Cells of the innate immune system can recognize pathogens via a number of extracellular and intracellular receptors, such as Fc, mannose or Toll-like receptors. Recognition of these pathogens leads to the synthesis of inflammatory lipid mediators, the eicosanoids. The eicosanoids derive from arachidonic acid (AA), an ω-6 polyunsaturated fatty acid that mammals can incorporate directly through dietary sources or synthesize from linoleic acid. In cells, AA seldom occurs in free fatty acid form, and is almost always found esterified at the sn-2 position of glycerophospholipids. Thus, it has to be removed from there before any eicosanoid synthesis can occur. The enzymes involved in such a removal are the phospholipase A2s (PLA2s). The signaling pathways that mediate the production of eicosanoids by cells involved in innate immunity are not completely understood, but it is now clear that the calcium-dependent cytosolic group IVA PLA2 (cPLA2α) is a critical enzyme in this process, and that, depending on cell type and stimulation conditions, regulatory cross-talk mechanisms exist between cPLA2α and other PLA2 enzymes present in the cells.
Las células del sistema inmune innato pueden reconocer patógenos mediante una serie de receptores extracelulares e intracelulares, tales como los receptores para Fc, receptores de manosa y receptores tipo Toll. El reconocimiento de estos patógenos conduce a la síntesis de mediadores lipídicos de la inflamación conocidos bajo el nombre colectivo de eicosanoides. Los eicosanoides derivan del ácido araquidónico (AA), un ácido graso ω-6 poliinsaturado que los mamíferos pueden incorporar directamente a través la dieta o sintetizar a partir de ácido linoleico. El AA no se halla nunca en forma libre, sino esterificando la posición sn-2 de los glicerofosfolípidos de membrana. Por ello, antes de que se produzca la síntesis de eicosanoides, el AA tiene que ser liberado de los fosfolípidos. Las enzimas involucradas en tal liberación son las fosfolipasas A2 (PLA2). Aunque las rutas de señalización que median la producción de eicosanoides por células involucradas en inmunidad innata no están bien caracterizadas, se ha demostrado de modo concluyente que la fosfolipasa A2 citosólica de grupo IVA (cPLA2α) es una enzima fundamental en este proceso y que, dependiendo del tipo celular y de las condiciones de estimulación, existen mecanismos reguladores de cross-talk entre la cPLA2α y otras enzimas con actividad PLA2 presentes en las células.
arachidonic acid
cyclooxygenase-2
Ca2+−dependent cytosolic phospholipase A2
complement receptor 3
C-type lectin receptor
dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin
fragment c receptor
formyl-methionylleucyl-phenylalanine
nitric oxide synthase
Ca2+−independent cytosolic PLA2
lipopolysaccharide
leukotriene
lipoxygenase
mitogen-activated protein kinase
mannose receptor
NOD-like receptor
nucleotide oligomerization domain
platelet-activating factor
pathogen-associated molecular pattern
phosphatic acid phosphatase-1
prostaglandin
phosphoinositides
phospholipase A2
pathogen recognition receptor
RIG-I-like helicase
Ca2+−dependent secreted PLA2
scavenger receptor
Toll-like receptor
uridin 5'-diphosphate.
The phospholipase A2S (PLA2s) are enzymes that catalyze the hydrolysis of the ester bond at the sn-2 position of glycerophospholipids, generating a free fatty acid and a lysophospholipid. This reaction constitutes the major pathway through which arachidonic acid (AA) is released from glycerophospholipids during cellular stimulation. Free AA is the precursor of the eicosanoids, which include the prostaglandins, generated through cyclooxygenasecatalized reactions, and the leukotrienes and lipoxins, generated through lipoxygenase-catalyzed reactions(1). Additionally, the PLA2 reaction generates a platelet-activating factor (PAF) precursor when the lysophospholipid product possesses a choline headgroup and an alkyl linkage at the sn-1 position. Thus, PLA2s are important signaling enzymes, which regulate the generation of lipid second messengers of different types with key roles in regulating innate immune responses. In turn, direct inhibition of PLA2 would have the potential of blocking multiple kinds of lipid-signaling pathways at once, which could be of therapeutic advantage in certain settings. Targeting and inhibiting the PLA2 reaction has proved problematic since numerous PLA2 enzymes exist in cells with overlapping activation properties. Only in human, at least 22 proteins possessing PLA2 activity have been described(2, 3). Thus, the first step for a rational PLA2 drug design strategy should be to define the involvement of the different PLA2 classes present in cells and elucidate their roles in lipid mediator biosynthesis during signaling triggered by multiple innate immunity receptors.
