T lymphocyte antigen activation is facilitated by clustering of membrane glycosphingolipid-enriched microdomains (GEMs, lipid “rafts”) at the T cell/APC contact that is linked to changes in actin cytoskeleton and is one major mechanism of CD28 costimulation. Ligation of CD28 alone, or ligation of the CD28-like molecules CTLA-4 (CD152) and ICOS (CD278) induces actin polymerization with cell elongation and generation of lamellipodia and filopodia in T cells. These changes are dependent on Src, PI3-kinase, Vav, and Rho family GTPases. Whereas CD28 and CTLA-4 have been shown to be functional and physically associated with lipid rafts, the presence of ICOS in lipid rafts or its effect in raft clustering is not known. In this work, we have activated the T cell line D10 with anti-ICOS antibodies, alone or combined with anti-CD3 antibodies, bound or unbound to polystyrene microbeads or glass coverslips. The possible relationship of ICOS-induced changes in actin cytoskeleton to the ICOS localization in membrane rafts was then analyzed by fluorescence microscopy, or by immunoblot of detergent insoluble (“raft”) or soluble (“non-raft”) fractions of cell lysates. Our data show that ICOS promotes TCR/CD3 induction of raft clustering at the site of activation. However, ICOS, which, on its own, can induce accumulations of polymerized actin, is undetectable in membrane rafts, even when using CD3 or ICOS, ligands capable of inducing clear changes in the actin cytoskeleton.
La activación de linfocitos T se facilita por la concentración, en el sitio de interacción con el ligando, de microdominios de membrana enriquecidos en glicoesfingolípidos (GEM, o “balsas” lipídicas). Este fenómeno está unido a, y es dependiente de cambios en el citoesqueleto de actina, siendo uno de los principales mecanismos implicados en la coestimulación por CD28. El entrecruzamiento de CD28 aisladamente, o de moléculas de su familia como CTLA-4 (CD152) e ICOS (CD278) inducen en linfocitos T polimerización de actina acompañada de elongación celular y aparición de lamelipodia y filopodia. Estos cambios son dependientes de Src, PI3-cinasa, Vav, y GTPasas de la familia Rho. Se han descrito relaciones funcionales y físicas de CD28 y CTLA-4 con balsas lipídicas, pero se desconoce si ICOS se encuentra en estos dominios, o su efecto sobre la agrupación de balsas inducida por ligandos.
En este trabajo se han activado células T de la línea D10 con anticuerpos anti-ICOS, solos o combinados con anticuerpos anti-CD3, y unidos o no a microesferas de poliestireno o a cubreobjetos de vidrio. En estas células se ha determinado la posible relación entre los cambios en el citoesqueleto de actina y la localización de ICOS en las balsas lipídicas mediante microscopía de fluorescencia, o mediante “inmunoblot” de las fracciones de lisados insolubles (“balsas”) o solubles (“no-balsas”) en detergente. Nuestros datos muestran que ICOS incrementa el agrupamiento de balsas lipídicas inducida por anticuerpo anti-CD3 en el sitio de contacto con el estímulo. Sin embargo, ICOS, que por sí solo induce acumulación de actina polimerizada, es indetectable en las balsas de membrana, incluso empleando ligandos (CD3 o ICOS) capaces de inducir cambios claros en el citoesqueleto de actina.
The Inducible Costimulator (ICOS, CD278) is a 50–65kDa surface disulfide-linked homodimeric glycoprotein of the CD28 family characteristically expressed by activated T lymphocytes1–5 (reviewed in6–10). After activation, ICOS expression is clearly detectable in the surface of T cells by 24h, and peaks by day 7.1,5,11 Optimal expression of surface ICOS in activated naïve T cells needs CD28 costimulation, which is mediated in part by IL-2 and IL-4.12,13
CD28 is unique among T cell costimulatory molecules because of its ability to support antigen-induced proliferation of naïve T lymphocytes, while preventing antigen-induced anergy.14,15 CD28 costimulation fosters naïve T cell proliferation, differentiation and effector functions by the coordinated activation of a program of gene transcription, leading to secretion of cytokines like IL-2, cell cycle progression, enhanced survival, and changes in cell metabolism and motility (reviewed in10,16).
CD28 potentiates TCR-specific signalling, and its 41 amino acid-long cytoplasmic tails have sequence motifs that allow association of a number of adapter proteins or enzymes that confer CD28 their specific signalling properties.10,16 Particularly, the YMNM motif binds the SH2-domains of Class IA phosphatidyl inositol-3 kinase (PI3-K) regulatory subunits upon phosphorylation of the tyrosine residue, and the same motif binds the Grb2 adapter SH2 domain. One polyproline motif (PRRPGP) binds a SH3 domain of the IL-2-inducible T-cell kinase (Itk); whereas a second proline motif (PYAPP) binds the SH3-domains of the Lck tyrosine kinase, Grb2, or the scaffolding protein Filamin-A (FLNa). A second C-terminal tyrosine-containing motif (YRS) can also bind regulatory subunits of Class IA PI3-K.17 Of note, other members of the CD28 family such as CTLA-4 and ICOS share with CD28 one YxxM motif that binds PI3-K regulatory subunits upon phosphorylation, but not the other motifs or binding proteins.
