The group of major histocompatibility complex (MHC) alleles known as shared epitope (SE) is to date the strongest rheumatoid arthritis (RA) genetic risk factor. Many studies have shown that the measurement of anti-citrullinated peptides antibodies would be useful in the diagnosis and follow-up of RA.
Our aim is to determine the magnitude of the association between the possession of SE alleles and serum positive titres of antibodies against citrullinated peptides.
Our selection criteria included case–control or cohort studies, where data involving antibodies against citrullinated peptides and SE in RA patients were available. No date or language restrictions were imposed.
Bibliographical databases MEDLINE, Cochrane Database of Systematic Reviews and EMBASE were searched for pertinent literature. Two reviewers independently identified relevant citations and extracted data. Data extraction was then checked by two different reviewers.
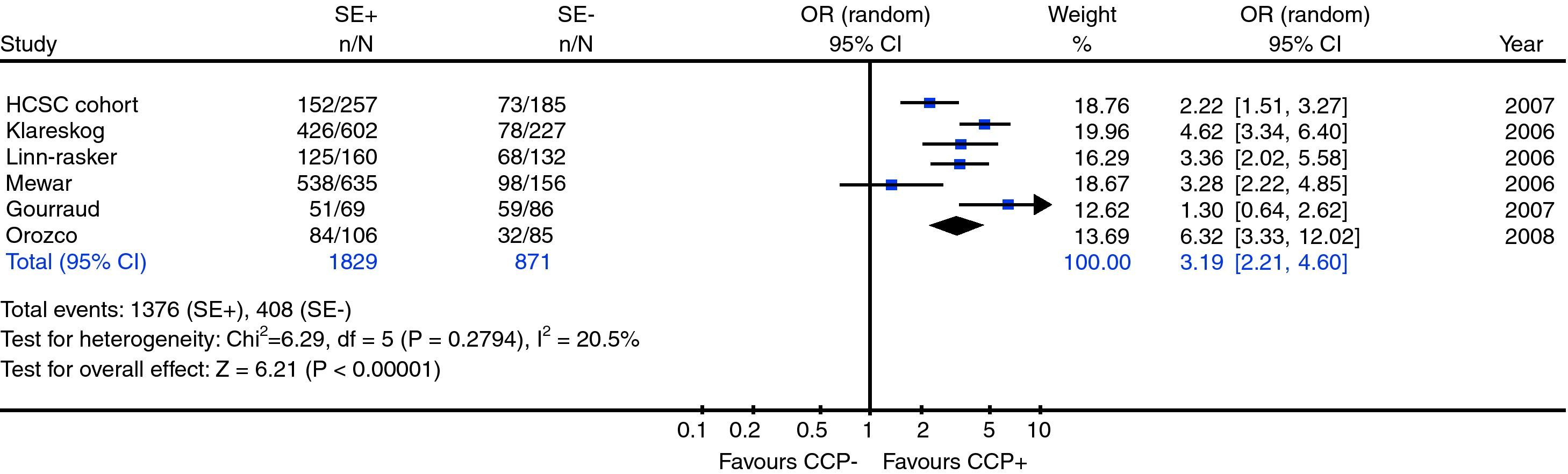
Five published and one unpublished (own data) studies were included in the final meta-analysis. Overall, 2700 European descent RA patients were included in this meta-analysis. A significant association between SE and positive titres of serum antibodies against citrullinated peptides [OR(95% CI)=3.19 (2.21–4.60)] was found.
Positive titres for antibodies against citrullinated peptides are threefold more frequent in RA patients who carry SE alleles than in those patients lacking them.
El grupo de alelos del complejo mayor de histocompatibilidad (MHC) conocidos como epítopo compartido (EC) es a día de hoy el más fuerte los factores de riesgo genético a artritis reumatoide (AR). Diferentes estudios han puesto de manifiesto que la presencia de autoanticuerpos contra péptidos citrulinados sería útil en el diagnóstico y seguimiento de la artritis reumatoide.