PHOSPHOLIPASE A2The PLA2 enzymes have systematically been classified into several group types according to their primary structure(3, 4). The latest update to this classification, published in 2009(2) included fourteen groups, most of them with several subgroups. Only PLA2s whose nucleotide sequence has been determined should be included in the classification. From a mechanistic point of view, the PLA2s can be grouped into two major families, namely the low-molecular mass enzymes (>20kDa), which utilize a catalytic histidine, and the high molecular mass enzymes (<40kDa), which utilize a catalytic serine(4, 5). For detailed information on the biochemistry and functioning of PLA2 enzymes, the reader is kindly referred to recent reviews on the subject(2-8).
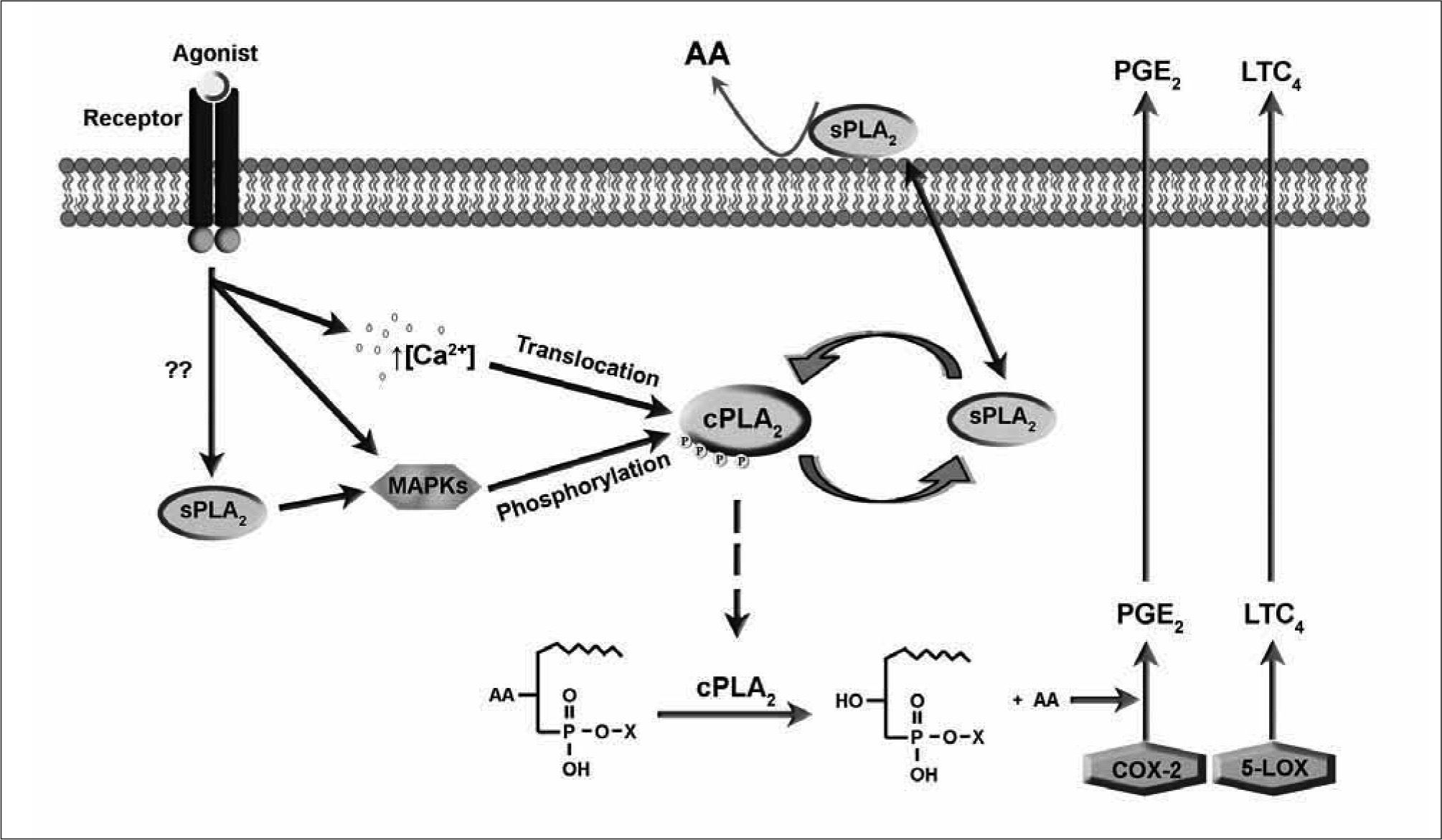
From the point of view of cellular regulation, mammalian PLA2 enzymes involved in AA metabolism are frequently classified into three families, namely the Ca2+-dependent cytosolic PLA2s (cPLA2s), the Ca2+-dependent secreted PLA2 (sPLA2s), and the Ca2+−independent cytosolic PLA2s (iPLA2s)(5, 7). Of these families, the first two have been repeatedly implicated in AA mobilization in response to a variety of immunoinflammatory stimuli. Today, it is firmly established that the calcium-dependent cytosolic group IVA PLA2α (cPLA2α) is the critical enzyme in AA release (Figure 1)(9, 10) and that, depending on cell type and stimulation conditions, a secreted PLA2 may also participate by amplifying the cPLA2α-regulated response(11-15). There is no compelling evidence that iPLA2 enzymes play an effector role in AA mobilization in response to stimuli of the innate immune response(5), but these enzymes may participate in the formation of cellular AA pools by regulating basal AA reacylation reactions(16-18).