There are data showing that early events in the activation of T cells by antigen are facilitated, on one hand, by the coalescence of plasma membrane glycosphingolipid-enriched microdomains (GEMs, lipid “rafts”) at the T cell/APC contact.18–20 On the other, there is abundant evidence of the involvement of changes in the actin cytoskeleton in these events.18–21 Both phenomena are essential to the coordinated recruitment of signalling components ending in the formation of mature immunological synapses. In this regard, GEMs are enriched in proteins involved in early antigen receptor signalling, including Src family tyrosine kinases. There are also data supporting a prime role of GEMs as sites of inositol lipid metabolism because of the preferential localization within GEMs of phosphatidyl inositol phospholipids PIP2.18–21
The signalling mechanisms that coordinate the reorganization of GEMs are not exactly known, but protein clusters located both inside and outside lymphocyte GEMs interfaces with the actin cytoskeleton.22,23
It has been long known that CD28 costimulation facilitates activation of naïve T lymphocytes by rapid redistribution and clustering of raft microdomains at the site of T cell receptor (TCR) antigen recognition engagements.24,25 This results in enhanced and more stable tyrosine phosphorylation of substrates, and requires CD28-mediated redistribution of the actin cytoskeleton.26,27 As mentioned above, the CD28 cytoplasmic domain binds filamin-A (FLNa), an actin-binding protein that functions as a scaffold coupling the cell cytoskeleton to surface receptors (reviewed in28,29). In addition, CD28–FLNa interaction is required to induce the T-cell cytoskeletal rearrangements that recruit lipid microdomains and signalling mediators into the immunological synapse.30 Thus, it seems that the enrichment of rafts at the T cell/APC contact and the changes in the actin cytoskeleton are mechanistically linked.
Rapid polarization of lipid rafts at the T cell–bead interface can be also achieved using microbeads coated with anti-CD28 antibodies alone, with simultaneous activation of Vav-1 and enhanced intracellular Ca2+ levels, suggesting a direct relationship of CD28 to membrane rafts.25 Interestingly, another member of the CD28 family like CTLA-4 (CD152) can be found in membrane rafts.31,32
In parallel, it has been shown that ligation of CD28, CTLA-4, or ICOS alone induces profound changes in the actin cytoskeleton of T cells. Thus, CD28 engagement induces the formation of cytoplasmic elongations (microspikes or filopodia) enriched in filamentous actin (F-actin). These CD28-induced cytoskeletal changes required Src family kinase activity, and promoted phosphorylation and membrane localization of the Rho family GEF Vav. Because of CD28-induced PI3-kinase activation, it is likely that the phosphatidyl-inositol binding PH domain of Vav is involved in its membrane localization, which then recruits and activates Rho family GTPases like Rac1 and Cdc42 that modify the actin cytoskeleton.25,33–36 Ligation of CTLA-4 or CD3/CTLA-4 ligation (but not CD3 ligation) also induced T cell polarization within 15–30min, with increased formation of lamellipodia, filopodia, and uropod37. Importantly, PI3-kinase activity was essential to cell polarization that was also dependent on the Rho family GEF Vav1, or Rho GTPase like Cdc42, but not on PKB (Akt, one major substrate of PI3-kinase).37
Lastly, ICOS ligand bound to flat surfaces induces PI-3K-dependent re-organization of the actin cytoskeleton producing elongation of T cells expressing ICOS.38–40 However, there are data either against38,39 or in favour40 of a role for Akt. There are also data showing a role for Rho family members like Rac and Cdc42, in ICOS-mediated T cell elongation (40; Acosta, Y., Rojo, J.M. unpublished data). According to Franko and Levine, ICOS-mediated T cell elongation also depends on the PI-3K-dependent inhibition of RhoA accumulation involved in uropod retraction.39
In this work, the possible relationship of ICOS-induced changes in actin cytoskeleton to the ICOS localization in membrane rafts has been analyzed. Our data show that ICOS is not present in detectable amounts in rafts, even under conditions (CD3 or ICOS ligation) that induce clear changes in the actin cytoskeleton. Moreover, actin polymerization induced by ICOS failed to induce lipid rafts clustering.
Materials and methodsCellsCells from the CD4+ICOS+ Th2 cell line SR.D10 (D10) were used throughout the study.41,42 SR.D10 is a subclone of the D10.G4.1 CD4+ T cell line.43
Antibodies and other reagentsThe monoclonal antibodies (mAbs) used were one armenian hamster anti-mouse/human ICOS (CD278) (C398.4A1,3), a mouse anti-D10 clonotypic antibody (3D343), rat anti-mouse CD3 (YCD3-144) and rat anti-mouse CD11b (M1/7045). All were purified from hybridoma supernatants by protein A- or protein G-affinity chromatography. Polyclonal anti-mouse CD3¿ antibodies were obtained by immunization of rabbits with a fusion protein of the extracellular domain of mouse CD3¿ and GST.46 Rabbit antiserum against a peptide comprising residues Gly50 to Gly63 of mouse CD4 was raised by immunizing with the peptide coupled to ovalbumin.47 Anti-Lck antibodies were obtained by immunizing rabbits with a fusion protein comprising the residues between Gly2 and Ser59 of human Lck and GST. Rabbit anti-ICOS was obtained by immunizing rabbits with a fusion protein comprising most of the extra-cellular domain of mouse ICOS (residues Thr18 to Ser132) and GST; anti-Vav antibodies were raised in rabbits immunized with a fusion protein of mouse onco-Vav1 residues 738–845 and GST. The antibodies were affinity purified over columns of the immunizing antigen coupled to CNBr-Sepharose. Streptavidin-Sepharose and horseradish peroxidase-coupled Protein A were from Sigma; Protein A-Sepharose was from GE Healthcare. Cytochalasin D, Wortmannin, PI3-Kα Inhibitor VIII (PIK-75), PI3-Kβ Inhibitor VI (TGX-221), PI3-Kγ Inhibitor, and PI3-Kγ Inhibitor II were from Calbiochem; the PI3-kinase inhibitor LY 294002 was from Sigma. Cholera toxin B subunit coupled to FITC and FITC-Phalloidin were from Sigma; AlexaFluor-568-Phalloidin was from Molecular Probes.
