Plasmacytoid dendritic cells (pDC) are also known as natural type-I-interferon-producing cell (IPC) owing its name to an outstanding capacity to secrete large amounts of type I interferons (IFN) upon viral infections, thus constituting important mediators in antiviral immunity. This review aims to summarize some of the human pDC attributes, such as their origin, migration, as well as recent findings on interaction of pDC with other cells within the immune system. In addition, we will review the differences and similarities between pDC and their leukemic counterparts (LpDC), with a special focus on the validity of using cell lines derived from leukemic pDC as a model to study normal pDC.
Las células dendríticas plasmacitoides (pDC), conocidas también como “las células que espontáneamente producen interferón de tipo I” (IPC, interferon producing cells), deben su nombre a su notable capacidad de secretar grandes cantidades de los interferones de tipo I (IFN) cuando se producen infecciones virales, lo que las convierte en importantes mediadores de la inmunidad antiviral. Esta revisión resumirá algunos de los atributos de las pDC humanas, como su origen y migración a órganos linfáticos, así como los recientes hallazgos sobre la interacción de pDC con otras células del sistema inmune. Evaluaremos también las diferencias y similitudes entre pDC y sus homólogos leucémicos (LpDC), discutiendo especialmente sobre la validez de la utilización de líneas celulares derivadas de pDC leucémicas como modelo para estudiar pDC normales.
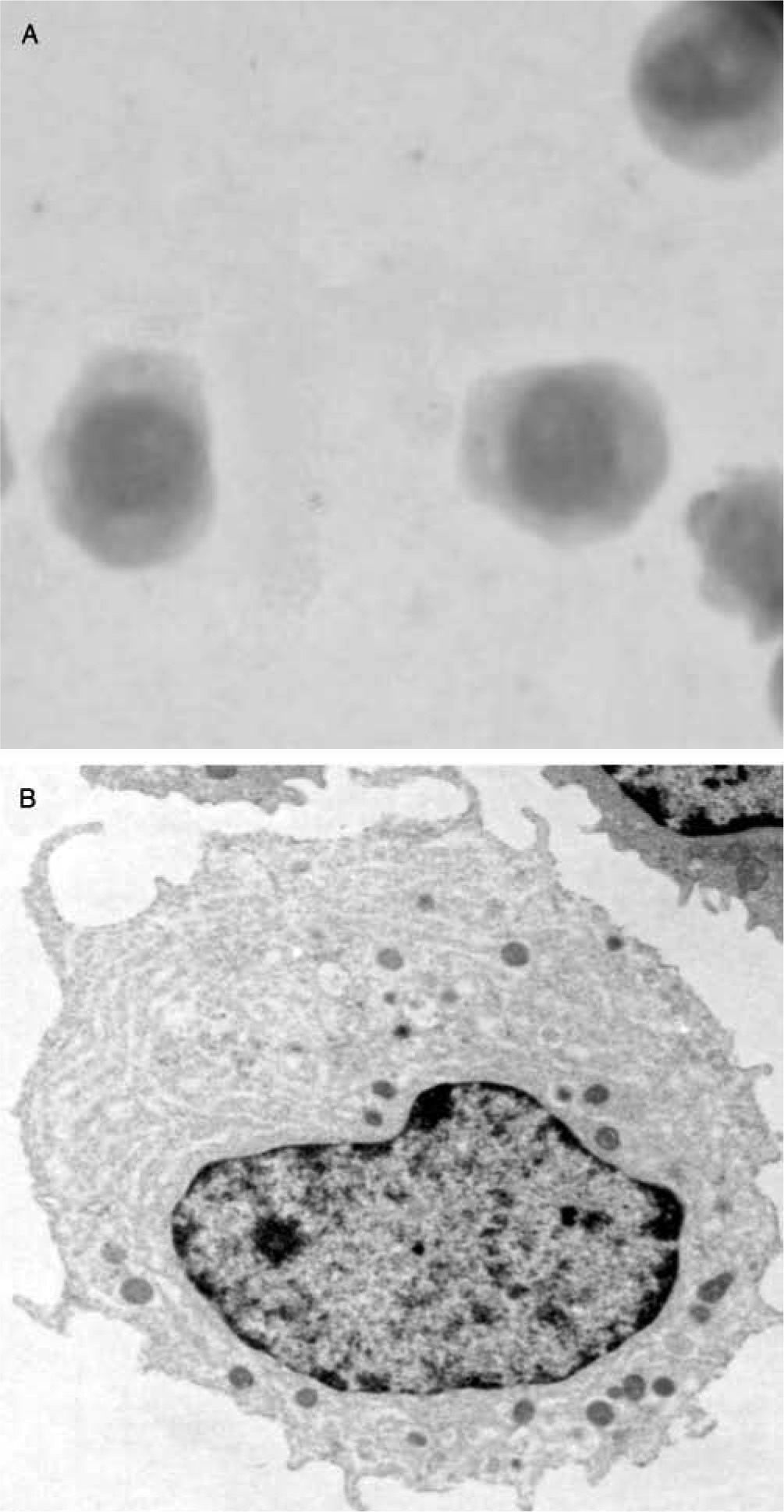
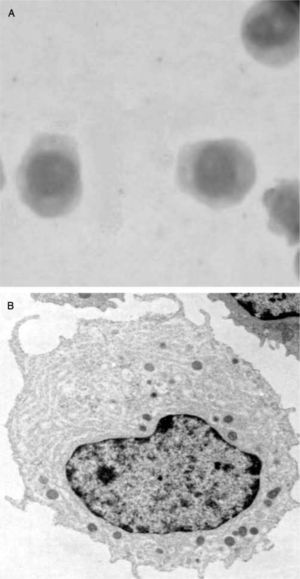
Circa 1950, pathologists K. Lennert and W. Remmele1 described a small cell population with plasma cell-like morphology in the T cell areas of human reactive lymph nodes. Later, these plasma cells were named as plasmacytoid T cells or plasmacytoid monocytes2 because of their expression of CD43, and monocytic markers such as the alpha chain of the interleukin-3 receptor (IL-3R/CD123) and CD684-6. Although the function of the so-called plasmacytoid T cell/plasmacytoid monocyte population was initially unknown, the presence of a large endoplasmic reticulum on these cells suggested a potential function in cytokine secretion (fig. 1). Later, such cells were named pre-pDCs (resting pDCs), plasmacytoid dendritic cells (name currently reserved for activated pDCs)7 or interferon-producing cells (IPCs), referring to the most relevant feature of pDCs: the production of massive amounts of type I IFNs (IFNα, β, χ, and κ)8 in response to in vitro exposure to unmethylated CpG DNA or viral infections. Although all nucleated cells can produce type I IFNs upon infection by an appropriate virus, this blood cell has the ability to produce more type I IFN than any other cell type. This feature makes them unique.
