Las Eph son la mayor familia de receptores tirosina quinasa presentes en la mayoría de tipos celulares. Junto con sus ligandos, las ephrinas, las Eph participan en la organogénesis de muchos tejidos regulando numerosos procesos, como el posicionamiento y la migración celular, los cuales son claves para el correcto funcionamiento del timo, un órgano linfoide primario implicado en la maduración de las células T. En el presente trabajo, revisamos diferentes resultados sobre el papel que estas moléculas juegan en la biología del timo. La mayoría de las Eph y ephrinas se expresan en el timo adulto y fetal, tanto en los timocitos como en las células del estroma. Estas moléculas tienen un papel esencial regulando el tamaño del timo, a través del control de la supervivencia de los timocitos y de las células epiteliales tímicas (TEC). Además, estudios in vivo e in vitro demuestran que modificaciones en la señalización de Eph y ephrinas resultan en fenotipos tímicos específicos, concluyendo que dicha señalización determina finalmente tanto el patrón de maduración y diferenciación de los timocitos como el de las TEC. El papel de Eph y ephrinas en la función del timo aparece pronto en la ontogenia. En este sentido, varios resultados apoyan su relevancia en procesos claves para la organización del órgano, tales como el reclutamiento de los progenitores linfoides al primordio tímico, el patrón de ramificación del epitelio tímico y el posicionamiento de los timocitos y las TEC en el timo en crecimiento. Algunas de las alteraciones fenotípicas observadas en el timo de ratones deficientes en Eph y ephrinas se observan también en los órganos linfoides periféricos, pero no hay evidencias de una alteración funcional en sus sistemas inmunes.
Eph are the largest family of protein tyrosine kinases, which are described in most cell types. Together with their ligands, ephrins, Eph participate in the organogenesis of many tissues mediating numerous processes, such as cell positioning and cell migration, which are key for the functioning of the thymus: a primary lymphoid organ involved in T-cell maturation. In the present study, we review available data on the role played by these families of molecules in the biology of the thymus gland. Most Eph and ephrin are expressed in adult and fetal thymus, frequently in both thymocytes and thymic stromal cells. They appear to play an essential role in governing thymus size through the control of survival of thymocytes and thymic epithelial cells (TEC). Furthermore, studies in vivo and in vitro demonstrate that altered Eph/ephrin signalling results in specific thymus phenotypes and conclude that the balance of Eph/ephrin signals finally determine the pattern of maturation/differentiation of thymocytes and TEC. Indeed, the role of Eph/ephrin in thymus function appears early in ontogeny. In this regard, several results emphasize their relevance in key processes for thymus organization, such as the recruitment of lymphoid progenitors to the thymic primordium, the branching pattern of thymus epithelium and the positioning of thymocytes and TEC in the growing gland. Some of the phenotypic alterations observed in the thymus of Eph/ephrin deficient mice are reflected in the peripheral lymphoid organs, but there is no evidence for alterations in the function of their immune systems.
The thymus is a primary lymphoid organ which is essential for the functional maturation of T lymphocytes during fetal and adult life. To perform these functions, the thymus contains thymic epithelial cells (TEC) and thymocytes, which mature and differentiate intimately associated. Thus, impaired or altered development of either TEC or T-cell progenitors severely affects the maturation of the other thymic component(1). Histologically, the thymus is organized into two distinct compartments, the cortex and the medulla, containing phenotypically different TEC and thymocyte subsets. In both regions, the interactions between epithelial cells and developing thymocytes organize a 3D network that ensures the migration and topological distribution of T-cells that are key for their correct final maturation. A huge number of molecular and cellular factors, which act both autonomously and non-autonomously to the two main thymic components, seem to be involved in determining the cell fate of distinct thymic cells since the appearance of the thymic primordium to the adult life. However, we are far from understanding the true role played by each thymic cell type and the global interactions between them in the final product named functional thymus.