Activation with antibody-coated beadsFor visualization of lipid raft clustering or actin polymerization, cells were stimulated either with antibody-coated polystyrene microspheres (Polybeads, 4.5μm diameter, Polysciences Europe GmbH, Eppelheim, Germany) as described previously,46 or activated with ligand-coated coverslips. The microspheres (20–40×106/ml in PBS) were coated with antibodies by overnight incubation at 4°C with anti-CD3 mAb (YCD3-1, 2μg/ml) or M1/70 as a negative isotype control antibody (M1/70, 2μg/ml) plus anti-ICOS antibody C398.4A or control antibody at 10μg/ml, unless stated otherwise. The beads were washed with PBS and eventually mixed with T cells (1:1 cell:bead ratio, 108cells/ml), and incubated at 37°C for 20min at 37°C. The stimulated cells were seeded on washed, poly-l-lysine-coated coverglasses (1mg/ml overnight at 4°C). After brief incubation at room temperature, the unattached cells were washed with PBS, and the cells remaining were fixed with 4% paraformaldehyde in PBS for 5min at 37°C, and washed. For detection of membrane rafts, ganglioside monosialic acid (GM1) was stained with CTB-FITC (5μg/ml in PBS/0.1% bovine serum albumin) for 30min at 4°C, washed with PBS, and mounted using Vectashield antifading. To stain polymerized actin, paraformaldehyde-fixed cells were permeabilized with 0.1% saponin in PBS/0.1% bovine serum albumin (PBS saponin, 5min at 20°C), and then stained with 50nM FITC- or Alexa-Fluor-568-coupled phalloidin in PBS saponin. After washing with PBS saponin, the coverglasses mounted in Vectashield as above.
In some experiments, the cells were mixed with anti-ICOS- or poly-l-lysine (PLL)-coated beads (10μg/ml) at a 4:1 cell:bead ratio, 108cells/ml, and incubated 5min on ice. Then, 0.25–0.3×106 D10 cells were taken, seeded on 24-well plates containing poly-l-lysine-coated coverglasses (0.1mg/ml overnight at 4°C) in 0.5ml of 10mM HEPES, 0.1% glucose PBS, pH 7.2 and briefly spun. The cells were incubated for 30min at 37°C, washed with PBS, fixed with 4% formaldehyde and stained as described above.
Confocal microscopy analysis was performed in an Axiomat135 Zeiss microscope equipped with a MRC1024 Bio-Rad confocal sytem (Bio-Rad) or a Leica TCS-SP2-AOBS-UV ultraspectral confocal microscope. Immunofluorescence analysis was done using a 63×1.4 objective lens at 0.5μm intervals. Signals from different fluorescent probes were taken in parallel. One hundred cells were analyzed for each labelling condition, and representative results are shown. Image processing was performed with Adobe Photoshop (Adobe Systems, Mountain View, CA). Alternatively, the images were acquired at room temperature at a magnification of 40 or 40×1.25 using an Axioplan Universal microscope (Carl Zeiss, Jena, Germany) and a Leica DFC 350 FX CCD camera.
Adhesion and elongation assayTen mm diameter glass coverslips were placed into 24-well tissue-culture plates and incubated overnight at 4°C with 0.3ml of anti-ICOS antibody, or poly-l-lysine (10μg/ml in PBS). The coverslips were washed with PBS, and 0.25–0.3×106 D10 cells were added in 0.5ml of 10mM HEPES, 0.1% glucose PBS, pH 7.2. After a brief centrifugation, the cells were incubated for 20–40min at 37°C, washed with PBS, and fixed for 5min with 0.5ml of 4% formaldehyde warmed at 37°C. After washing with PBS, the coverslips were mounted on Vectastain (Vector). Alternatively, to stain F-actin, the cells were further permeabilized for 5min with PBS/0.1% Saponin. Then, the cells were stained for 30min with 50nM FITC-Phalloidin (Sigma) or Alexa Fluor-568-Phalloidin (Molecular Probes) in PBS/0.1% Saponin. After further washing with PBS/0.1% Saponin, the cells were washed once with PBS and the coverslips mounted on Vectastain (Vector). Cell images were acquired with an Axioplan Universal microscope (Carl Zeiss, Jena, Germany) and a Leica DFC 350 FX CCD camera, as described above. The images were recorded on disk and analyzed using Adobe Photoshop 6.0 (Adobe). Quantification of elongation and cell size was performed using ImageJ 1.38× public domain software (National Institutes of Health). For each cell, the elongation index was calculated as the ratio of the longest axis to the longest segment perpendicular to the first one. The adhesion surface was measured by the projected contour of the cells. In every experiment, 10–20 cells/field in six different fields were quantified.