Nuestro objetivo es determinar la magnitud de la asociación entre la posesión de alelos del SE y la presencia de títulos positivos de anticuerpos contra péptidos citrulinados.
Nuestros criterios de selección incluyeron estudios de cohortes y caso-control en los que hubiera datos disponibles acerca de los anticuerpos contra péptidos citrulinados y el epítopo compartido en artritis reumatoide. No se hicieron restricciones de fecha ni de idioma.
Se realizaron búsquedas en las bases de datos bibliográficas MEDLINE, Cochrane Database of Systematic Reviews y EMBASE. Dos revisores identificaron de manera independiente las citas relevantes y extrajeron los datos. La extracción de datos fue comprobada posteriormente por dos revisores diferentes de los anteriores.
En el meta-análisis definitivo se incluyeron cinco estudios publicados y uno no publicado (datos propios). En total se incluyeron 2700 casos de RA de ascendencia europea en el meta-análisis. Se encontró una asociación significativa entre los títulos de anticuerpos positivos y el hecho de ser portador de alelos del epítopo compartido [OR(95% CI)=3.19 (2.21–4.60)]. Los títulos positivos de anticuerpos contra péptidos citrulinados son tres veces más frecuentes en pacientes de AR portadores de alelos del EC que en los no portadores.
Rheumatoid arthritis (RA) is a polygenic complex trait with a strong immune inflammatory component. Its global prevalence ranges from 0.5 to 1% around the world.1 Nowadays RA aetiology is not completely elucidated although it is considered an autoimmune disease where both cellular and humoral components are affected.
Genetic background has been estimated to contribute up to a 60% to RA pathogenesis.2 Some well-defined genetic susceptibility factors are widely accepted. The 6p21 cytogenetic region, encoding the Main Histocompatibility Complex (MHC, HLA), was the first one described. This genetic region is characterized by high genetic variability and high linkage disequilibrium. Carriage of at least one haplotype comprising risk alleles: DRB1*0101, *0102, *0401, *0404, *0405, *0408, *1001 or *1402 results in an increased susceptibility to develop RA. This group of class II HLA alleles is known as shared epitope (SE).3 However, the HLA region is responsible for just a 40% of the genetic component of RA,1 meaning that SE is not the only genetic risk factor for this disease.
It is well established that levels of circulating antibodies, such as rheumatoid factor (RF) and antibodies against citrullinated peptides, correlate with RA outcome. Titres of antibodies against citrullinated peptides provide perhaps the most significant prognostic index.4 Citrullination is a posttranslational modification of arginine. Citrullinated peptides act as self-antigens and are present in the synovial compartment of RA patients. These self-antigens drive B cell maturation and production of antibodies against citrullinated peptides.5,6 Antibodies against citrullinated peptides have been reported to arise even before the first symptoms of RA.4 Additionally, these antibodies against citrullinated peptides are highly specific for RA and their titres are closely correlated with the severity of radiological damage.7
Currently both SE and antibodies against citrullinated peptides are routinely studied in clinical practice. Much literature can be found on the association of SE or antibodies against citrullinated peptides with RA, and about their value as prognostic and diagnostic factors for this disease. In the last years, antibodies against citrullinated peptides have been analysed together with RA outcome variables7–10 and the same applies to SE,11,12 but very few has been written on their relationship to each other, and usually as marginal data in studies, as will be seen in this report.
The aim of this study is to perform a systematic review of literature and a meta-analysis in order to determine whether antibodies against citrullinated peptides and SE are associated in RA patients and the magnitude of such association. This was carried out by analysing case–control and cohort studies reporting data on the association between antibodies against citrullinated peptides and SE.
Materials and methodsData sources and searchesWe performed a comprehensive search strategy of various electronic databases (MEDLINE (1966–March 2008), Cochrane Database of Systematic Reviews (1991–March 2008) and EMBASE (1980–March 2008). We additionally searched within the references list from the selected original studies. The Medical Subject Headings and text words selected for our searches were: Rheumatoid, citrullinated OR anti citrulline antibodies, shared epitope, arthritis. Search strategy was limited to adults and humans.
