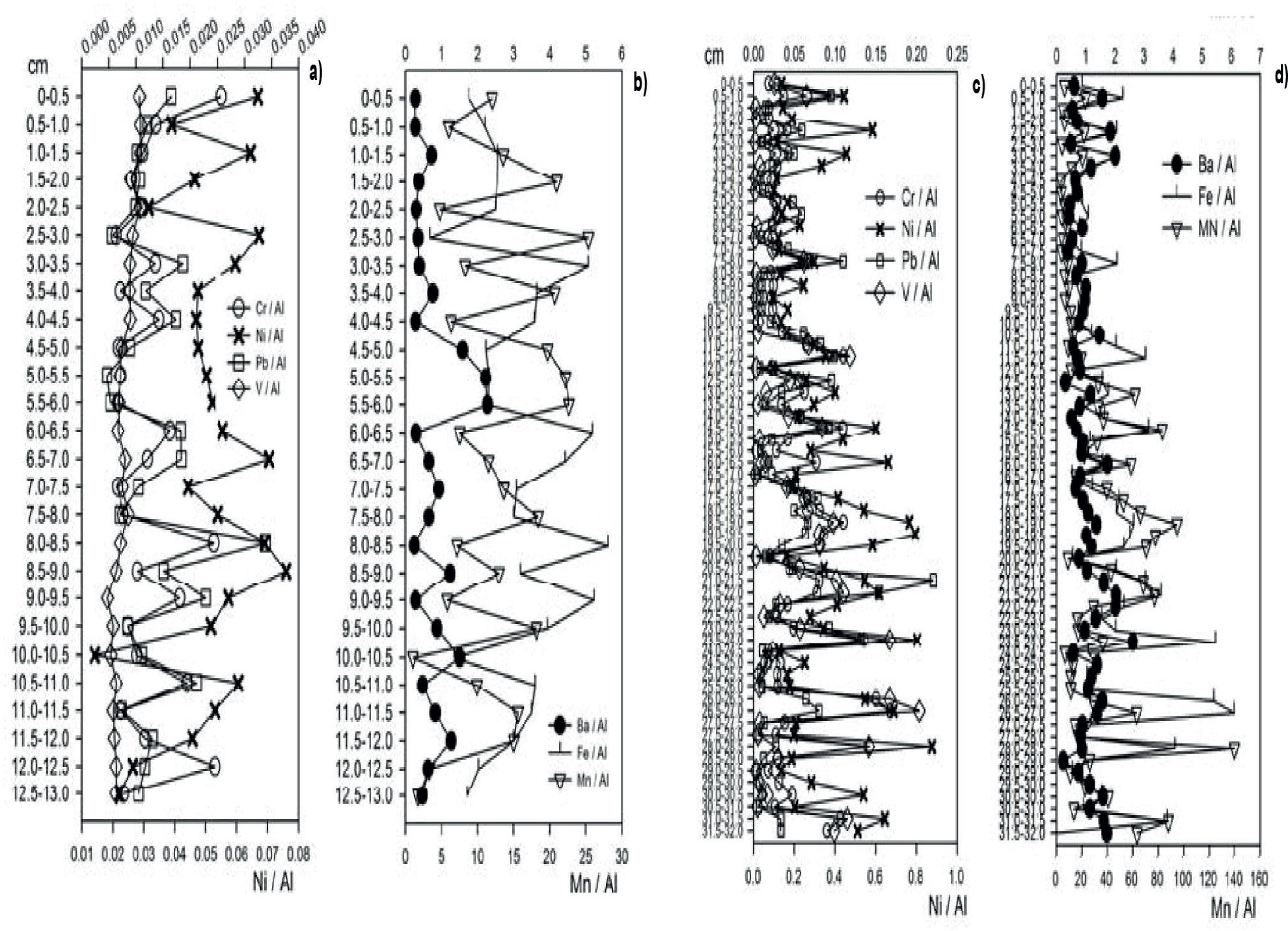
Core N2. Cluster and principal components analyses of normalized data for total metals in N2 are explained because prevailing similarities between geochemical processes with depth. Data conglomerates become evident (S4, Supplementary Information Section). Normalization on the accumulation of total metals show similar changes for all metals with depth (Figure 3) A high correlation of normalized-total metals, except for BaT and MnT (p<0.05, N=64) (Table 1) (PEP-UNAM, 2008) was observed.
Normalization of data trends for sorbed metals show significant changes along the core. Data variability becomes more notorious compared to N1. This is particularly true for the cases of Baa and Fea, The close proximity of natural “chapopoteras” and high concentrations of oil derivatives is thought to influence the compartmentalization of metals.