A number of agents that exert effects on cells via innate immune receptors elicits a series of signals that ultimately lead to increased PLA2 activity. Elucidation of these signals has been the subject of much effort for the last twenty years. Figure 2shows the PLA2 signal transduction mechanism developed over the years for major immunoinflammatory cells such as macrophages and mast cells. The model depicted in Figure 2 contemplates a scenario where the concerted action of two distinct PLA2s leads to a full AA release response. cPLA2α appears to be responsible for the bulk of the AA release, whereas the sPLA2 effects the AA release as well, but may primarily act to amplify the response by providing additional signals for full activation of the cPLA2α. The cells respond to two different kinds of signals that generate either immediate (min) or delayed (h) responses. In the first case, the agonist acts on pre-existing proteins, whereas in the latter, synthesis of new protein effectors is a key event. In either case, the foremost event is the translocation and activation of cPLÁ2α in an intracellular compartment. The mechanism of activation of this enzyme has been the subject of many studies, and generally involves the concerted action of the mitogen-activated protein kinase (MAPK) cascade and transient elevations of the intracellular Ca2+ concentration(7-10). Examples have been provided indicating that cPLA2 activation may also take place in the absence of intracellular Ca2+ elevations(19-21).
Activation of cPLA2α may precede or follow the activation of a sPLA2 which, depending on the type of cell, may belong to groups IIA, V, or X. Depending on the stimulation conditions, the cPLA2α-modulation of sPLA2 cellular activity may occur at a gene regulatory level (delayed responses) or at the level of regulation of enzyme activity itself (immediate responses). In the latter case, a variety of cellular mechanisms may account for this activation, from rearrangement of membrane phospholipids that enables further PLA2 attack, to more sophisticated biochemical mechanisms such as inactivation of endogenous inhibitors or Ca2+ fluxes. The evidence that the sPLA2 may also act to regulate cPLA2α by various mechanisms, including the regulation of phosphorylation reactions further complicates the situation. Specific examples are provided in the following sections.
While it is clear that cPLA2α acts predominantly on perinuclear membranes, the precise site of action of the sPLA2 has been the subject of numerous studies. The enzyme appears to be released to the extracellular medium, from which it re-associates with the outer cellular surface where it hydrolyzes phospholipids. However, recent studies have suggested that the enzyme is re-internalized deep into the cell, probably via the caveolae system to the vicinity of nuclear membranes(22, 23). Whether the enzyme is still active in the cellular interior or this represents a signal termination mechanism is unclear at present. This is currently an area of active study. Recent data have suggested that sPLA2 acts intracellularly just prior to secretion of the enzyme to the extracellular medium(24, 25).
RECEPTORS OF THE INNATE IMMUNE RESPONSEThe immune system can be divided into two categories: the innate or non-specific immunity and the adaptive or specific immunity. The latter is based on lymphoid cellsurface receptors generated by gene rearrangements that undergo somatic hypermutations in order to recognize an infinite variety of antigens. This response is more complex than the innate response, as the antigen has to be processed first. The innate or non-specific immunity is present in almost all multicellular organisms and constitutes the first line of defense against invading pathogens.
The innate immune response system has the capacity to directly recognize a broad range of pathogens using a repertoire of receptors, the so-called pathogen recognition receptors (PRRs)(26). These receptors are encoded in the germ line of each individual and do not undergo somatic mutations, as is the case of the receptors of adaptive immunity. PRRs recognize conserved molecular patterns of foreign organisms such as viruses, bacteria, fungi and parasites, which are denominated pathogen-associated molecular patterns (PAMPs), although they are present on both pathogenic and non-pathogenic microorganisms. They comprise sugars, proteins and lipids, as well as distinct nucleic acid motifs. There are several hundred of these PAMPs, that directly activate immune cells, triggering a signaling cascade that lead to the expression of a variety of genes involved in the inflammatory and immune responses.