In healthy individuals, pDCs constitute only 0.2-0.8 % of peripheral blood mononuclear cells (PBMCs), but induce 95 % of the IFN produced by PBMCs in response to viruses sensed through Toll-like receptors (TLR), in particular the selectively expressed TLR-7 and TLR-9 9, and thus constitute critical mediators of antiviral immune responses. Interestingly, several studies have reported a significant age-related reduction in pDC numbers10,11 and functional impairment12, which could relate among other factors to the higher susceptibility to infection and deficient response to vaccination often observed in aged individuals.
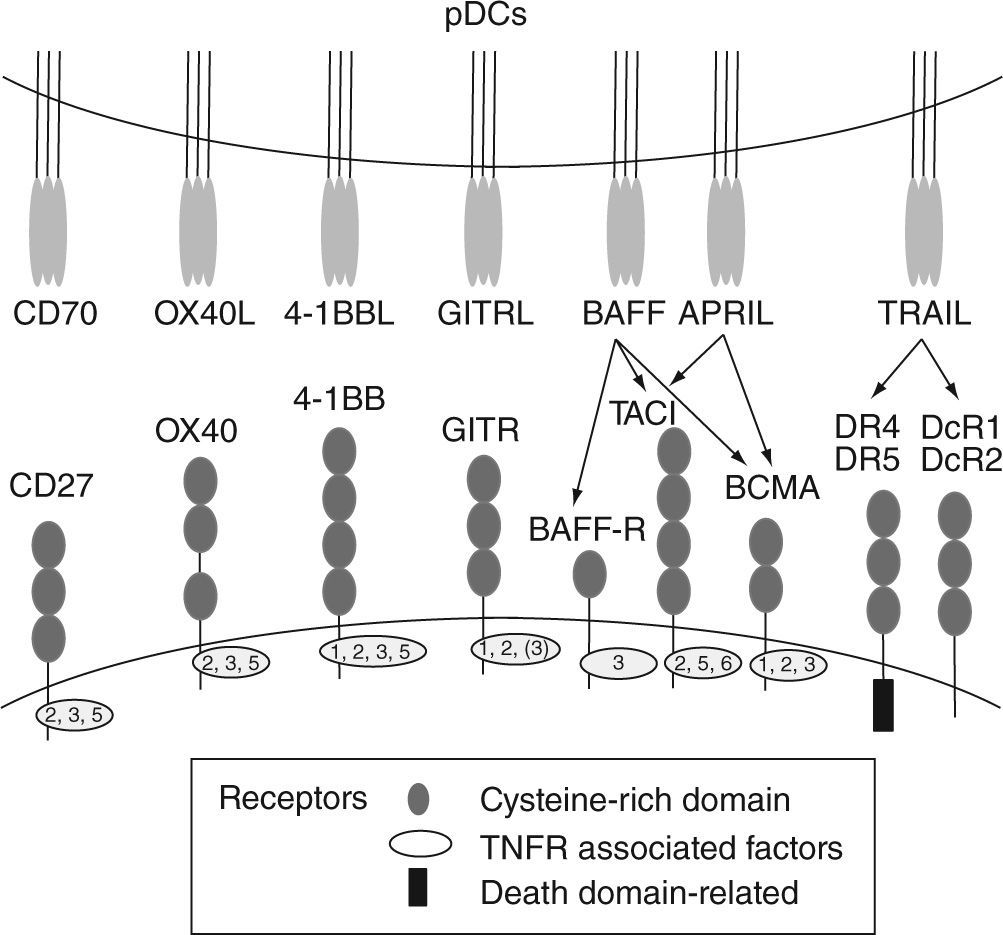
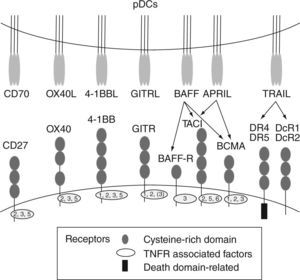
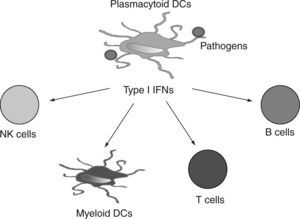
Human pDCs can be distinguished from other blood cells based on their selective expression of blood dendritic cell antigen-2 (BDCA-2) (also called CD303)13 and immunoglobulin like transcript 7 (ILT7) 14. BDCA4 (neuropilin-1), which is extensively used for positive-isolation of human pDCs, is also expressed on cultured myeloid cells15. Human pDCs also express CD4, MHC class II, CD123, and CD2. CD2 is a cell adhesion molecule that distinguishes two human pDC subsets16. One of these subsets (CD2hi) expresses lysozyme and displays cytolytic capacity. Additionally, human pDCs express two intracellular proteins, granzyme B5,17,18 and the CD2-associated protein (CD2AP)19. In contrast, human pDCs lack the lineage markers CD3, CD19, CD14, CD16, and the myeloid marker CD11c. Upon activation, pDCs upregulate the expression of "classical" co-stimulatory molecules such as CD40, CD80 and CD86 and also receptors such as CD2520 that contribute to the cellular response. In addition, activated pDCs also express high levels of different cytokines, and other co-stimulatory and accessory molecules, including TNFα, LTα, 0X40L, 4-1BBL, CD27L (CD70), GITRL, TRAIL, BAFF, APRIL (fig. 2)21-24 that together with type I IFNs, can strongly promote the activation of myeloid DCs (mDCs), natural killer (NK) cells, T cells and B cells in anti-viral and anti-tumor immune responses.