In the last few years, we have extensively studied the role played by the Eph tyrosine kinase receptors in numerous processes that occur within the thymus from its formation and development to the organization and functioning in the adult thymus. In the following pages, we will review the state of the art on Eph/ephrin and thymus, emphasizing the key role played by these molecules in thymus biology.
EPH AND EPHRIN ARE INVOLVED IN MORPHOGENESIS, CELL POSITIONING AND CELL MIGRATION IN NUMEROUS ORGANSEph is the largest family of tyrosine kinase receptors (16 members) known to occur in animal cells. They promiscuously bind ligands called ephrin (9 members) which are also extensively represented in numerous cell types. Both Eph and ephrin are divided into two families, A and B, based on gene sequence similarities of the molecules and ligand binding preferences. EphA (10 members) bind GPI-anchored ligands, the ephrinA (6 members), whereas EphB (6 members) bind transmembrane proteins, the ephrinB (3 members). As previously mentioned, each Eph kinase can bind several ephrin and vice versa, and both receptors and ligands transmit cytoplasmic signals to the expressing cells. Therefore, they constitute a very plastic system in which different affinities and expression patterns determine a high number of distinct cell interactions that allow these molecules to participate in a wide spectrum of cellular functions(2). Despite this promiscuity, the Eph/ephrin system exhibits a remarkable fine specificity.
These molecules determine when and where a cell type must move, attach or detach itself, contributing to the regulation of numerous morphogenetic processes as well as to cell positioning and cell migration mediated by different mechanisms. Thus, they can specifically restrict cell movement, guide the cells to specific positions and/or establish tissue domains and boundaries(2). It is important to remark that these processes are capital to the organogenesis and functioning of the thymus, indirectly implying a presumptive role for these molecules in governing the organ. Both Eph and ephrin not only contribute to these processes by regulating cytoskeleton dynamics, cell adhesion and integrin activity, but also through the modulation of gene expression or the regulation of different intracellular pathways(2).
EXPRESSION OF EPH AND EPHRIN IN THE THYMUSWe, and other authors, have determined a wide pattern of expression of Eph and ephrin of the two families, A and B, in the thymus of rats, mice and humans. However, contradictory results have been published with respect to the thymic cell types expressing Eph/ephrin within the thymus and/or their topological location in the distinct thymus compartments. These differences presumably reflect the diverse methods used for detection and/or speciesspecific differences.
Expression of EphA1, A2, A3, A4, A7 and A8 has been reported in thymocytes, whereas EphA1, A2, A4 and A8 are also expressed on TEC(3-5). EphA2 has been described to be expressed in immature dendritic cells(6) and cortical rat macrophages(3). Topologically, EphA1 occurs principally in the mouse cortex and rat cortico-medullary border whereas EphA2 and EphA3 are largely restricted to the thymic medulla. EphA4 expression has been described in the medulla of both rat and mouse but also in rat cortex, connective tissue trabeculae and blood vessels.
EphrinA expression is largely restricted to thymocytes but ephrinA1, A2 and A5 are also expressed by TEC(3). Subcapsulary thymocytes, presumably CD4-CD8- (DN) cells, express ephrinA1, A2, A3 and A5 whereas medullary thymocytes express all ephrin types studied(3). In murine thymus, all thymocyte subsets express ephrinA1(5). EphrinA1 is expressed in rat subcapsulary and inner cortex and ephrinA2 in the medulla, showing a pattern of expression partially overlapping with that of ephrinA5(3). In murine thymus, the expression of ephrinA2, occurs throughout the organ, whereas those of ephrinA1 and A5 are mainly restricted to subcapsule and cortex(5).