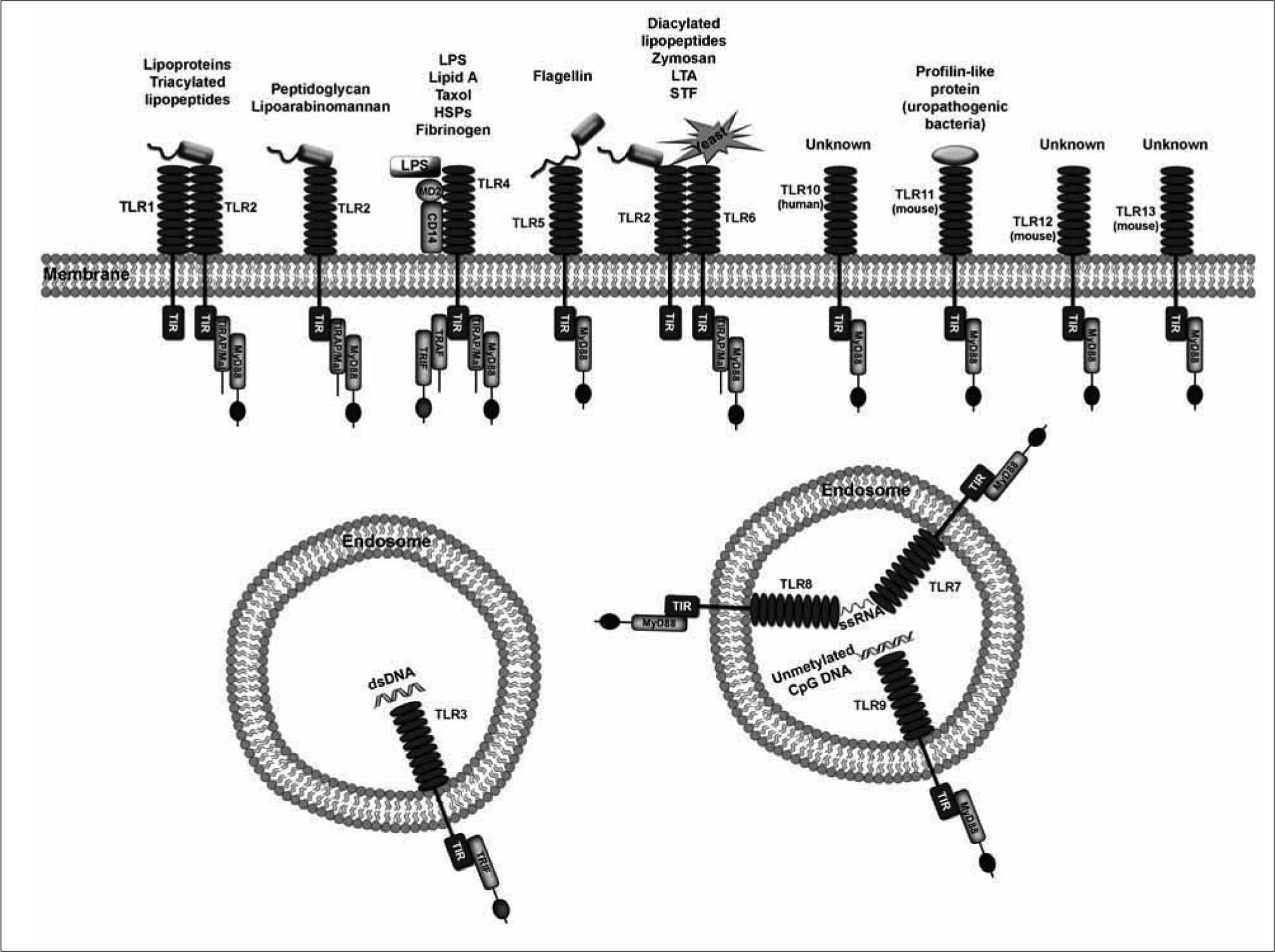
Among the PRRs, the best characterized are the TLRs(27-32) (Figure 3). Up to date, thirteen TLRs have been described in mice and human, TLR10 being expressed exclusively in human and TLR11-13 in mice(33). In humans, the TLRs can be sub-divided into five subfamilies based on amino acid sequences, namely TLR2 (including TLR1, TLR2, TLR6 and TLR10), TLR3, TLR4, TLR5 and TLR9 (including TLR7, TLR8 and TLR9)(34). In mice, TLR11, 12 and 13 comprise another additional subfamily(35). The TLR3 and TLR9 families are located exclusively in endosomal compartments and are considered intracellular receptors(36).