The murine counterpart of human pDCs, displaying similar phenotype and function, was identified in 200125,26. However, this review aims to summarize some human pDC attributes, such as their origin, migration to lymphoid organs, as well as recent findings on interaction of pDCs with other cells within the immune system. We will describe the differences and similarities between pDCs and the plasmacytoid dendritic cell leukemia/lymphoma or blastic plasmacytoid dendritic cell neoplasm (their leukemic counterparts), focusing on the validity of using cell lines derived from leukemic pDCs as a model to study normal pDCs. Finally, we provide a short view of the current treatments for this pathology.
pDCs origin: Lymphoid or myeloid?The lineage origin of pDCs is controversial. Based on the finding that pDCs display features commonly associated with lymphoid but not myeloid cell lineages, several studies support their lymphoid origin in humans27-29. Expression of CD2, CD5 and CD7 and lack of CD11c and the fact that GM-CSF does not promote pDCs/IPCs development30 among other extensively described patterns, contribute to the conceptual idea of an early segregation with lymphoid development, and therefore that pDCs are lymphoid-lineage derived31,32.
However, more recent studies revealed that Flt3+ cells within both the common myeloid precursors (CMP) and common lymphoid precursors (CLP), can generate pDCs in cultures and in vivo32,33. In particular, Shigematsu et al concluded that the gene expression pattern of pDCs is unique and includes activation of both myeloid-and lymphoid-related genes. Studies have also shown that although GM-CSF and Flt3L both play a critical role in the development of conventional DCs (cDCs), only Flt3L is critical for the development of pDCs27. Interestingly, in a recent paper, Sekar et al described the ex vivo generation of human pDC-like cells 34 by adding Flt3L to cultured CD14+Flt3+ monocytes isolated from human PBMCs. Although the differentiated monocyte-derived pDCs (thus called mo-pDCs) retained some of the characteristics of monocytes (i.e., CD14 expression) that are lost during long-term culture, they showed a pDC profile, such as high CD123, BDCA-4 and upregulation of BDCA-2 expression, as well.
Finally, the ability of Flt3L to promote pDCs/IPCs development in vivo was confirmed by experiments showing that administration of Flt3L into human volunteers led to an increase in the number of peripheral blood pDCs/IPCs8.
pDCs distribution and migrationpDCs are found at a very low frequency at the steady state in the blood, thymus, bone marrow, and secondary lymphoid tissue7.
Considering that the initiation of immune responses occurs mainly at the secondary lymphoid organs, spleen and lymph nodes, one may wonder how the main artifices of these well-orchestrated mechanisms mobilize to these organs. After leaving the bone marrow, pDCs appear to migrate into the T cell-rich areas of the secondary lymphoid tissues through high endothelial venules (HEV) in lymph nodes and mucosa-associated lymphoid tissues, as well as through marginal zones of the spleen under steady-state conditions. Although afferent lymphatics is the most well-established route of entry of DCs in lymph nodes, pDCs are known to enter by passing across the specialized HEVs within lymph nodes, the same route accessed by naive lymphocytes35. Accumulation of pDCs just beneath HEVs in human inflamed lymph nodes supports this assumption36.
pDCs express a set of specific chemokine receptors such as chemokine-like receptor 1 (CMKLR1), CD62L and CCR7 that interact sequentially with L-selectin ligands expressed by HEV and chemokines ELC/CCL19 and SLC/CCL21 expressed by HEV and stromal cells within the T cell-rich areas8. This specific cytokine pattern appears to mediate their recruitment across HEVs37.
Under steady state conditions, pDC migration to peripheral organs occurs infrequently in humans35, but dramatically increases in inflammatory autoimmune diseases. In systemic lupus erythematosus (SLE)38,39, psoriasis40 and allergic diseases of the airway41 pDC activation is commonly dysregulated, hence contributing to the development of the lesions through type I IFNs. In rheumatoid arthritis pDCs are recruited to the synovium where, though they appear in numbers and phenotypes similar to those in normal peripheral blood pDCs, they may contribute significantly to the local inflammatory environment42,43.
Recent studies show that pDCs also infiltrate many types of human tumors, including melanoma44, metastatic ovarian carcinoma45 head and neck cancer46, non-small cell lung carcinomas (NSCLC)47 and carcinoma of the breast, where pDCs infiltration is associated with a poor prognosis48. The capacity of type I IFNs production by pDCs was found to be inhibited by tumors via downregulation of TLR-946.
In conclusion, the migratory patterns and distribution of pDCs notably differ from those in cDCs conferring an immunological advantage or disadvantage to the host since it appears to be related to normal conditions or a particular disease.
pDCs interactions with other immune cellspDCs and T regulatory cellsIn HIV-1-infected human thymus, pDCs were shown to be fully capable of producing type I IFNs that exerts antiviral effects49 and upregulates MHC class I expression on thymocytes50. However, whether thymic pDCs play a role in the differentiation and/or selection of T cell subsets was unclear until recently. Liu's laboratory first reported that thymic stromal lymphopoietin (TSLP), a member of the hematopoietic cytokine family produced by the epithelial cells of the Hassall's corpuscles in thymus, educates thymic mDCs to induce differentiation of thymocytes into regulatory T cells (Tregs) within the medulla of human thymus51. Interestingly, TSLP appears to also play an important role in the communication between pDCs and thymocytes in the human thymus. Hanabuchi et al demonstrated that upon activation through TLR-7 or TLR-9, peripheral blood pDCs rapidly expressed the TSLP receptor and IL-7 receptor alpha complexes and therefore became responsive to TSLP52. Such TSLP-activated pDCs induced the generation and expansion of F0XP3+ Treg cells from thymocytes with a different pattern of cytokine production (more IL-10 and less TGFb) than that on the Tregs induced by TSLP-mDCs. This finding suggests that in thymus two subsets of Tregs with different cytokine production potential can be selected by TSLP-activated mDC or pDCs, respectively, and points to an active participation of thymic pDCs in Treg development. In support of this finding, similar observations were reported by Martin-Gayo et al in a system where a functional cross-talk between thymic pDCs and thymocytes may depend on CD40-CD40L interaction53.