Study selectionAll selected studies included RA patients diagnosed following the American College of Rheumatology (ACR) criteria.13 Neither date nor language restrictions were imposed. The studies considered for further analysis were required to hold information about RA patients with both antibodies against citrullinated peptides and SE data. Papers dealing on non-RA rheumatoid traits, such as juvenile idiopathic arthritis, or describing isolated subsets of patients were discarded. Asiatic RA cohorts were not considered, due to the different frequencies of HLA alleles in these populations as compared with European/Caucasic populations. Those studies including more than one patient from the same family were discarded.
We also included non-published data from Hospital Clinico San Carlos (HCSC) cohort, with non-related white Spanish RA patients (76% women), consecutively recruited from a single center (HCSC, Madrid) between 2004 and 2008; the inclusion criteria were based on the ACR criteria.13 All patients were of European descent and all were over 18 years old. The cohort comprises recently diagnosed patients and those with several years of treatment, the average follow-up being of 8 years. No distinction according to therapy status was made.
Data extraction and quality assessmentTwo reviewers (JV and MCM) independently identified relevant citations, which were included when describing original research on subjects with both SE and antibodies against citrullinated peptides data. Once studies were identified, data were first extracted by JV and MCM and then checked by SC and MF. Regarding findings of more than one publication on the same or overlapping groups of patients, the largest of the available published data sets was the one included to avoid double counting.
Study qualityThe quality of the included studies was independently assessed by two reviewers (JV and MCM), using the quality evaluation tool from STROBE (Strengthening The Reporting of Observational studies in Epidemiology; www.strobe-statment.org).14
Data synthesis and analysisThe review was conducted according to the adapted Quality of Reports of Meta-Analyses of Randomized Clinical Trials (QUOROM) recommendations. Reviewer Manager 4.2 (2000 Cochrane Collaboration, Oxford, United Kingdom) and Epidat 3.0 were used.
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by assessing the association between carriage of SE alleles and presence of antibodies against citrulline peptides, using raw data for each study and for the pooled population.
The Der Simonian and Laird, random effects or fixed effect models, were used according to the results of the tests of heterogeneity. The combined effect for heterogeneity was calculated by taking the inverse variance estimated. The effect of each study was weighted for its number of patients. p values <0.10 define a significant degree of heterogeneity. We also used I2 statistic, with a cut-off point of 25% to assess heterogeneity between the studies.
A sensitivity analysis was performed to test the relative influence of each study on the results. Studies were sequentially dropped, and the effect on the change in the overall size was determined.
Evaluation for publication biasWe used Review Manager 4.2 and Epidat 3.0 to generate the forest plot of pooled Odds Ratio with 95% confidence interval. We used the Egger test to assess funnel plots for evidence of publication bias.