Magnitude values for Fea serves as an indication of metal mobility with depth. By contrast, obtained values for Cra and Nia suggest variations to a lesser degree and, thereby, a lower mobility. Particularly so, for the case of Nia. Hence, natural “chapopoteras” have an effect on the environmental mobility and compartmentalization of Cra and Nia. Variations in Va magnitude values determined along the core is in agreement with vanadium input originated from natural “chapopoteras”, arguably in the form of either vanadium-porphyrin or vanadium-non-porphyrines complexes (Fish and Komelenic, 1984). The behavior of Pba denotes little changes along the core, with accumulation in the deep zones only (Balsam et al., 2007).
Our results show a lack of correlation between normalized absorbed metals data trends (p<0.05; n=64). However, data trends for Va show a significant correlation with data trends for Cra and Fea, in consistency with the idea of supplies in the deep zone originated from the Grijalva River (Table 1; PEP-UNAM, 2008; Vázquez et al., 2000).
Core N3. Normalization of data trends for total metals show variations for the Fe/Al and Mn/Al ratios, in consistency with normalized data trends for N1. These findings can be explained in part because both zones receive water supply from the Grijalva River (Figure 1). Perhaps the decreases in magnitude values observed can be attributed to reactions between Fe and Mn with HS-, S=, and/or other metals (Stumm and Morgan, 1996).
The Ba/Al ratio shows variations probably because the formation of Ba-carbonates (e.g., BaCO3) or sulfates (BaSO4; Hurtgen et al., 1999). The Cr/Al ratio presents similar variations, with no indication of the presence of Cr in the beaches of Tabasco (Vázquez et al., 2006; Fuschsman et al., 2007). The Ni/Al and V/Al ratios present similar variations along the core and in agreement with those observed for N1. The Pb/Al ratio in the top (5 to 6cm) show high values that may be a result of an unusual Pb supply originated from either the Grijalva River, the atmospheric supplies, or the oil-industry related activities (Varela, 2007). Furthermore, a correlation analyses of normalized data for total metals, namely Fe/Al with Mn/Al, Ni/Al with V/Al, and Ni/Al with V/Al were found to be statistically significant (p<0.05; n=24; Table 1). These results further confirm an influence of sediments originated from oil wells. Additional cluster and principal components analyses for normalized data of total metals reveal four conglomerates formed by the Fe/Al, Mn/Al, Ni/Al and V/Al ratios, while those presenting no statistical significance were found to be the Pb/Al and Ba/Al ratios (Balsam et al., 2007), (S4, Supplementary Information Section).
Cores N1, N2, and N3. Principal components analyses of total metals data sets for the three cores show three different groupings (S5, Supplementary Information Section). The core N3 shows a metal grouping, with the exception of Pb/Al and Ba/Al. A second group of conglomerates in N2 is observed, with the exception of Ba/Al. A third metal conglomerate becomes evident for N1, with the exception of Mn/Al. The later results are explained in part by a higher input of Mn to N1 relative to N2 or N3 originated from heterogeneous-industrial activities at the Coatzacoalcos River vicinity (Vázquez et al., 2000; PEP-UNAM, 2008; Geissen et al., 2009).
Finally, normalized data trends obtained for N1 were found to be similar to those reported for the time series of stations near the influence of the Coatzacoalcos River (PEP-UNAM, 2008). Likewise, normalized data trends obtained for N2 were found to be similar to those reported for the time series for stations near the “chapopoteras” (PEP-UNAM, 2008). Normalized data trends obtained for N3 were found to be similar to those reported for the time series of stations near the Grijalva River (Vázquez and Virender, 2004; Vázquez et al, 2006).
Metal enrichment factors in marine sedimentsDiverse methods have been proposed for evaluating the degree and enrichment of metals in marine sediments (Salomons and Förstner, 1984; Abrahim and Parker, 2008). The simplest method for estimating the level of pollution factor involves the normalization of concentration values to grain size (Rubio et al., 2000). The Enrichment Factor (EF) is calculated on the basis of the AIT (where AlT is total aluminum and AlL is lixiviate aluminum) values according to supplementary information (S6 Supplementary Information Section).
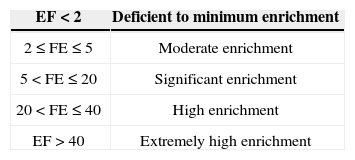
Calculated EF values are classified according to Table 2 (Kumar and Edward, 2009).