Isolation of glycosphingolipid-enriched microdomains (GEM)Detergent-insoluble membrane microdomains, or GEM was separated essentially as described in,31 with minor modifications. For each experimental condition, 40–50×106cells were activated, or not, with the indicated antibodies (10μg/ml, 15min at 37°C), washed, and then lysed in 1ml of 25mM MES, 150mM NaCl, 1mM EDTA, 0.5% Triton X-100, 1mM PMSF, 10μg/ml Aprotinin, 1mM Na3VO4, pH 6.5. The lysate was mixed v:v with 85% sucrose in 25mM MES, 150mM NaCl, pH 6.5 (MBS) and set on a ultracentrifuge tube. The lysate was carefully overloaded with 5ml of 35% sucrose in MBS and then with 5ml of 5% sucrose in MBS. The samples were centrifuged 16–19h at 4°C at 200,000×g. Fractions of 1ml were taken from above and were analyzed by dot-blot for GM-1 content. Raft (GM1-positive fractions, usually fractions 3–6) and non-raft (usually fractions 10–12) were pooled. Proteins in the pooled raft and non-raft fractions were precipitated with cold acetone and resuspended in 1× SDS-PAGE reducing sample buffer before SDS-PAGE separation and immunoblot analysis.
ImmunoblotSamples were separated by SDS-PAGE in 10% acrylamide gels. Proteins were transferred to PVDF membranes (Immobilon-P, Millipore); these were blocked for 2h with PBS/0.1% Tween (PBST) containing 3% non-fat dry milk. The membranes were washed with PBST and incubated overnight with rabbit polyclonal antibodies in PBST/0.2% gelatin/0.1% NaN3. After further washing with PBST, PVDF membranes were incubated for 1h at room temperature with HRP-coupled Protein A in PBST-containing 3% non-fat dry milk. The membranes were washed with PBST and eventually the blots were visualized on X-ray films using the Supersignal West Pico chemiluminiscence substrate (Pierce).
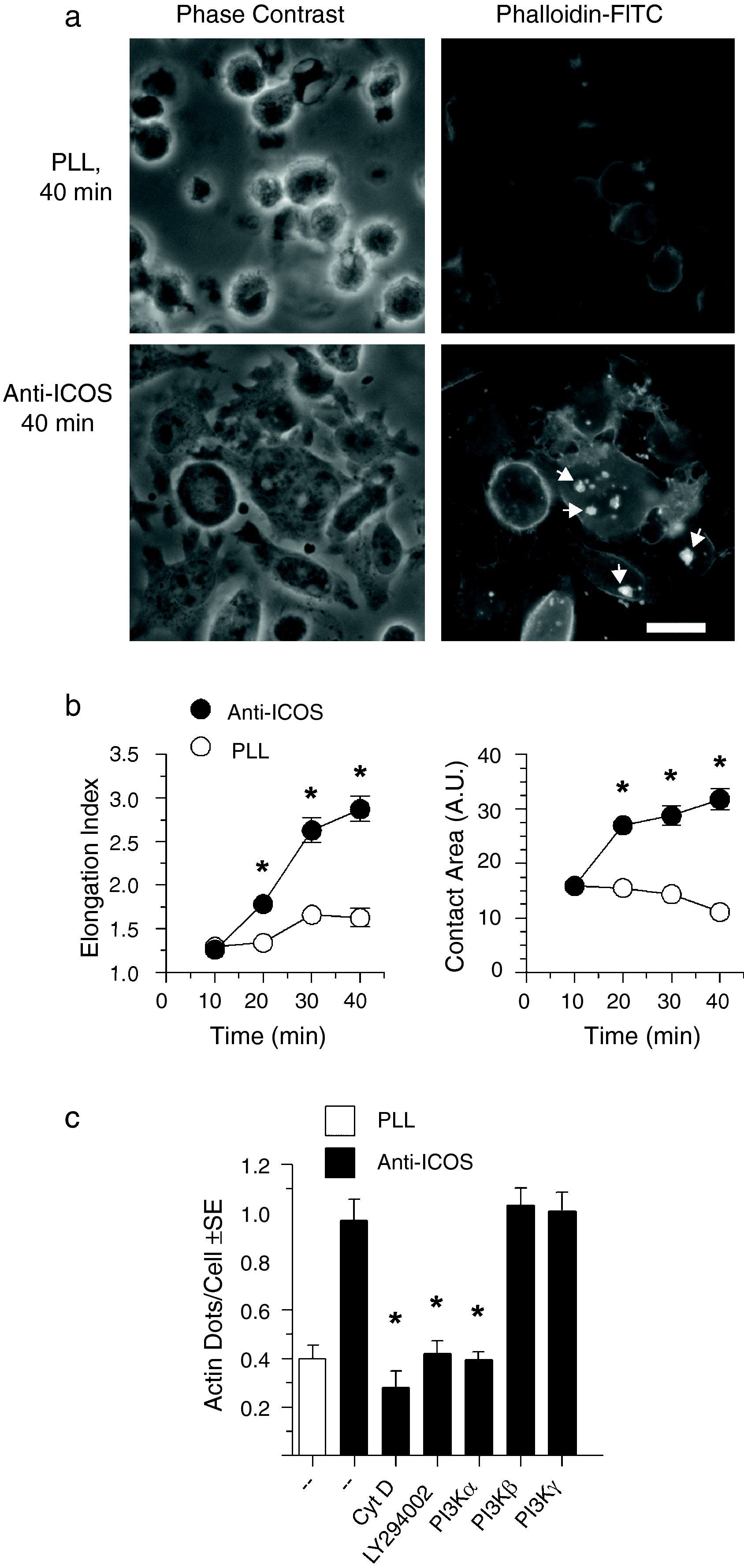
Results and discussionPI3-kinase dependence of ICOS induced cell spreading and actin polymerizationSeveral reports show that, in the absence of other stimuli, ICOS ligands (i.e. B7h, anti-ICOS antibodies) adsorbed to solid substrates induce PI-3 kinase-dependent changes in the actin cytoskeleton leading to T cell elongation and filopodia formation.38–40 Furthermore, our previous data show that it is the p110α catalytic isoform of PI3-kinase that is primarily involved in this phenomenon.38 We show here that in D10 cells, elongation is accompanied by cell spreading with distinct lamellipodia- and filopodia-like structures (Fig. 1a). ICOS-induced cell spreading and elongation are fast processes, reaching a