All EphB family receptors seem to be represented in the human and murine thymus. EphB2 and EphB3 and their main ligands, ephrinB1 and ephrinB2, are expressed by total thymocytes(7-10). The four molecules are expressed in all thymocyte subsets and the TEC of adult and fetal mice(11). In agreement with these data, Yu and colleagues(12) found by flow cytometry, expression of ephrinB1 and B3 in all Tcell subsets whereas EphB2 expression occurred in all thymocytes, except DN cells. However, Coles and colleagues(13) stated that about 65% of murine DN cells express EphB2 while Shimoyama et al.(10) have reported on the interaction between ephrinB1-Fc and fetal DN thymocytes. We demonstrated expression of EphB2, B3, ephrinB1 and B2 throughout the thymus(11) but other authors restrict ephrinB1 expression to the subcapsulary region(13). Expression of ephrinB1 and B2 has been reported in the cortex, where they co-localize with EphB6; this was largely detected on CD4+CD8+ (DP) thymocytes(9,10). In the human thymus, both EphB6 and ephrinB2 have also been detected although ephrinB2 expression is weaker than in mice(14). We also demonstrated a strong expression of both EphB6 and ephrinB1 on rat thymic dendritic cells.
On the other hand, Eph/ephrinA(3,15) and B(11,16) are expressed during thymus ontogeny in both thymocytes and TEC. In addition, preliminary results obtained in our laboratory using quantitative PCR have demonstrated significant variations in the expression of EphB in TEC during ontogeny.
Together, these results suggest that, frequently, the same cells or thymic compartments express several Eph/ephrin. This makes difficult to assign a specific role to individual members of these families in a discrete cell type. Quantitative differences in the expression of distinct molecules and/or in their affinity for specific ligands thus become important, particularly in thymic processes that require specific combinations of cell-to-cell interactions to occur. This is the case in the adult thymus in which mobile developing thymocytes need to interact in a temporal and topological sequence to reach full functional maturation. During ontogeny, the organization of specific microenvironments also requires specific migration and positioning of lymphoid and epithelial cell progenitors.
ROLE OF EPH/EPHRIN AND THE CONTROL OF THYMUS CELLULARITY: CORRELATION WITH APOPTOSIS AND PROLIFERATION RATEWe, and other authors(17-19), have demonstrated the involvement of different Eph and ephrin together with other factors in the balanced cellularity of many embryonic and adult tissues, including the thymus.
In a first in vitro approach, the absolute numbers of cells were significantly reduced in fetal thymic organ cultures (FTOC) supplied with EphA1-Fc, EphA2-Fc, EphA3-Fc or ephrinA1-Fc fusion proteins, with a more severe phenotype of cell survival in those receiving ephrinA1-Fc proteins that presumably block the signalling of most EphA members(3). In addition, all CD4/CD8 T-cell subsets decreased significantly, but this was specially the case in the immature CD4-CD8+ cells and in DP thymocytes. Remarkably, there were no significant variations in the proportions of γδ T-cells. When the proportions of cycling cells and apoptotic cells, two parameters directly related to the cellularity of lobes, were analyzed, increased proportions of apoptotic cells appeared in the treated lobes; these largely occurred in those T-cell compartments with the lowest cell contents, namely immature CD4-CD8+ cells and DP thymocytes. No significant variations were found, however, in the proportions of cycling cells yielded by the treated lobes. More recently, similar results were obtained with FTOC receiving soluble EphB2-Fc or ephrinB1-Fc fusion proteins(20). Treated FTOC showed decreased numbers of cells in correlation with increased proportions of apoptotic DP cells. Other authors have obtained similar results using similar approaches. EphrinB1-Fc, but not ephrinB2-Fc or ephrinB3-Fc fusion protein treatment of FTOC, caused decreased cellularity and significant increase of cycling cells indirectly indicating the occurrence of increased apoptosis(12).