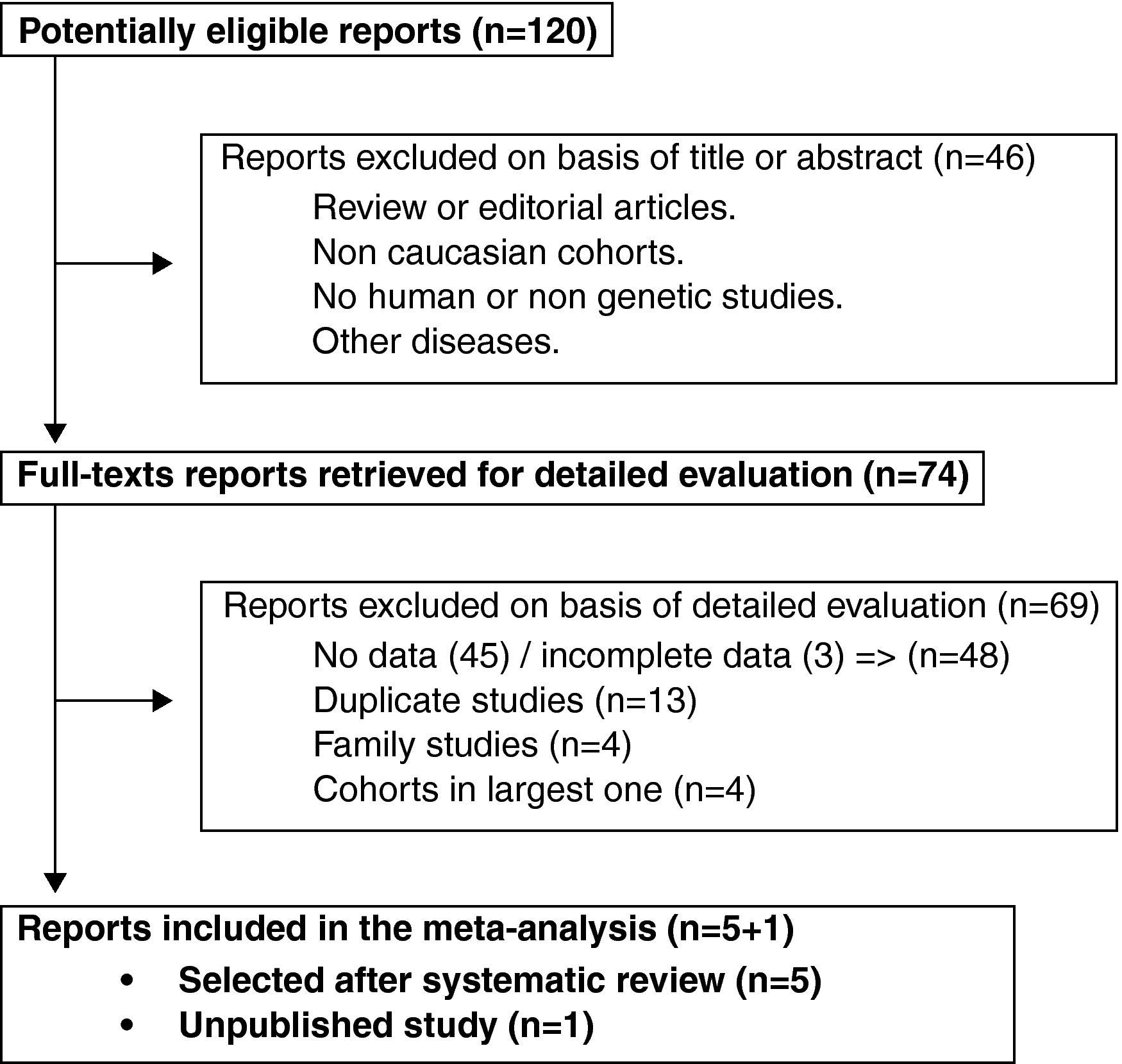
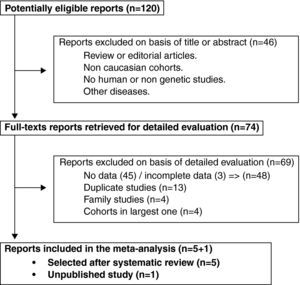
ResultsTrial flow and characteristics of the studyThe initial strategy search identified 120 articles. Seventy five of them met our inclusion criteria. Thirteen redundant studies were excluded.7,8,11,15–24 Forty six were eliminated because they either did not show numeric data or lacked simultaneous antibodies against citrullinated peptides and SE-genotyping data.1,7–9,11,15,16,18–20,22–57 Three original articles held incomplete data58–60 and were therefore discarded. Four family studies included more than one sibling or data from an indexed patient that could not be ascertained21,61–63 and were not included for those reasons. We additionally removed 4 studies of small cohorts for being included within other larger ones selected.64–67 Only five studies remained accurate for further mathematical analysis.16,68–71 (Fig. 1). We also added one unpublished study for the final meta-analysis.