Other types of PRRs include (i) CD14, co-receptor for bacterial LPS(37), (ii) C-type lectin receptors (CLRs) such as the mannose receptor or CD206(38), dectin-1, the primary macrophage receptor for the phagocytosis of yeast(39), or DC-SIGN(40); (iii) scavenger receptors (SRs), a group of
receptors involved in receptor-mediated endocytosis of polyanionic ligands such as low density lipoproteins (LDL) modified by oxidation or acetylation(41); (iv) the formylmethionyl-leucyl-phenylalanine (fMLP) receptor, mainly present in neutrophils(42); and (v) a series of intracellular receptors such as the nucleotide oligomerization domain (NOD)-like receptors (NLRs)(43) or the RIG-I-like helicases (RLHs)(36, 44).
On the other hand, the innate immune system also includes a series of receptors that do not directly recognize the pathogens, but do it so when the pathogens are coated with opsonins. These are the complement receptor type 3 (CR3 or CD11b/CD18), and the Fc receptors (FcRs), and are generally considered the link between innate and adaptive immunities(45, 46). CR3 is the main receptor mediating type II phagocytosis of complement-coated particles, but it has been also implicated in nonopsonic phagocytosis of zymosan and Mycobacterium kansaii through engagement to distinct sites(47). Thus, in a broad sense CR3 could also be considered a PRR.
TLRS AND AA MOBILIZATIONTLR2. Little information is available on TLR2-mediated stimulation of PLA2 leading to AA mobilization. Suram et al. (48) showed that, in RAW264.7 macrophages, TLR2 acts together with dectin-1 to activate cPLA2α and cyclooxygenase- 2 expression upon stimulation with soluble lipopeptide-2 (MALP-2) and particulate β-glucan (P-βG), respectively. In bone marrow-derived mast cells of sPLA2-V-null mice stimulated with the TLR2 agonist Pam3CSK4, the release of AA and the production of leukotriene C4 and prostaglandin D2 were diminished, as well as the phosphorylation of ERK1/2 and cPLA2α, demonstrating a role of endogenous group V sPLA2 in TLR2-mediated stimulation through the activation of ERK and cPLA2α(49). Activation of cPLA2α via MAPK, with enhancement of AA release in mouse peritoneal macrophages, COX-2 induction and eicosanoid production through stimulation of TLR2 with the bacteria Listeria monocytogenes has also been reported(50).
TLR3. Pindado et al.(51) studied the mechanism of activation of TLR3 with viral dsRNA in RAW264.7 macrophages. TLR3 stimulation with dsRNA was found to lead to a signaling cascade involving activation of cPLA2α, induction of COX- 2, with the subsequent production of PGE2 which in turn promotes an enhanced iNOS expression. Remarkably, no role for sPLA2 enzymes along this cascade of activation was found.
TLR4. The stimulation of AA mobilization by TLR4 agonists has been extensively studied. The classic TLR4 agonist is LPS, a major component of Gram-negative bacterial cell walls. Although LPS on its own is a poor stimulus of AA release in primary cells of human and mice, it possesses a remarkable potency as a primer of the AA mobilization response. Thus, LPS potentiates by several-fold the AA release response of a second stimulus that is added shortly after(52, 53). In macrophage-like cell lines, however, LPS is able to elicit a robust AA mobilization on its own, although the response takes several hours to fully develop. This strong activating effect of LPS on macrophage cell lines has been extensively used as a model system for studies aiming at understanding cellular regulation of PLA2 and AA mobilization for eicosanoid biosynthesis.
Well before the TLR4 receptor was identified, Balsinde et al.(11) demonstrated by the use of antisense oligonucleotide approaches that approximately 60-70% of [3H]AA released by murine macrophage-like P388D1 cells primed with LPS and activated with PAF was under the control of a sPLA2 enzyme. Further studies utilizing specific inhibitors(54) corroborated these findings and also indicated the participation of cPLA2α in the process, thus providing the first direct evidences that two distinct PLA2 enzymes were involved in receptor-regulated AA mobilization reactions. A crosstalk between cPLA2α and sPLA2, where a prior activation of cPLA2α was necessary for sPLA2 to act was established(13). Subsequent work demonstrated that the sPLA2 involved in these processes was a novel group V enzyme, which had been discovered only two years before(55). Activation by LPS/PAF induced sphingomyelin synthesis, with accumulation of ceramide and diacylglycerol, leading to activation of group V sPLA2, probably via some sort of perturbation in the membrane(56).
In other series of studies, the delayed response of P388D1 macrophage-like cells to LPS alone was investigated(57). LPS stimulation activates cPLA2α, which regulates sPLA2-V expression and in turn mobilizes AA leading to PGE2 production via COX-2, an enzyme whose induction was found to depend on the activity of group V sPLA2(57, 58). These results were confirmed by Murakami et al.(12), who studied the effect of various sPLA2 enzymes in AA metabolism when over-expressed in human embryonic kidney 293 (HEK 293) and Chinese hamster ovary (CHO-K1) stimulated with calcium ionophore (immediate response) or interleukin-1 (delayed response). In these studies it was demonstrated that cPLA2α, group IIA sPLA2 and group V sPLA2 all mediate AA release, and that a synergism exists between cPLA2α and the sPLA2 enzymes in AA release; cPLA2α was necessary for sPLA2 but not the opposite(12).