pDCs andB cellsThe important role of pDCs in the regulation of plasma cell differentiation and immunoglobulin (Ig) secretion has been extensively described54-56. Interferon and IL-6 produced by pDCs mediate the differentiation of B cells into plasmablasts and promote their subsequent development into Ig-secreting plasma cells. However, in vitro cultures of CD40L-activated B cells in the presence of IL-2, IFNa and IL-6 produce levels of IgG lower than those observed in a pDC/B cell co-culture, suggesting a requirement for additional factors. In a recent paper, we addressed the role of CD70, a TNF family ligand, expressed by pDCs following CpG activation. After binding of CD70 to its receptor CD27 expressed on memory B cells, plasma cell differentiation and Ig secretion are promoted. This occurs independently of IFN and residual CpG, and requires physical contact between pDCs and B cells. CpG stimulated pDCs can induce the proliferation of both naive and memory B cells, although Ig secretion is restricted to the memory subset. Blocking the interaction of CD70 ligand-CD27 receptor using an antagonist anti-CD70 antibody reduces the induction of B-cell proliferation and IgG secretion by CpG-stimulated pDCs, pointing to CD70 as an important factor in the regulation of B-cell growth and differentiation by pDCs57.
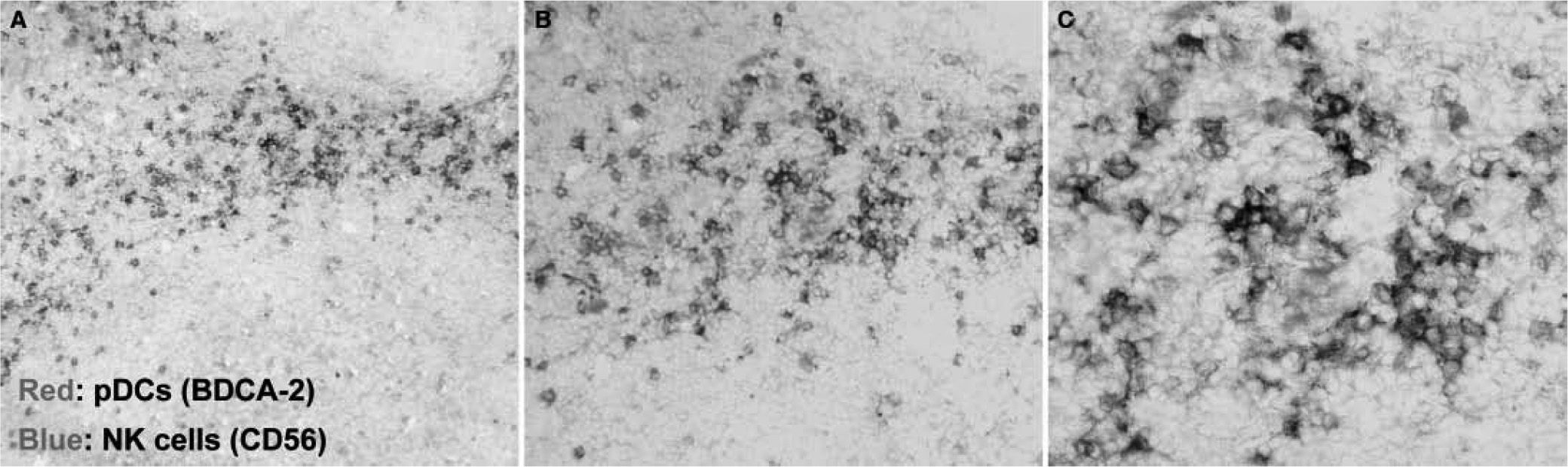
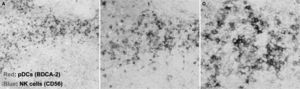
pDCs and NK cellsSeveral works demonstrated a contribution of pDCs to the migration and activation of NK cells during antiviral and antitumor responses. pDCs and NK cells are found in T cell areas in human tonsils (fig. 3) . Also, supernatants from HSV-stimulated pDCs were shown to recruit NK cells and induce their migration, driven by the chemokines CCL4 and CXCL1058. Another study demonstrated the ability of human plasmacytoid pre-dendritic cells to activate NK cells through glucocorticoid-induced TNF receptor-ligand (GITRL)24. Also, type I IFNs secreted by pDCs upon influenza A virus or CpG-ODN activation increased NK cytolytic activity and CD69 expression59. Type I IFNs has also been implicated in being responsible for pDC-stimulated NK lysis of HIV-1-infected CD4+ T cells60. In fact, pDCs have an impaired IFN-a production in HIV+ patients, leading to defective pDC/NK interplay61,62. This may be explained, at least in part, by the inhibition of type I IFNs production due to the HIV protein Vpr63.
Conversely, a controversial study showed that HCMV-infected pDCs induced CD69 expression, migration and increased of TNFa and IFNg by NK cells, but no cytotoxic activity64. Another cytokine involved in the cross-talk between pDCs and NK cells is IL-18, which induces the activation of NK cells by virally-activated pDCs, making that stimulation more potent than that led by cDCs65. Reciprocally, IL-2-activated NK cells are able to induce maturation of mDCs and pDCs59.