Some papers on this matter have been published after March 2008.72,73 Those articles have been peer-reviewed as well but none of them held complete data and were so far discarded. A third article74 has been published that is not included in our meta-analysis for the cohort analysed overlaps with the one in 71.
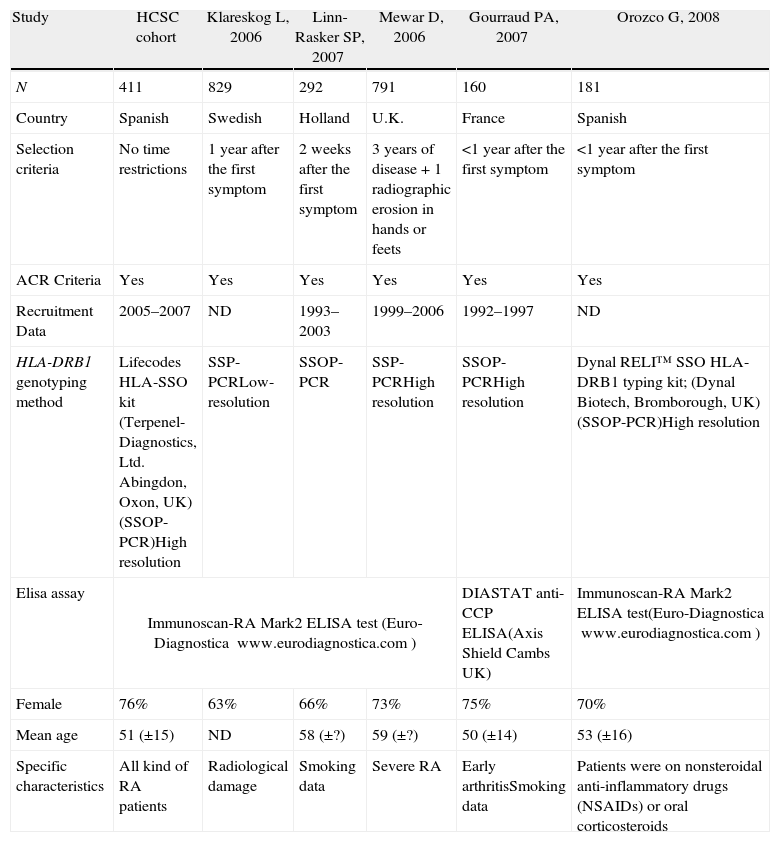
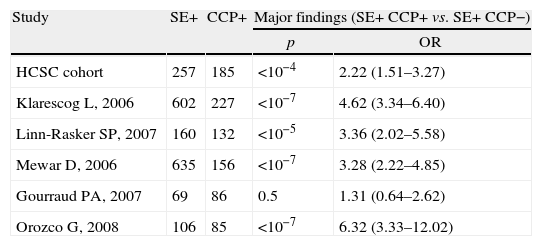
Meta-analysis baseline characteristicsTable 1 shows the characteristics of the five cohort studies and the case–control study included in the statistical analysis. Overall, 2700 RA patients were included. All studies were undertaken in Europe and all were published after 2000. Patients mean age ranged between 50 and 59 years. ORs ranged from 1.31 to 6.32 (Table 2). Only one study reported no statistically significant association between SE and antibody against citrullinated peptides status, but this was the only study with a compromised statistical power.
Cohorts’ characteristics (ND: no data).