In agreement with these results showing increased apoptosis in the absence of Eph/ephrin signalling, the activation of the Eph/ephrin pathway might have opposite effects resulting in decreased apoptosis. In this respect, Freywald and colleagues(21) demonstrated that the stimulation of murine thymocytes with EphA1-Fc reduced TCR-mediated apoptosis. Previously, the same group had reported that crosslinking of Eph with ephrinB1 protected murine thymocytes from apoptosis induced by anti-CD3 antibody(22). Also, Yu and colleagues(12) described protection of thymocytes from anti-CD3 antibody-induced apoptosis by co-stimulation with ephrinB1-Fc proteins. On the contrary, EphB6 crosslink induces apoptosis of Jurkat cells(9), and both EphB2-Fc and ephrinB1-Fc immobilized proteins modulate anti-CD3 antibody-induced apoptosis of DP thymocytes(20). The latter study also demonstrated that Eph/ephrinB effects were dependent on the density of immobilized protein on the plate surface, a remarkable fact that could explain the different results obtained. Thus, growing concentrations of immobilized fusion proteins resulted in a gradual increase of the proportions of apoptotic DP thymocytes that would later decrease(20).
In vivo results also support the view that lack of one or more Eph courses with profound thymic hypocellularity, increased apoptosis, and changes that are more or less significant in the rate of cell proliferation. The phenotypic analysis of EphA4 null mutant mice demonstrated thymic hypoplasia that became more evident as mice grew so that, at 4 weeks, thymocyte numbers were up to 100 times lower than those of control thymi(15). In correlation, higher proportions of apoptotic cells, mainly DP thymocytes occurred in mutant as compared to wild type (WT) mice. The cell cycle was also reduced, and the percentage of proliferating cells diminished in all thymocyte subsets(15). In EphB2 and/or EphB3-deficient thymi, the reduction of cycling cell proportions only affected the DN cell compartment(11). Other authors, however, have described normal cellularity in the thymi of both EphB6 and EphB2 knockout (KO) mice(13,23,24) and EphB6 overexpression under a CD2 promoter apparently results in important hypocellularity(13).
Analysis about the underlying mechanisms determining the final size and cell content of thymus, including the possible role played by Eph/ephrin, are few and inconclusive. Recent results suggest that the available thymic stromal niches determine the thymus size and the number of its cells rather than the total cellularity of the organ(25,26). TEC would undergo cellular expansion to ensure a sufficient number of niches within the thymus. During ontogeny, the proportion of cycling murine TEC peaks at E14, which is then followed by a gradual reduction leading to a small proportion of proliferating epithelial cells in the neonatal thymus(25), although medullary TEC have been reported as a mitotically active population in the adult thymus(27). Nevertheless, other authors have pointed out that TEC have a finite capacity for expansion and the number of endodermal progenitors that form the thymus primordium is key for further determining the size of both embryonic and adult thymus(25).
Flow cytometry analysis of the CD45- thymic cell fraction from fetal (E15.5) and adult EphB2- and/or EphB3-deficient mice demonstrates decreased proliferation rate in these cells in the deficient thymi(16). Furthermore, although normal T-cell differentiation is observed in dGuo-treated fetal thymus lobes from EphB2- and/or EphB3-deficient mice grafted under the kidney capsule of WT mice, they show decreased cell numbers that suggest a role for EphB2 and EphB3 expressed on TEC in controlling thymic cell content (García-Ceca et al. submitted, 2009). SCID mice recovered with bone cell progenitors isolated from EphB-deficient mice also exhibited hypocellular thymi. In correlation, the SCID chimeras established with EphB2 and/or EphB3 mutant cells showed increased proportions of apoptotic DP thymocytes, whereas those receiving cells which express a truncated form of EphB2 that does not transmit forward signals but induces reverse signalling in the neighbouring ephrinBexpressing cells -largely TEC-, maintain WT values (Alfaro et al. submitted, 2009). Thus, this reverse signal could target the production of survival factors from the ephrinB-expressing TEC to protect developing thymocytes from cell death.