maximum at 30–40min of incubation (Fig. 1b). Besides, a few dense grains or dots of polymerized actin usually appear in the attached cells (Fig. 1a, lower right); they are located very close to the cell-coverslip contact surface, as determined by confocal microscopy (data not shown). Then, we checked whether the appearance of these actin dots was also dependent on PI3-kinase. Indeed, actin grains were depleted by depolymerization of actin by Cytochalasin D, but also by the PI3-kinase inhibitor LY294002 (Fig. 1c). Furthermore, inhibitors of the p110α catalytic isoform, but not of the p100β or p110γ isoforms significantly inhibited actin grains (Fig. 1c), in full agreement with our previous data concerning ICOS-induced cell elongation.38
ICOS ligation induces cell elongation and spreading as well as actin condensation that depends on PI3-kinase activity. (a) D10 cells were incubated (40min at 37°C) on coverslips coated with 10μg/ml anti-ICOS antibody or poly-l-lysine. Fixed cells were permeabilized and F-actin stained with Phalloidin-FITC. ICOS ligation induced a clear elongation and spreading of the cells, with numerous microspikes and a few dots of polymerized actin as those indicated with arrows (lower right). Bar: 10μm. (b) Time-course of ICOS-induced D10 cell elongation and spreading measured as elongation index (left panel) and cell-surface contact area (right panel). (c) Effect on ICOS-induced actin dots of actin polymerization inhibitor Cytochalasin D (10μM), or PI3-kinase inhibitor LY294002 (20μM), or inhibitors specific for PI3-kinase p110α (PIK-75, 1μM), p110β (TGX-221, 1μM), or p110γ (PI3-Kγ Inhibitor II, 12μM). In each case, 10–20 cells/field in six different fields were examined. Asterisks in (a), (c) in indicate significant differences (p<0.05) as determined by the two-tailed Student's t test.
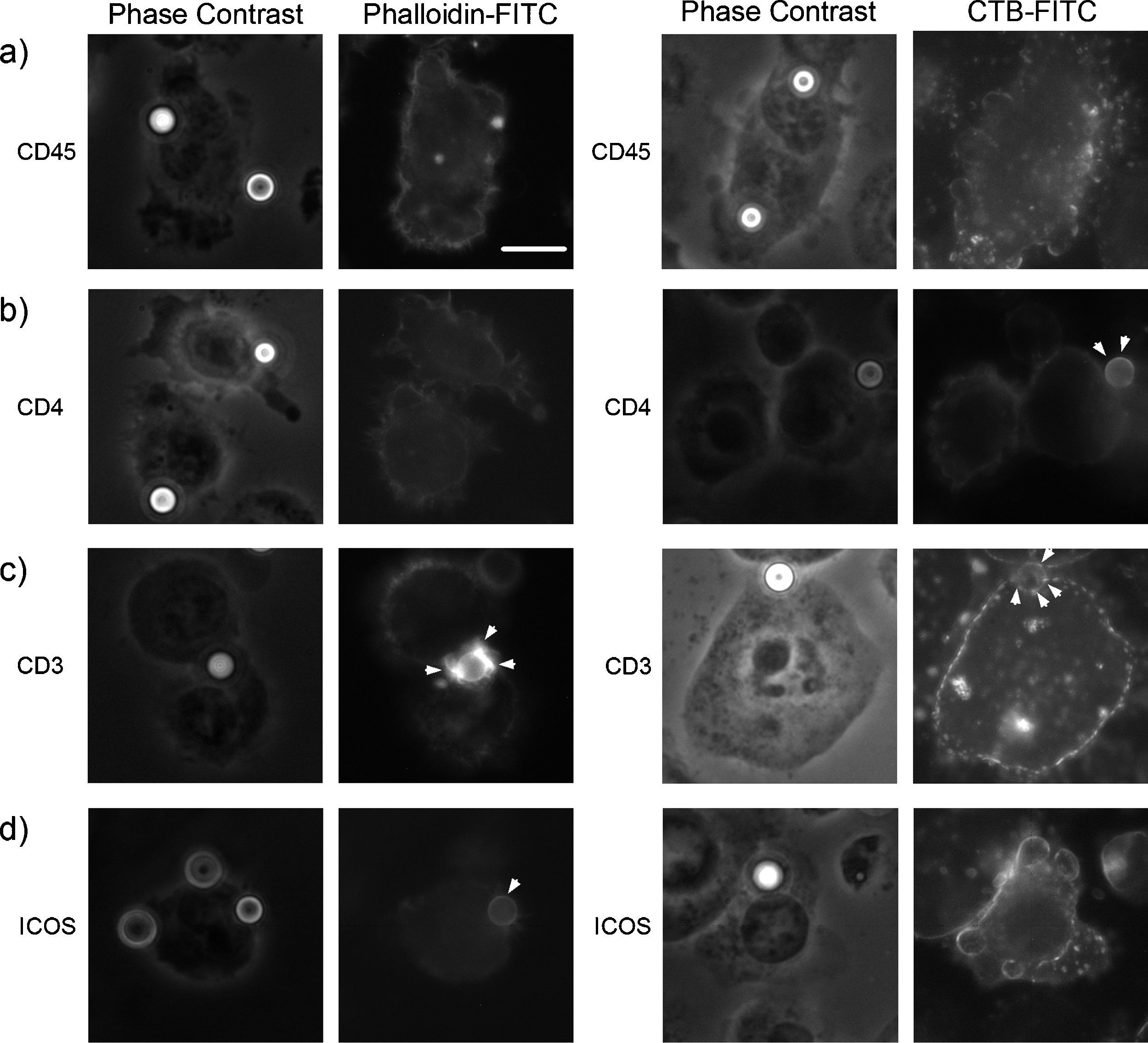
Our previous data show that ICOS-induced T cell elongation is dependent on the Lck tyrosine kinase38 which, like other Src family kinases, is a resident of membrane GEM.48 This suggested a functional link of ICOS to rafts. Furthermore, other previous results in human T cells showed partial co-capping between ICOS and another resident protein in GEM, namely the CD4 correceptor.3,49 In view of these data, we then determined whether changes in actin polymerization induced by ICOS were accompanied by redistribution of surface GEM, as shown by staining of GM1 gangliosides with cholera toxin B (Fig. 2). In this instance, the cells were mixed with