Classification of EF values according to Kumar and Edward (2009)
| EF<2 | Deficient to minimum enrichment |
|---|---|
| 2≤FE≤5 | Moderate enrichment |
| 5<FE≤20 | Significant enrichment |
| 20<FE≤40 | High enrichment |
| EF>40 | Extremely high enrichment |
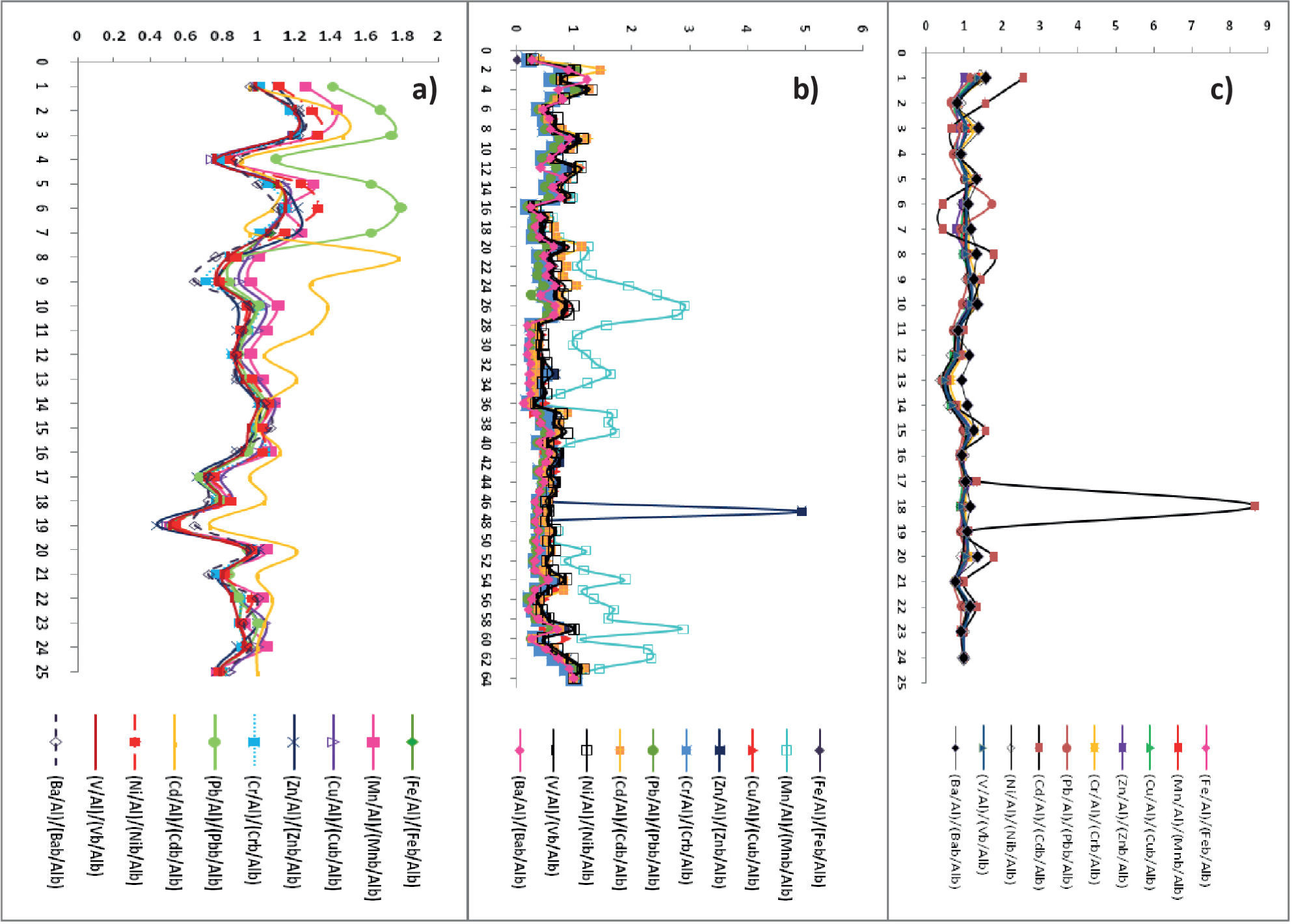
Our results for N1 (Figure 4) show that Cr and Ni present a minimum enrichment, Fe and V a moderate enrichment, and Ba, Mn and Pb a significant enrichment. The significant enrichment of Ba, Mn and Pb can be explained by industrial supplies and natural drainage received from the Coatzacoalcos River. Except for the case of Ba, data trends for all metals show decreases in EF values with core depth (Figure 4).
Results for N2 at ca. 0.5 to 7.5, 18.0–27.0, and 30.0–32.0cm depth indicate high EF values, except for the case of Mn (Figure 9). These results can be explained by the manganese oxides in organic matter mineralization or secondary environmental reactions (Luther et al., 1997; Hulth et al., 1999; Anschute and Blanc, 1995; Anschute et al., 2005).