On the other hand, profound alterations in TEC maturation observed in EphB2 and/or EphB3-deficient mice(16), that correlate with a reduction of the proportions of CD44-CD25+ (DN3) cells(11), course with in vitro decreased migration of bone marrow lineage negative (Lin-) cells into fetal thymus lobes(28). Remarkably, other authors have emphasized an important role for DN thymocytes, largely DN3 cells, in determining the number of available epithelial niches that, in turn, seem to control the lymphoid progenitor recruitment and their expansion within the thymus(29,30).
ALTERED EPH/EPHRIN SIGNALLING RESULTS IN DIFFERENT, SPECIFIC THYMUS PHENOTYPESAlthough some reports had described no changes in the thymus of mice deficient in EphA2(31), several years ago, we demonstrated that the in vitro blockade of Eph/ephrinA-Fc fusion proteins induced a significant decrease of the different T-cell subsets defined by CD4/CD8 expression that largely affected the immature CD8+CD4- thymocytes and the DP cells. The treatment did not affect, however, the progression of DP cells to TCRαβhi-expressing cells [both CD4+CD8- (SP-CD4) and CD4-CD8+ (SP-CD8)](3). The alterations of Tcell maturation were more severe when ephrinAl-Fc proteins, which block activation of most EphA, were used, and they did not affect the γδ T-cell lineage(3); a feature repeatedly observed in other Eph/ephrin deficient models(11,5) (Alfaro et al submitted, 2009).
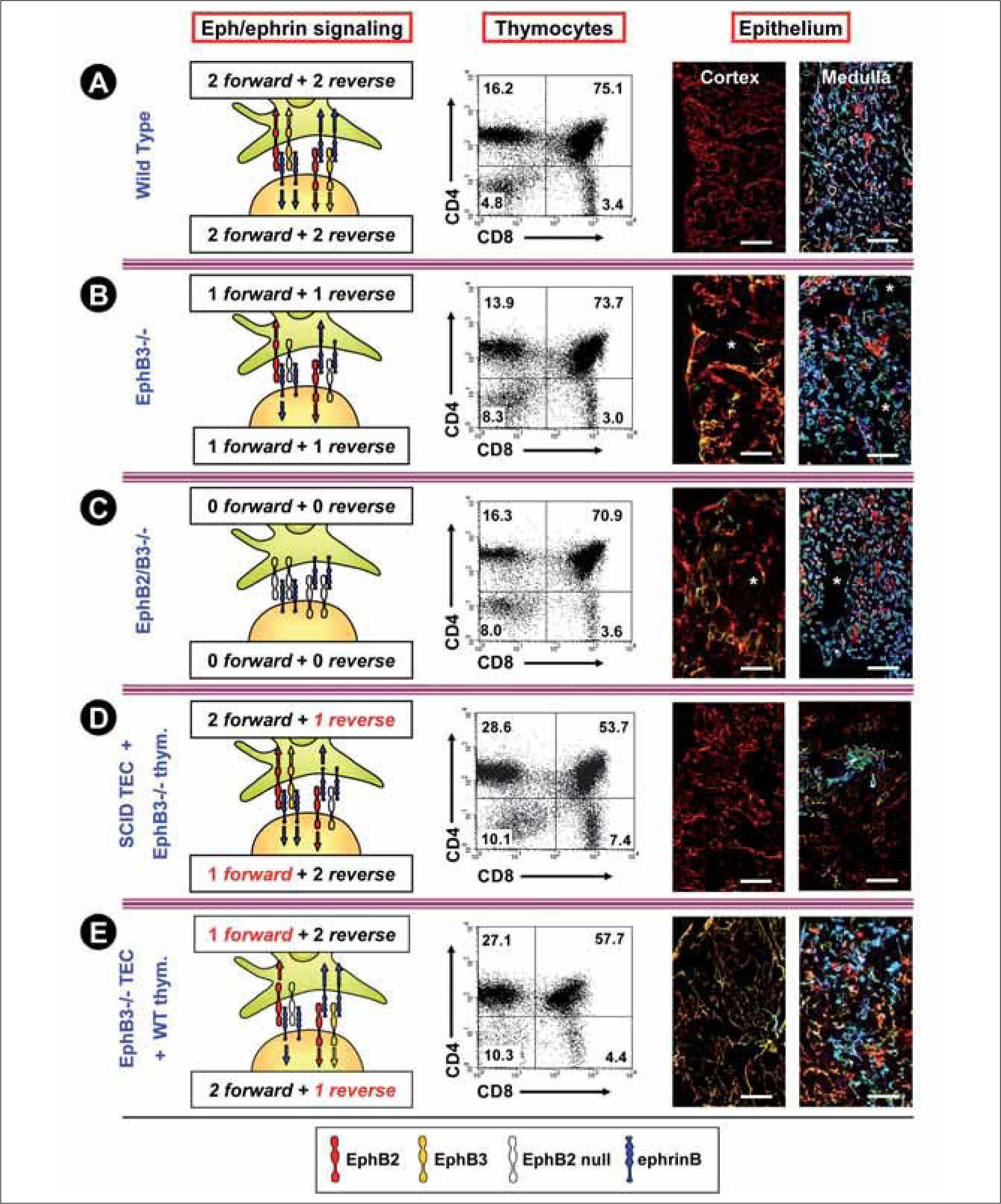
More recently, in vivo analysis of the thymus of EphA4 deficient mice showed an altered T-cell development in about 70% of 4 week-old animals, which largely consisted of extreme reduction of DP cell proportions(15). Most mice studied showed other alterations, including a slight increase in the percentage of DN thymocytes, which reflected largely increased DN3 cell proportions, and decreased percentage of mature TCRαβ hi cells. These results suggested that both DN to DP and DP to SP (CD4+CD8- or CD4-CD8+) progression could be affected. On the contrary, the analysis of EphB2 and/or EphB3 deficient mice also demonstrated increased percentages of DN cells but no significant variations in the proportions of other thymocyte subsets (Figure 1 A-C). In absolute terms, the number of all thymocyte subpopulations decreased significantly from the DN3 cell stage onwards(11).