polystyrene microbeads previously coated with monoclonal antibodies specific for ICOS, or other surface molecules that are present (CD4) or not (CD45) in GEM.48,49 As shown in Fig. 2, CD45-specific microbeads did not induce detectable increase in actin polymerization (Fig. 2a, left) or raft accumulation (Fig. 2a, right) at the site of cell–bead contact. CD4-specific beads did not induce enhanced actin polymerization near the site of contact with beads (Fig. 2b, left), yet raft accumulation was observed as rims around the beads (Fig. 2b, right). Cells incubated with beads coated with antibodies directed at the TCR/CD3 complex induced a strong accumulation of polymerized actin at the site of contact (Fig. 2c, left) that was accompanied by a minor but detectable fraction of the cells’ GEM (Fig. 2c, right). Lastly, actin polymerization was detected at the site of contact with ICOS-specific microbeads, which were often coated by a rim of F-actin (Fig. 2d, left). However, no parallel accumulation of rafts could be detected by CTB staining of cells with beads coated with anti-ICOS antibody (Fig. 2d, right), as has been described for some CD28 ligands.25
Effect of ligation of ICOS and other cell surface molecules on the localization of membrane GEM and actin polymerization. D10 cells were mixed with polystyrene microbeads coated with 10μg/ml monoclonal antibodies to (a) CD45; (b) CD4; (c) CD3, or (d) ICOS, seeded on PLL-coated coverslips and incubated for 40min at 37°C. Then, the cells were fixed and the localization of polymerized actin was determined by phalloidin-FITC staining (left panels). Alternatively, GEM distribution was determined by staining with CTB-FITC (right panels). One hundred cells were examined for each labelling condition by phase contrast and fluorescence microscopy, and representative results are shown. Actin or GEM accumulation at cell–bead interface is indicated by arrowheads, at least 15% of the cells had the pattern shown. Actin or GEM accumulation in the other conditions depicted was <2%. Bar: 10μm.
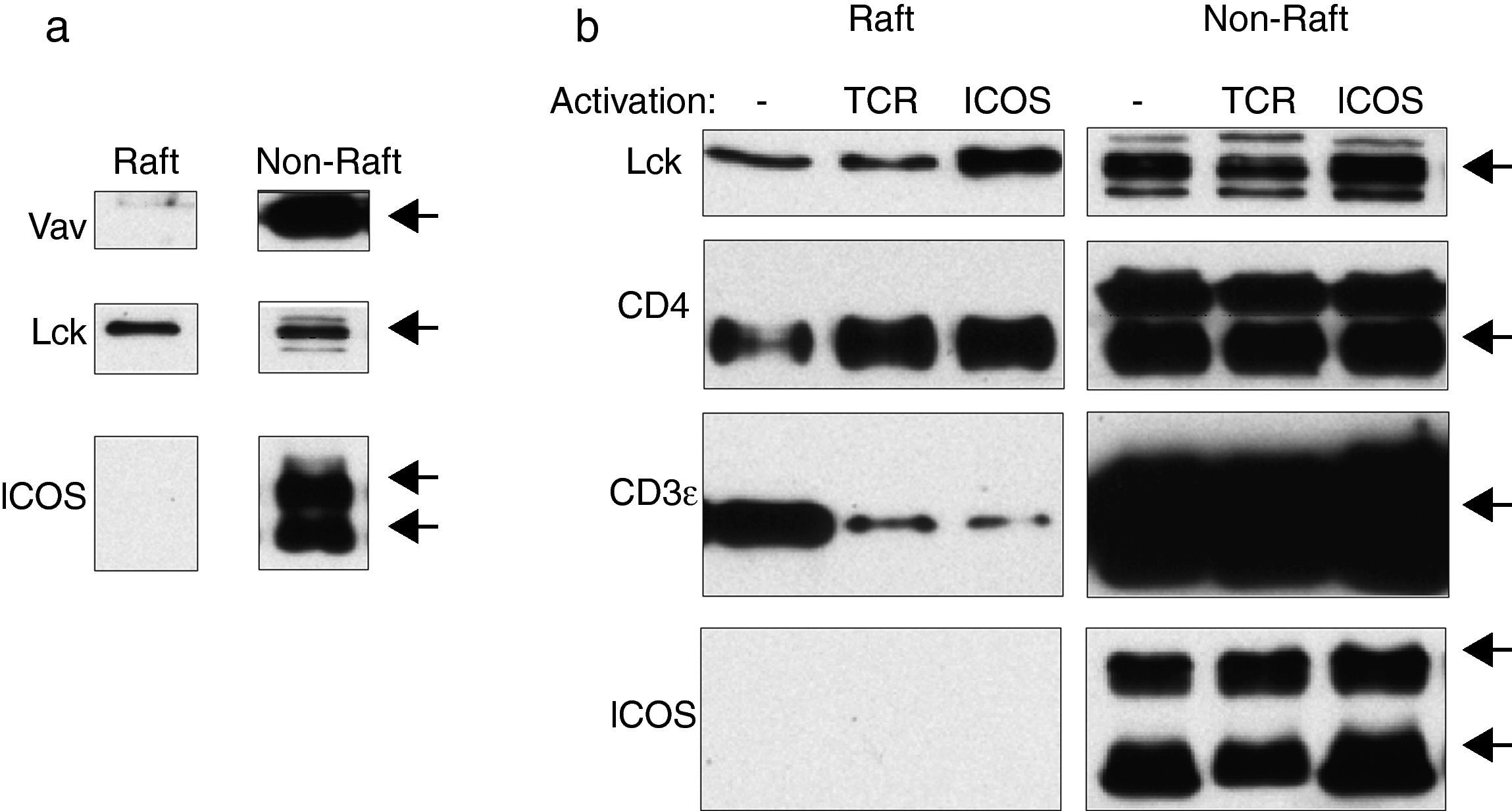
To confirm the absence of ICOS from lipid rafts, D10 cells were lysed in Triton X-100 containing buffer and the lysates were separated by ultracentrifugation into detergent-insoluble (i.e., “raft”) and soluble (non-“raft”) fractions that were then analyzed by immunoblot. As shown in Fig. 3, the raft-resident Src-family tyrosine kinase Lck was easily detected in the detergent-insoluble fraction (Fig. 3a and b). In contrast, ICOS was not detected in this fraction, even after very long exposure of the blot, whereas the 25 and 29kDa chains characteristic of reduced ICOS were readily detected in the soluble, non-raft fraction of the lysates (Fig. 3a). The same happened when the distribution of Vav was determined as a control protein50 (Fig. 3a).