In vivo experiments demonstrated that CpG-activated pDCs initiate a systemic antitumor response, partly by recruiting NK cells via the production of CCR5 chemokines and increasing their IFNγ secretion mediated by 0X40L expression on pDCs66. Recently, CXCL10 and CD62L were also involved in the recruitment and increased cytotoxicity activity of NK cells induced by TLR ligand-activated pDCs in vivo67. Finally, a regulatory activity has been defined between NK cells and their target cells, among them are pDCs, through the interaction between NKR-P1A on NK cells and LLT1 on target cells that inhibit NK-cell mediated cytotoxicity and cytokine production68.
The plasmacytoid dendritic cell leukemia/lymphoma or blastic plasmacytoid dendritic cell neoplasmThe World Health Organization (WHO)'s classification of hematologic neoplasic diseases is based on morphologic, immunophenotypic, genetic and clinical criteria1. Following these criteria, the French GEIL (Groupe d'Etude Immunologique des Leucemies) published in 2002 a broad study describing the clinical, biological and cytogenetic features of a newly defined leukemia69, which they named leukemia of early plasmacytoid dendritic cells (epDC). This study was based on previous observations made by several authors on the striking similarities between pDC and leukemic cells found in patients diagnosed with NK blast lymphomas or lymphomas of unknown origin70. Finally, blastic pDC neoplasms have been included as a new entity in the 4th edition (2008) WHO classification of myeloid neoplasms.
Yet being a different entity, blastic pDC neoplasm may show clinical and pathological features that overlap with some types of acute myeloid leukemia (leukemic and skin involvement, and expression of macrophage markers). A gene-expression profiling helped to distinguish these CD4+CD56+ hematodermic neoplasms from the cutaneous myelomonocytic leukemia71, which also showed differential chromosomal alterations. Moreover, a recent study72 has provided evidence that nucleophosmin —the immunohistochemical surrogate for NPM1 mutations— is consistently found in the cytoplasm in NPMc(+) AML (because of the presence of NPM1 mutations), whilst it is nucleus-restricted (predictive of a germline NPM1 gene) in blastic pDC neoplasm.
The majority of patients suffering from this type of leukaemia are elderly, but some young patients have also been reported (ages ranged between 5 and 86 years). The disease course is very aggressive, usually with primary skin involvement including nodular cutaneous lesions in nearly 85 % of cases. Approximately half of the patients show infiltration of bone marrow and/or lymph nodes. Among all cases reported, peripheral blood malignant cells may be present as broadly as 1 % to 94 %.
Leukemic pDCs versus pDCs. How different are they?The main characteristics of pDCs have been discussed before in this review. Here, a summary of the current knowledge of LpDCs would permit the reader to identify the similarities and differences between pDCs and LpDCs.
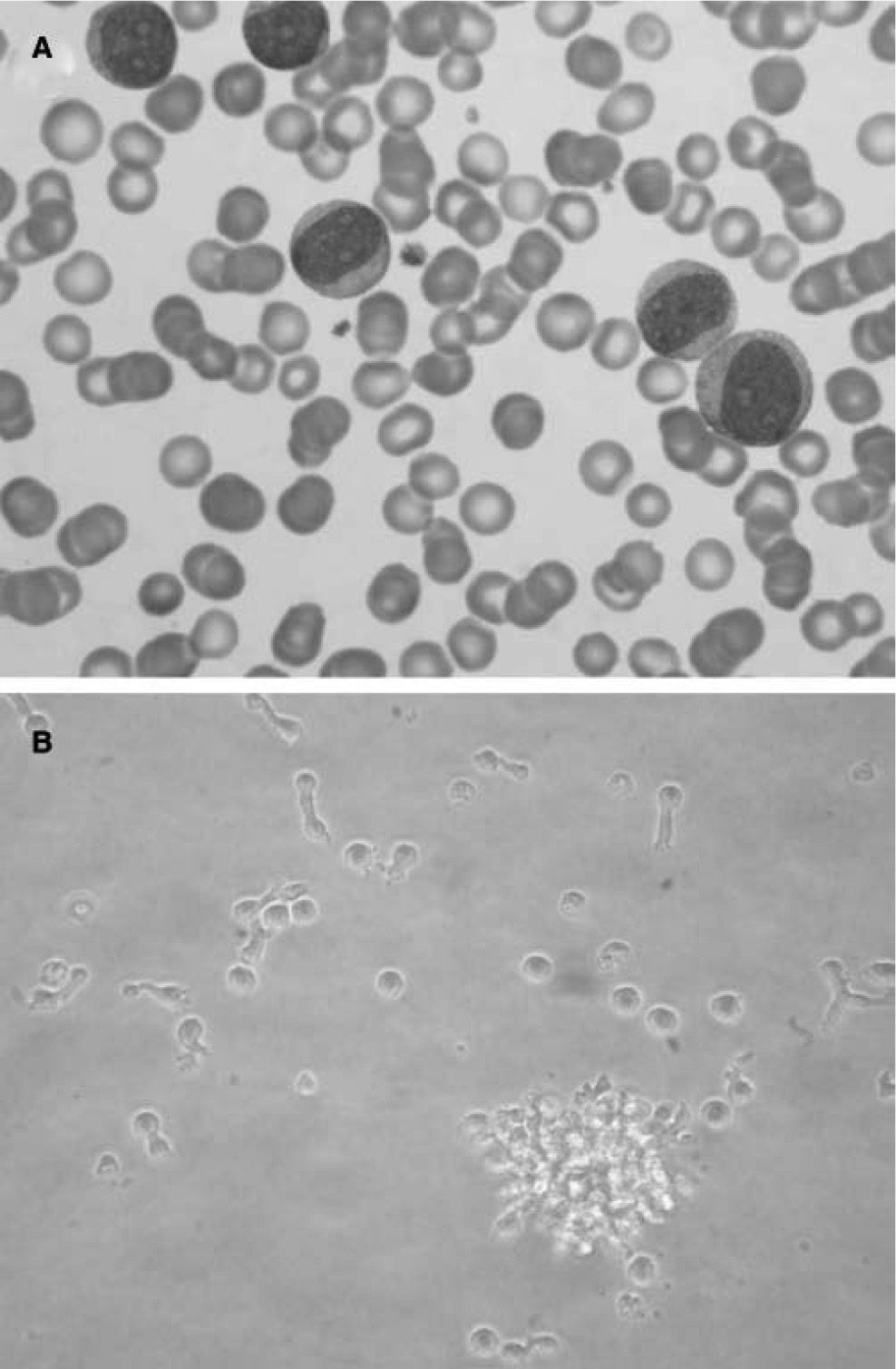
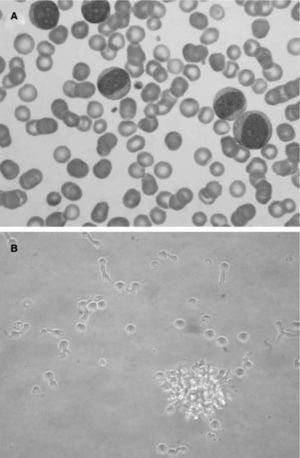
Morphologically, LpDCs have a large size and blastic-like appearance, occasionally exhibiting pseudopodia (fig. 4A) . A basophilic cytoplasm includes some granules and vacuoles. Cytochemistry reactions to myeloperoxidase and esterases are negative. A round-shaped and well-defined nucleus contains a prominent nucleolus and reticular chromatin. Cytogenetic studies have revealed a complex kariotype with partial and total deletions at different chromosomes73. Immunophenotyping of the LpDCs has been routinely negative for T cell markers (CD3, CD5), B cell markers (CD19, CD20), and myelomonocytic markers (CD13, CD14) among others. However, CD33, a prototypic myeloid marker, has been detected at different levels in some LpDC patients by different groups74,75. Leukemic pDCs are routinely CD4+CD56+, causing their previous consideration