Results for N3 show EF values corresponding to significant-to-high enrichment (Table 2; Figure 4). These results are all based on the proximity of the coast to the sampling site; therefore, EF values provide an indication of the influence from the Grijalva River on metal accumulation in sediments. Barium, Fe, Pb, Ni, and V showed EF values that correspond to the category of significant enrichment, and manganese to the category of high enrichment.
These results may not be attributed to anthropogenic activities, but to the diagenetic processes instead. In contrast, chromium showed and EF value that correspond to the category of high enrichment. We explain these latter values due to the presence of chromite in the coasts of Tabasco (PEP-UNAM, 2008). The EF values determined in N3 for Cr were found to be high relative to those determined in N1 or N2 (Figure 4).
ConclusionsThe metals were normalized with respect to Al, which maintains a natural change in the south of the Gulf of Mexico. The analysis of conglomerate and principal components allowed us to compare metal the three sediment cores obtained from in close proximity to the Coatzacoalcos River, a natural “chapopoteras” and the Grijalva River. Analyses of EF values showed that metals present with the same core can present diverse enrichment behavior. Value for EF of Mn in N2 is consistent with a significant enrichment not attribute to anthropogenic activities (PEP-UNAM, 2008). Values EF for Mn accounts only import to Mn distribution. Either is necessary to distribute between Mn in geochemical processes and those introduced by the anthropogenic activity. In N3, EF values for CrT were found to be high perhaps due to the precipitation-dissolution processes of chromite in the coast of Tabasco.
Contents of the supplementary information sectionS1 Box plots of changes of total metals (T) and absorbed (L). Change of the horizon of the depth of the total metals (Ba and Al). Change of the horizon of the depth of the total metals (Fe and Cr). Change of the horizon of the depth of the total metals (Ni and Mn). Change of the horizon of the depth of the total metals (V and Pb). Change of the horizon of the depth of the metal absorbed. Cluster diagram and major components of the total metals in the sediment cores (N1, N2 and N3) Principal component analysis for the three cores. Formulae 1 Location of sampling points in the southern Gulf of Mexico.
Holds a Master's in Engineering degree, is an expert on soil and aquifer pollution, water quality, sustainability, and environmental impact. He has more than 10 yrs of professional experience as a Professor at the Universidad Nacional Autonoma de Mexico (UNAM) and is appointed as a full-time professor (tenure) at the School of Engineering; has directed 10 B.Sc. theses and revised more than 30 theses. He is a Ph.D. candidate in the Earth Sciences Program at UNAM. His work has been presented and recognized in various national and international forums.
Chicago citation style De Lorenz-Santos, Fernando Jesús, Felipe Vázquez, Georgina Fernández-Villagómez, Javiera Cervini-Silva. Cluster and Principal Components Analyses on the Contents of (Total and Sorbed) 2 Trace Metals in Fresh Marine Sediments from the South of the Gulf of Mexico. Ingeniería Investigación y Tecnología, XIV, 04 (2013): 511–522.
ISO 690 citation style De Lorenz-Santos F.J., Vázquez F., Fernandez-Villagomez G., Cervini-Silva J. Cluster and Principal Components Analyses on the Contents of (Total and Sorbed) 2 Trace Metals in Fresh Marine Sediments from the South of the Gulf of Mexico. Ingeniería Investigación y Tecnología, volume XIV (issue 4), October-December 2013: 511–522
She is an expert on storage, production, transportation, distribution, treatment and disposal of materials and hazardous wastes; prevention and mitigation of chemical hazards, and water quality control. She has more than 34 yrs of professional experience as a Professor at the Universidad Nacional Autonoma de Mexico (UNAM) and is appointed as a full-time professor (tenure) at the School of Engineering; has directed 34 B.Sc. theses, 37 master's theses, and 5 underway; has also held several professional positions at the National Center for Disaster Prevention. The work of Dr. Fernández has received worldwide recognition.
Holds knowledge on environmental geochemistry, with more than 15 yrs of research experience, and is appointed as Titular Professor “C” (tenure) at Universidad Autonoma Metropolitana. She supervises thesis work at the Bachelor, Master, and Doctoral levels. Dr. Cervini has served on the editorial board of international journals. She is a member of the NASA Astrobiology Institute, Academia Mexicana de Ciencias, and SNI. She has been distinguished with the Perfil Deseable PROMEP recognition, and ad honorum positions at the Lawrence Berkeley National Laboratory (EUA) and the Laboratoire of Geophysique Interne et Technophysique-Observatoire des Sciences de la Terre de Grenoble (Francia).