| Study | HCSC cohort | Klareskog L, 2006 | Linn-Rasker SP, 2007 | Mewar D, 2006 | Gourraud PA, 2007 | Orozco G, 2008 |
| N | 411 | 829 | 292 | 791 | 160 | 181 |
| Country | Spanish | Swedish | Holland | U.K. | France | Spanish |
| Selection criteria | No time restrictions | 1 year after the first symptom | 2 weeks after the first symptom | 3 years of disease+1 radiographic erosion in hands or feets | <1 year after the first symptom | <1 year after the first symptom |
| ACR Criteria | Yes | Yes | Yes | Yes | Yes | Yes |
| Recruitment Data | 2005–2007 | ND | 1993–2003 | 1999–2006 | 1992–1997 | ND |
| HLA-DRB1 genotyping method | Lifecodes HLA-SSO kit (Terpenel-Diagnostics, Ltd. Abingdon, Oxon, UK)(SSOP-PCR)High resolution | SSP-PCRLow-resolution | SSOP-PCR | SSP-PCRHigh resolution | SSOP-PCRHigh resolution | Dynal RELI™ SSO HLA-DRB1 typing kit; (Dynal Biotech, Bromborough, UK)(SSOP-PCR)High resolution |
| Elisa assay | Immunoscan-RA Mark2 ELISA test (Euro-Diagnostica www.eurodiagnostica.com) | DIASTAT anti-CCP ELISA(Axis Shield Cambs UK) | Immunoscan-RA Mark2 ELISA test(Euro-Diagnostica www.eurodiagnostica.com) | |||
| Female | 76% | 63% | 66% | 73% | 75% | 70% |
| Mean age | 51 (±15) | ND | 58 (±?) | 59 (±?) | 50 (±14) | 53 (±16) |
| Specific characteristics | All kind of RA patients | Radiological damage | Smoking data | Severe RA | Early arthritisSmoking data | Patients were on nonsteroidal anti-inflammatory drugs (NSAIDs) or oral corticosteroids |
Statistical characteristics of single studies included in meta-analysis.
| Study | SE+ | CCP+ | Major findings (SE+ CCP+ vs. SE+ CCP−) | |
| p | OR | |||
| HCSC cohort | 257 | 185 | <10−4 | 2.22 (1.51–3.27) |
| Klarescog L, 2006 | 602 | 227 | <10−7 | 4.62 (3.34–6.40) |
| Linn-Rasker SP, 2007 | 160 | 132 | <10−5 | 3.36 (2.02–5.58) |
| Mewar D, 2006 | 635 | 156 | <10−7 | 3.28 (2.22–4.85) |
| Gourraud PA, 2007 | 69 | 86 | 0.5 | 1.31 (0.64–2.62) |
| Orozco G, 2008 | 106 | 85 | <10−7 | 6.32 (3.33–12.02) |
Most studies did not clearly point out their objectives and hypotheses. It was remarkable that none of the studies estimated the sample size. All studies but one16 failed to report the existence of missing data along the follow-up.
Only two studies16,70 reported about possible limitations or biases in their respective analyses.
Meta-analysis heterogeneityUnder a random effect model, the Chi-square value (5 degrees of freedom) for the Q statistics from the 6 included studies was 6.29 (p=0.279), showing no evidence for heterogeneity.
Meta-analysis relationship between carriage of shared epitope alleles and presence of anticitrulline antibodiesRandom effects meta-analysis revealed statistically significant association between SE and anticitrullinated antibodies (p<0.00001; pooled OR (95% CI)=3.19 (2.21–4.60)) (Fig. 2)
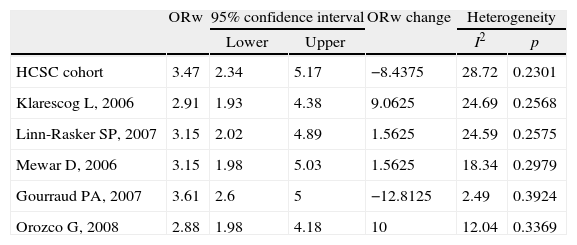
Meta-analysis sensitivity analysisIn order to analyse the consistency and robustness of the results, a sensitivity analysis was performed (Table 3).