Studies by confocal microscopy of P388D1 cells stimulated with LPS concluded that sPLA2-V is internalized in caveolinenriched granules through the perinuclear membrane, thus colocalizing with COX-2 and promoting AA release for subsequent production of prostaglandins(22). Shirai et al.(59) also studied the localization of fusion proteins of cPLA2α and sPLA2-V in P388D1 stimulated with PAF/LPS (primed immediate response) or LPS (delayed response). In the short-term stimulation, translocation of cPLA2α to perinuclear/Golgi membranes was observed, whereas no significant changes in the localization of group V sPLA2 were detected. In the long-term stimulation, both cPLA2α and group V sPLA2 translocated to intracellular membranes at 3h, with release of AA being observed in a cPLA2α-dependent manner.
In TLR4 knock-down experiments, Qi et al.(60) investigated the role of TLR4 in LPS-mediated stimulation of RAW 264.7 macrophages. In this system, activation of cPLA2α for eicosanoid production was found to be regulated via TLR4 through MyD88 and TRIF adapter proteins, with the subsequent activation of MAPK and the PI3K/Akt cascade, although only the first one leads to phosphorylation of cPLA2α. In human U937 macrophage-like cells and murine P388D1 macrophages stimulated with LPS it has been described that the enhanced COX-2 expression and PGE2 release is inhibited by the phosphatidate phosphohydrolase- 1 inhibitors BEL and propranolol. Moreover, diacylglycerol, a product of phosphatidate phosphohydrolase-1, increases the inflammatory response of LPS, thus implicating phosphatidate phosphohydrolase-1, and more specifically its product, diacylglycerol, in the signaling pathway promoted by TLR4 stimulation(61). Similar results have been obtained in murine macrophage RAW264.7 stimulated with the TLR4 agonist Kdo2-lipid A, where activation of cPLA2α is dependent on phosphatidate phosphohydrolase-1, which in turns activates protein kinase C through diacylglycerol production. However, neither phosphatidate phosphohydrolase-1 nor protein kinase C appear to be involved in the synergistic response promoted by TLR4 stimulation together with ATP (sustained-Ca2+ influx)(62).
TLR 7 and 8. One study has been reported in human polymorphonuclear leukocytes stimulated by the synthetic agonist of TLR7 and 8 imidazoquinoline resiquimod (R- 848)(63). Priming cells to further stimulation with fMLP promotes cPLA2α phosphorylation and 5-LO translocation, with the subsequent enhancement of AA release, biosynthesis of PAF and leukotriene and increase of COX-2 expression.
TLR9. TLR9 has been also implicated in AA release in RAW264.7 macrophage-like cells RAW264.7(64). In this system, stimulation of TLR9 with CpG (a compound that is similar to bacterial DNA) leads to activation of cPLA2α and subsequent AA release, in a process that is dependent on p38 and Akt pathways. In addition, cPLA2α is crucial for enhanced expression of iNOS under TLR9 stimulation. Furthermore, it was further demonstrated in the same system that activation of cPLA2α through TLR9 agonist promoted induction and JNK-mediated release of monocyte chemoattractant protein MCP-1(65). Importantly, AA release in TLR-mediated stimulation was found to be independent on sPLA2 enzymes, as judged by the use of the cell-permeable inhibitor scalaradial.
To finish this section, two groups have comparatively studied the release of AA with multiple agonists for TLRs (TLR1 to 9). Buczynski et al.(66) characterized the eicosanoid profiles produced after activation of RAW264.7 macrophagelike cells by a variety of TLR agonists, but no striking differences
were appreciated among the various agonists used. Ruipérez et al.(67) utilized P388D1 and RAW264.7 macrophage-like cells to study the cross-talk between cPLA2α and group V sPLA2 in the release of AA under stimulation with agonists of TLR1 to 9. In this study it was found that all TLR agonists but TLR5 and TLR9 were able to induce AA mobilization by a mechanism involving both cPLA2α and sPLA2-V. The latter enzyme was reported to regulate phosphorylation of the former via ERK1/2.
AA MOBILIZATION IN RESPONSE TO NON-TLR AGONISTSAlthough much of the recent work on AA mobilization mainly focuses on stimulation via TLR, there is also significant data on the stimulation of AA mobilization via non-TLR receptors of the innate immunity.