as NK lymphomas. These cells also express CD36, CD38, CD45RA, CD68, CD123 and HLA-DR, a phenotype that resembles normal pDCs. A more extended phenotype and an estimated incidence of the disease (approximately 1 %) have been reported76,77. In addition, it was shown that LpDCs may also express the specific pDC antigens BDCA-2 and BDCA-4. Importantly, the expression of the c-type lectin BDCA-2 antigen in CD4+CD56+ hematodermic neoplasms correlates positively with CD7 and negatively with the expression of terminal deoxynucleotidyl transferase, a marker of immaturity/blast stage78. These findings suggest BDCA-2 CD7+ cell expression on LpDCs is similar to non-neoplastic pDCs, indicating a relatively mature differentiation state. More interestingly, the clinical follow-up data confirm the aggressiveness of these tumors and suggest that BDCA-2 immunoreactivity as a potential marker of poor prognosis.
Leukemic pDCs (A) detected in a patient's peripheral blood sample (Hematoxilin staining) have a large size and blastic-like appearance, some of them with pseudopodia. A basophilic cytoplasm includes some granules and vacuoles. In B, isolated LpDCs cultured in vitro in the presence of IL-3 acquire a typical plasmacytoid morphology and form cellular aggregates as their normal counterparts.
In vitro cultured LpDCs exhibit typical pDC morphology and showed a rapid formation of clusters in the presence of IL-3, as expected for this cell type (fig. 4B). Although not as sensible as their normal counterparts, which die in a few hours of culture when deprived of IL-3, LpDCs do require this factor to survive in culture.
Regarding their migratory capabilities, LpDCs express CXCR3, CXCR4, and CCR7, and also CCR679. They have been shown to migrate in response to CXCR4, CCR2, CCR5, CCR6, and CCR7 ligands, and they have the ability of CXCR3 ligands to increase the responsiveness to CXCL12, which may have implications in the metastatic activity of the cells. Activation of LpDCs leads to downregulation of CXCR3 and CXCR4 and upregulation of CCR7, associated with the loss of response to CXCL12, and the acquisition of sensitivity to CCL19.
Functionally, in vitro experiments demonstrated that LpDCs behave similarly to their normal counterparts77. These cells become fully competent antigen-presenting cells (APC) after a short maturation induced by IL-3+CD40L or viral stimulation. This maturation is revealed, among other markers, by the high expression of CD83 and of the co-stimulatory molecules CD40, CD80, CD86, and also CD25 as shown for normal pDCs (Naranjo-Gómez M, unpublished observations). Similar to that described for normal pDCs, IL-3+CD40L-activated LpDCs prime a Th2 phenotype on naive CD4+ T cells, while virus-activated LpDCs preferentially drive a Th1 profile. In addition, LpDCs also endow pDCs' ability to produce IFNa upon stimulation.
LpDC generated cell linesThe scarcity of DC subsets, particularly of pDCs (around 10 cells/mL in healthy blood donors11), has often hampered the study of the biology of this cell type. Then, the identification of a leukemic counterpart and the possibility to derive LpDCs with a similar phenotype is of great interest, giving the possibility to perform studies that would not be possible with peripheral blood pDCs as a source of cells. To date, three different groups have been able to derive a cell line from pDC neoplasms.
Maeda et al80 reported the generation of a cell line derived from a patient with typical blastic NK cell lymphoma. This cell line, named CAL-1, showed characteristics of pDCs, such as morphology assessed by light and electron microscopy; negative results for CD11c and lineage-associated markers of CD3, CD14, CD19, and CD16; positive results for HLA-DR, CD4, CD56, CD45RA, and CD123; and negative results for TCR and IgH gene rearrangements. Short-term culture induced the appearance of long dendrites, and the cells were able to secrete TNFα but not IFNα.