ICOS cannot be detected by immunoblot in GEM (raft) fractions of cell membrane lysates. (a) D10 cells were lysed in Triton X-100-containing MES buffer and the lysates separated by ultracentrifugation. Proteins from pooled GEM (raft) and non-raft fractions were separated by SDS-PAGE and analyzed by immunoblot with ICOS-specific antibodies, or antibodies against molecules that reside (Lck) or not (Vav) in GEM. (b) D10 cells were incubated for 15min at 37°C with 10μg/ml anti-TCR (3D3) or anti-ICOS monoclonal antibodies and washed. Then, the cells were lysed, separated in raft and non-raft fractions as described in (a), and analyzed by immunoblot for the presence of ICOS, or of molecules resident (Lck, CD4), or weakly present (CD3) in GEM.
The possibility that activation by TCR/CD3 or ICOS ligands might induce ICOS transfer into membrane rafts was then analyzed. As shown in Fig. 3b, the GEM-resident proteins Lck and CD4 were readily observed in the GEM fraction in all conditions, whereas ICOS could not be detected in the raft fraction after either stimulus, even after very long exposure of the blots. In contrast, a small fraction of CD3 was readily detected in the rafts, as expected from its known weak association to rafts.50
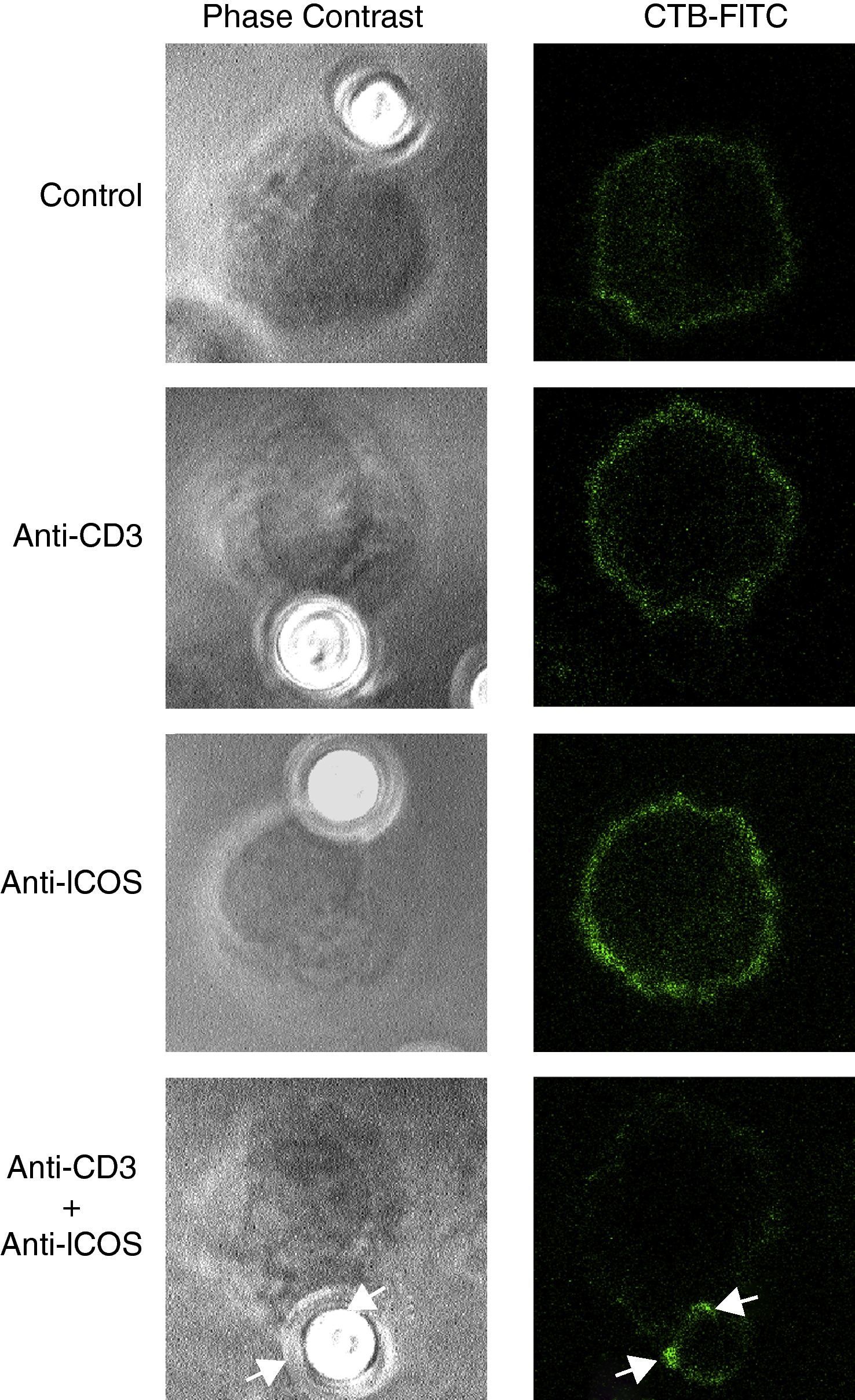
ICOS synergizes with TCR/CD3 signals to induce GEM accumulationIn the case of CD28, raft clustering and actin cytoskeleton redistribution are two interdependent phenomena involved in effective costimulation of naïve T lymphocytes24–27 that depends on filamin-A interaction with CD28.30 Although ICOS lacks the cytoplasmic PYAPP sequence involved in CD28-Filamin-A interactions, we checked whether ICOS might foster the accumulation of rafts at the site of interaction with TCR/CD3 ligands (Fig. 4). We used microbeads coated with amounts of anti-CD3 antibody that did not induce detectable accumulation of the rafts, as determined by confocal microscopy (Fig. 4). Beads coated with anti-ICOS antibody also failed to induce raft clustering (Fig. 4). In contrast, beads coated with both anti-CD3 and anti-ICOS antibodies induced a clear accumulation of GEM at the cell-contact site (Fig. 4, bottom row).