As early as 1982, the release of AA in response to IgE complexes was demonstrated in mouse peritoneal macrophages(68). With the establishment of gene knockdown and cloning technologies, the mechanisms underlying this phenomenon have been characterized in detail at a molecular level. Fujishima et al.(14) sensitized bone marrowderived mast cells (BMMC) from cPLA2α+/+ and cPLA2α−/− mice with monoclonal mouse IgE anti-trinitrophenyl (TNP) ascites and then stimulated them with TNP-BSA in order to mimic a FcωRI-dependent activation. Under these experimental conditions they failed to observe detectable production of PGD2 and LTC4 in cPLA2α−/− mice, but robust generation of the two eicosanoids was found in cPLA2α+/+ mice, demonstrating the essential role of cPLA2α for the immediate production of eicosanoids via engagement of FcωRI. Work by the same group(69) also demonstrated that group V sPLA2 amplifies the response of cPLA2α, inducing a FcωRI-dependent COX-2 production and delaying the production of PGD2, but not of LTC4, in mouse BMMC.
The classical model for AA mobilization studies in the human neutrophil is via activation of the fMLP receptor, which interacts with f-Met-Leu-Phe, a bacterial chemotactic tripeptide. Stimulation of neutrophils with fMLP induces phosphorylation of cPLA2α and AA release(70). Interestingly, the phosphorylation of the enzyme was MAPK-independent, but AA release was inhibited by inhibitors of p38 MAPK and ERK, suggesting that these proteins play a role downstream cPLA2α activation, perhaps by affecting the translocation of the enzyme where its substrate is located, or by reducing the increase in the intracellular levels of Ca2+(70). Two recent studies from the Rubin laboratory described in detail the importance of sPLA2 in the human neutrophil response to agonists of the fMLP receptor. In the first paper(71) a central role in governing the AA release was attributed to cPLA2α and an accessory secondary role was found for an unidentified sPLA2 enzyme, and the suggestion was made that such sPLA2 activity was somehow regulated by cPLA2α. In a second study(72) the authors observed that production of leukotriene B4 by fMLP- stimulated neutrophils was insensitive to sPLA2 inhibitors, suggesting that production of this particular eicosanoid depends only on cPLA2α activation.
THE AA MOBILIZATION RESPONSE TO ZYMOSANFor years, zymosan has constituted the classical stimulus for studies of AA mobilization in immunoinflammatory cells. Consequently, a wealth of data has accumulated on the role of PLA2-mediated lipid pathways in response to this agonist. Zymosan is a preparation of yeast cell walls consisting of protein-carbohydrate complexes, mainly formed by β -glucan and mannan. Zymosan has the ability to bind to different receptors in the macrophage surface depending on whether the particles are opsonized or not. Opsonized zymosan binds mainly to FcR and CR3, and although CR3 has also the capacity of binding nonopsonized zymosan by different sites, it has been reported not to induce AA release in murine peritoneal macrophages(73). On the other hand, unopsonized zymosan binds preferentially to dectin-1 and, to a lesser extent, to TLR2/TLR6.
Some three decades ago the release of AA and the production of prostanglandins and leukotrines in murine peritoneal macrophages stimulated with unopsonized zymosan was demonstrated(74-76). Dissection of the lipid pathways involved indicated a preeminent, if not exclusive, role for a PLA2-like activity in effecting the AA release(77-79). Such a PLA2 activity was identified to correspond to that of cPLA2α, and its cellular regulation by Ca2+ transients and phosphorylation was established(80, 81).
At that time, the different PLA2s present in cells and the receptors responsible for the recognition of zymosan were poorly characterized. With the cloning, characterization and better understanding of the function of the different PLA2 groups, it was possible to study of stimulation mediated by zymosan in a much more detailed way. By using mice lacking cPLA2α by genetic ablation, Gijón et al.(21) unambiguously demonstrated that this enzyme is essential for the AA release and eicosanoid production in response to unopsonized zymosan. Phosphorylation of cPLA2α on Ser 505 in response to zymosan was found to be mediated by ERK and p38 MAPK, although the molecular significance of such a phosphorylatiom reaction remains poorly understood(82). Subsequently, it was shown that in addition to translocating to perinuclear membranes, cPLA2α also translocates to the phagosome during the phagocytosis of particles of unopsonized zymosan in mouse peritoneal macrophages(83). Utilizing opsonized zymosan particles, Casas et al.(84) demonstrated in human monocyte-derived macrophages that translocation was regulated by the activation of JNK, which is responsible for the phosphorylation of cPLA2α on Ser 505. In this study no translocation of other PLA2 enzymes present in the cells to the phagosome could be observed(84).