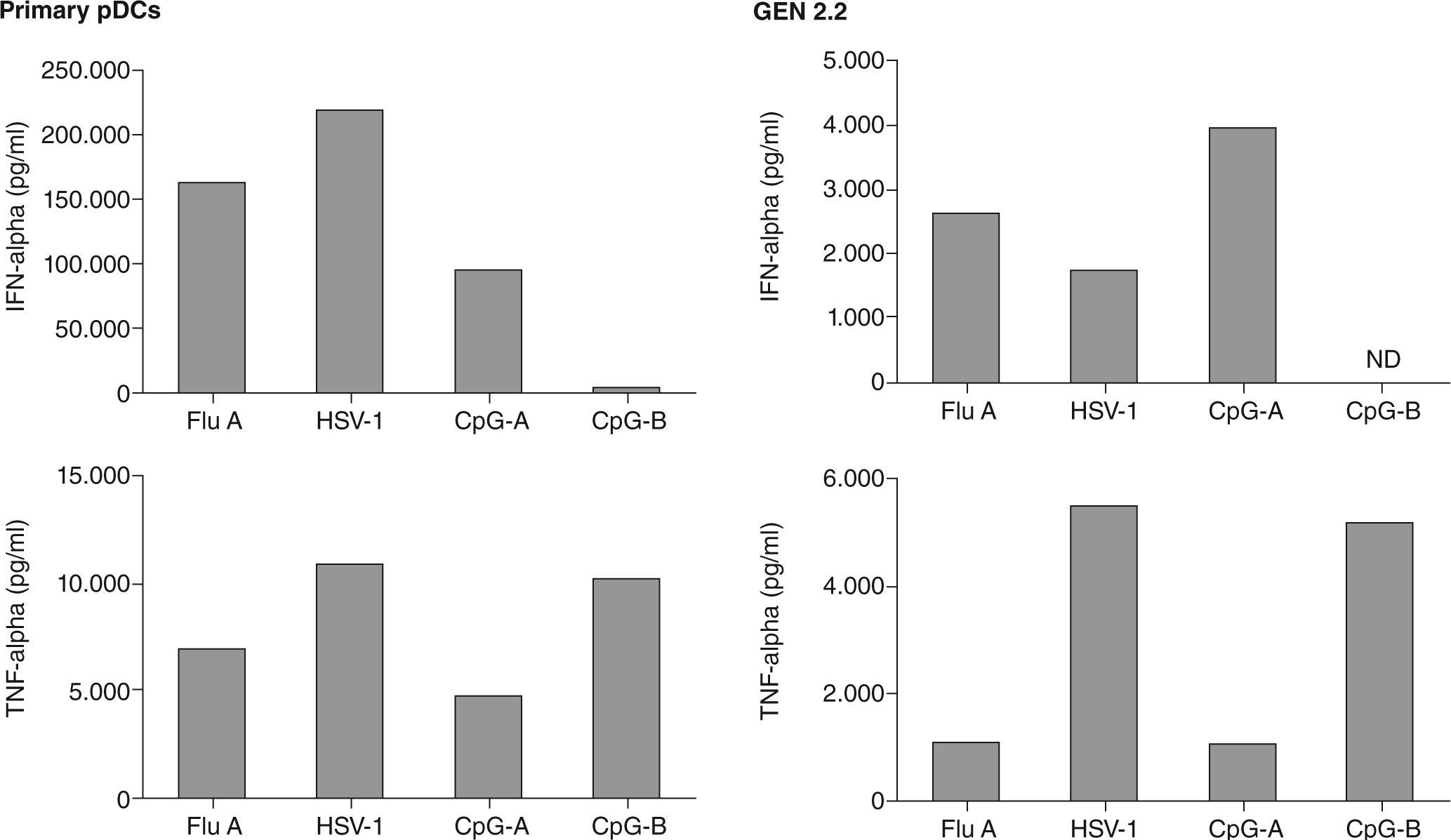
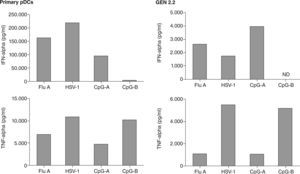
The French group who first identified the leukemic counterparts of pDCs, described the generation of a human pDC cell line called GEN2.2, derived from leukemic pDC, that shares most of the phenotypic and functional features of normal pDC81, including the secretion of IFN-a upon viral engagement. A direct comparison of IFNa and TNFa secretion by GEN 2.2 and primary pDCs has been conducted (fig. 5), demonstrating functional similarities between both cell types. Moreover, one of the most relevant results obtained with this cell line was the up-regulation of TRAIL expression on both GEN2.2 cells and also in normal pDC, and also their functional capacity to induce cell death in TRAIL-sensitive targets.
Finally, another leukemic pDC line, PMDC05, with the ability to secrete IFNa by stimulation via TLRs, has been reported82. As before, PMDC05 cells were positive for CD4, CD56, CD33, HLA-DR, CD123 and CD86 and showed no expression of lineage markers. TLR-1, TLR-2, TLR-4, TLR-7 and TLR-9 mRNA were detected and stimulation with CpG-A leads to the secretion of IFNa. Curiously, the authors also reported that PMDC05 cells could secrete IL-12, which is not produced by normal pDCs, and had the ability to respond to LPS, a feature not endowed by normal pDCs. In addition, this cell line can also transform to a myeloid phenotype83.
Thus, although it is clear that LpDC-derived cell lines are a useful tool to investigate the pathophysiology of LpDCs, their particular behaviour may preclude direct extrapolations to normal pDCs, and parallel experiments with peripheral blood normal pDC should be performed when investigating the biology of these cells.
Treatment approaches for the plasmacytoid dendritic cell neoplasmTo date, no optimal treatment has been found for this malignancy. Despite a high rate of initial response to chemotherapy, early and widespread relapses occur and a fatal outcome is expected by two-three years, when patients die of disease progression. It is therefore considered that this aggressive disease requires a radical approach that may consist of multi-agent chemotherapy followed by allogeneic hematopoietic stem cell transplantation. Classical treatments such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) or CHOP-like chemotherapy are rather uneffective, thus stressing the need for new drug designs and/or combinations. However, to date there is no consensus on the first-line treatment for these patients.