Sensitivity analysis.
| ORw | 95% confidence interval | ORw change | Heterogeneity | |||
| Lower | Upper | I2 | p | |||
| HCSC cohort | 3.47 | 2.34 | 5.17 | −8.4375 | 28.72 | 0.2301 |
| Klarescog L, 2006 | 2.91 | 1.93 | 4.38 | 9.0625 | 24.69 | 0.2568 |
| Linn-Rasker SP, 2007 | 3.15 | 2.02 | 4.89 | 1.5625 | 24.59 | 0.2575 |
| Mewar D, 2006 | 3.15 | 1.98 | 5.03 | 1.5625 | 18.34 | 0.2979 |
| Gourraud PA, 2007 | 3.61 | 2.6 | 5 | −12.8125 | 2.49 | 0.3924 |
| Orozco G, 2008 | 2.88 | 1.98 | 4.18 | 10 | 12.04 | 0.3369 |
To assess the influence of each single study included in the meta-analysis, each study was excluded at a time, and the analysis performed again to appraise the change in the weighted OR (ORw). The punctual estimators for ORw changed between 2.88 and 3.61, and the statistical association between SE and antibody against citrullinated peptides remained significant. None of the studies included in this meta-analysis showed a distinct impact on the global estimation of ORw.
Evaluation for publication biasQuantitative analyses testing for publication bias by regression of normalized effect estimate showed no evidence for bias (p=0.308).
DiscussionBased on a meta-analysis of 6 studies, which included new results from a non-published study carried out in our center, we found evidence that antibodies against citrullinated peptides are associated with the presence of SE. The overall magnitude of the effect was high (OR 3.19; 95% CI 2.21–4.60).
The results were consistent through the sensitivity analysis.
The discovery of antibodies against citrullinated peptides in RA sera was first observed in 1964 as the presence of auto-antibodies capable of binding to perinuclear granules in normal human oral mucosa cells.75 These antigens were identified as citrullinated moieties some years later.76
The first ELISAs (enzyme linked immunoassays) used to determine the presence of antibody against citrullinated peptides were not quite reproducible.5 A second generation of ELISAs eased measurement and standardization of serum antibody against citrullinated peptides.77 A third generation of tests are nowadays being introduced in clinical routine, anyway these third generation ELISAs are quite similar to those of second but for a slight PPV (positive predictive value) and would not add additional advantages.78 Many studies have tried to evaluate the implication of antibody against citrullinated peptides in RA pathology. Evidence accumulates for abnormal citrullination of various peptides in a diverse set of human inflammatory diseases including RA. The production of high levels of antibodies recognizing citrullinated peptides in rheumatoid-like conditions seems to be specific for RA patients.79 A number of studies have documented the detection of antibody against citrullinated peptides prior to RA onset.17,25,30 These antibodies are also useful in identifying patients prone to clinically significant disease activity or radiological damage.30
The presence of these auto-antibodies has been associated with MHC class II alleles corresponding to those comprised in the “shared epitope” (SE). The SE hypothesis was described in 1987; this hypothesis assumes that certain HLA-DRB1 alleles encode a highly conserved aminoacid sequence associated with susceptibility to RA.3 Up to date, SE is the main genetic factor associated with RA.
The main strength of this meta-analysis is its large power, derived from pooling results on 2700 patients. The extensive searches allowed us to explore the association pattern of SE and antibody against citrullinated peptides, and to minimize the effects of search and publication bias.
All included studies were performed in European-descendent populations, given the fact that the distribution of HLA alleles is different from the one found in other populations.80,81 In this sight, we decided not to include non-Caucasian populations in this work, due to their different genetic background.
Further studies are warranted in order to explore the association between SE and antibody against citrullinated peptides in populations with different genetic backgrounds.
We can conclude that positive titres for antibodies against citrullinated peptides are three times more frequent in RA patients who carry SE alleles than in those patients lacking them in European populations. Medical implications that could underlie this association need to be elucidated. High-quality independent studies in populations bearing different genetic backgrounds would be recommendable to further characterize this association between SE and antibody against citrullinated peptides.
Conflict of interestJezabel Varadé is employee with support from the “Fondo de Investigaciones Sanitarias” (CA08/00194). Elena Urcelay works for the “Fundación para la Investigación Biomédica-Hospital Cínico San Carlos”.
We are most grateful to Carmen Martínez Cuervo and Carmen Poyo Falcon for her expert technical assistance.