With regard to the involvement of sPLA2 family members in the amplification of cPLA2α signaling, Satake et al.(85) generated mice lacking group V sPLA2 and observed that peritoneal murine macrophages from these mice produced lower quantities of leukotriene C4 and prostaglandin E2 in response to zymosan compared to cells from wild-type mice. Following this observation, the same group(86) showed in peritoneal murine macrophages that group V sPLA2 translocates to the phagosome during the phagocytosis of unopsonized zymosan particles. Staining of LTC4 during phagocytosis demonstrated that this leukotriene was synthesized at the phagosome itself. Furthermore, peritoneal macrophages from group V sPLA2-null mice presented a lower phagocytosis of zymosan particles. This suggested that recruitment of sPLA2 to the phagosome is induced in order to amplify the signal of the cPLA2α already present in the phagosome, where both enzymes co-localize.
Suram et al.(48) observed that the AA release induced by incubating mouse peritoneal macrophages with unopsonized zymosan is blocked by a highly purified preparation of soluble β -(1, 3)-glucan phosphate. It was demonstrated in RAW 264.7 macrophages overexpressing dectin-1 or SIGNR1, a homologous receptor to DC-SIGN, that the receptor implicated in the activation of cPLA2α and release of AA is dectin-1. In addition, zymosan induced the expression of COX-2 and the increase in the production of PGD2 only in cells overexpressing dectin-1. Similarly, in human monocyte-derived dendritic cells it has been shown that unopsonized zymosan binds dectin-1 and DCSIGN, and induces phosphorylation of Syk, activation of cPLA2α and the subsequent mobilization of AA and COX- 2 expression(87).
CONFLICT OF INTERESTThe authors declare no financial conflict of interest.




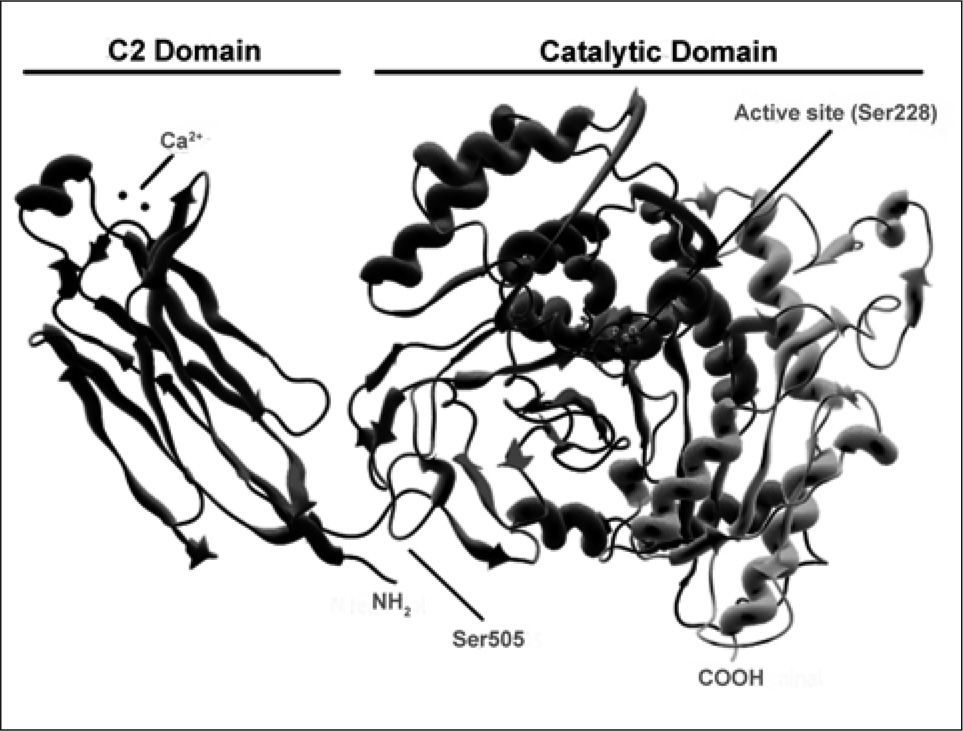
 cPLA2α. The C2 domain on the left mediates interaction of the enzyme with membranes via binding of two Ca2+ atoms. The active site Ser 228 on the catalytic domain is highlighted, as is the phosphorylation site Ser 505.' title='Ribbon diagram of
cPLA2α. The C2 domain on the left mediates interaction of the enzyme with membranes via binding of two Ca2+ atoms. The active site Ser 228 on the catalytic domain is highlighted, as is the phosphorylation site Ser 505.' title='Ribbon diagram of  PLA2 enzymes during activation of cells via innate immune receptors. For details, see text.' title='Cross-talk between
PLA2 enzymes during activation of cells via innate immune receptors. For details, see text.' title='Cross-talk between 