New combinations of therapies have been tested in individual patients in an effort to delineate an effective treatment. Martín et al84 reported two cases with different outcomes. A patient showing 25 % infiltration of the bone marrow at diagnosis received cytostatic treatment with Rituximab (anti-CD20 antibody, despite leukemic cells were CD20 negative) and six cycles of CHOP leading to a complete remission of the disease. The patient remained without symptoms 1 year after treatment. Interestingly, despite being CD20 negative as in the majority of LpDCs, this patient responded to the treatment, thus perhaps B-cell depletion may have a beneficial effect on the erradication of the leukemia. In this sense, it is worthy to mention another case of a patient suffering from a pDC neoplasm treated successfully with rituximab85. The other patient in the Martin study, who did not present bone marrow infiltration at diagnosis, received cytostatic treatment with six cycles of 'CHOP', leading to a complete remission of the disease. However, new cutaneous lesions appeared four months later and the patient suffered a progressive pancytopenia due to bone marrow infiltration. Additional chemotherapy was uneffective and the patient died 3 months later due to progression of the neoplastic process.
A case report showed a remarkable clinical response with regression of skin tumors using pralatrexate plus vitamin B12 and folic acid in a patient who had previously received two courses of conventional chemotherapy followed by a quick relapse86. In another case report, an early stage of the disease was successfully treated with an aggressive regime including methotrexate-asparaginase and local radiotherapy as first-line therapy87. Complete clinical remission was rapidly reached and the patient is alive after > 30 months of follow-up.
Despite the efforts in finding new drug combinations and treatments, skin lesions may be refractory to therapy. However, a recent report showed an excellent response to radiotherapy plus hyperthermia in an individual case88. This treatment would not avoid the systemic evolution of the disease, but may represent a therapeutic option to be used in combination with chemotherapy regimens.
Therefore, it is conceivable that an aggressive first line treatment at early stages of the disease, combined with other regimens, such as those used in acute leukaemia, may help to improve the outcome of these patients, but further investigations are needed to envisage adequate treatments.
Concluding remarks and future perspectivesIn 1997, Yong-Jun Liu and collaborators were the first to isolate pDCs and demonstrated that they represented a new cell type of the innate immune system, specialized in mounting robust type I IFN responses to viral infection.
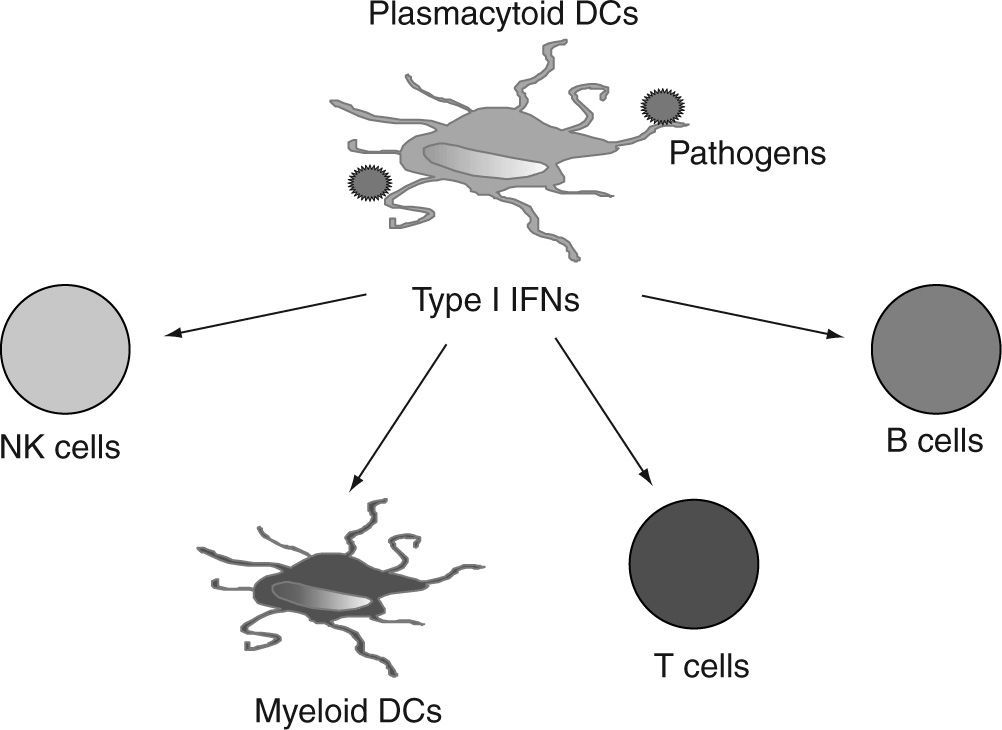
After this initial observation, several years of investigation demonstrated that activated pDCs expressing high levels of costimulatory molecules and type I IFNs, can strongly promote the activation of mDCs, NK cells, T cells and B cells in anti-viral and anti-tumor immune responses (fig. 6). Therefore, pDCs are not only the most important effectors in anti-viral innate immunity, but they also link innate and adaptive immunity. Importantly, pDCs have two functional faces: they can perform different and even opposing functions in immune regulation, depending on the signals from pathogens and tissue microenvironments that they receive. Understanding this functional plasticity of pDCs could help us to comprehend how normal pDCs (and their leukemic counterparts) react to different stimuli and may represent the biological basis for designing strategies to reprogram pDC function from "bad" or undesired immunity to "good" or controlled immune responses in the pathological microenvironment that they infiltrate.
FundingF.E.B.'s group is supported by grants (FIS 06/0453 and 09/0229) from the Instituto Carlos III (ISCIII) of the Spanish Ministry of Science and Innovation (MCIN). MNG is supported by a contract "Torres Quevedo" (PTQ 09/02-01750) from the MCIN. BPC is supported by a grant (FI 07/00054) from the ISCIII.
Conflict of interestsAuthors declare no conflict of interests.
Authors would like to thank Dr. Yong-Jun Liu for useful comments and Melissa Wentz for editing the manuscript.