Ligation of CD3 plus ICOS induce accumulation of GEM at the bead:cell interaction surface, as indicated by white arrows. D10 cells were mixed with polystyrene microbeads coated with monoclonal antibodies to CD3 (2μg/ml), or ICOS (10μg/ml) alone or together, as indicated. Then, the cells were seeded on PLL-coated coverslips and incubated for 20min at 37°C, fixed and GEM stained with CTB-FITC. One hundred cells were examined for each labelling condition by phase contrast and confocal fluorescence microscopy, and representative results are shown. GEM accumulation at cell–bead interface is indicated by arrowheads. At least 10% of the cells had the pattern shown; no accumulation was detected in the other conditions depicted. Bar: 10μm.
So, ICOS does not have a direct connection with the actin cytoskeleton, i.e. through Filamin-A, yet it is still capable of inducing clear changes in T cell shape mediated by p110α PI3-kinase activation of actin polymerization (see,38 and Fig. 1). Although they are usually linked, it has been also shown that actin polymerization and lipid raft clustering can be distinct and dissociated phenomena. For instance, actin polymerization without detectable raft clustering has been observed in T cells upon expression of high levels of the GEF Vav.51 It is tempting to speculate that, unlike CD28, ICOS crosslinking can induce actin polymerization through Src- and PI3-kinase-mediated activation of Vav, but that raft clustering needs additional mechanisms triggered through Filamin-A30 or other molecules.27 Small differences between the ICOS and CD28 might result in strong functional differences, as those observed, i.e. in cytokine secretion due to minor changes in their cytoplasmic sequences.52 Thus, these differences, plus the distinct expression pattern of CD28 and ICOS costimulatory molecules likely contribute to their non-redundant roles in immune responses.
FundingThis work was supported by Grants from Fondo de Investigación Sanitaria (Ministerio de Ciencia e Innovación, Spain) numbers PI070620 and PI10/00650 (to J.M.R.); PI070484, PI10/00648 (to P.P.) and by AIRC (Milan) (to U.D.). Y.Y.A. is the recipient of a Predoctoral Fellowship of the “Junta de Ampliación de Estudios” (JAE) Program (C.S.I.C., Ministerio de Ciencia e Innovación, Spain). P.P. is a Tenured Scientist of C.S.I.C. at the Centro Nacional de Microbiología, I.S. Carlos III.
Conflict of interestThe authors declare no financial conflict of interest.
The skilful technical assistance of Maria Luisa del Pozo is gratefully acknowledged.