The combined balance of Eph/ephrinB signals results in different thymus phenotypes.
A) In WT mice, in which both thymocytes and thymic stromal (largely epithelial cells -TEC-) cells received both forward and reverse signals through EphB2, EphB3 and ephrinB, the proportions of different CD4/CD8 thymocyte subsets are normal and the thymus gland shows the classical histological organization consisting of a regular K5-K8+ cortical epithelial network (red) and a well organized medulla that principally contains K8-K5+MTS10+ TEC (blue) with some K8+K5+MTS10- cells (yellow) and a few K8+K5–MTS10– cells (red). Scale bar: 50μm.
B) In the EphB3-deficient mice, thymocytes and TEC do not receive nor transmit signals through EphB3, but exhibit forward and reverse signals through, for example, EphB2 and ephrinB, respectively. In those conditions, thymocyte maturation is almost normal except for increased proportions of DN cells but the thymus parenchyma is profoundly altered with a cortical epithelial network that has lost its histological organization in palisade and exhibits K8+K5– TEC (red) with shortened processes and increased numbers of K8+K5+ cells (yellow) and K8–-K5– areas (asterisks). In the medulla, the cell types are similar to those of WT thymi, but the network is looser with K8– K5– areas (asterisks) and important shortening of the epithelial cell processes. Scale bar: 50μm.
C) In EphB2/B3 double mutant mice neither forward nor reverse signals are established between thymocytes and TEC throughout EphB2, EphB3 and ephrinB. Again, in these mice, the thymocyte maturation undergoes slight variations that result in increased proportions of DN cells. The changes in the histological organization of thymus are more important than in the EphB2 or EphB3 mutants. Here, the cortical meshwork is particularly disorganized with big K5–K8–areas (asterisks), appearance of K5+K8–medullary cells (green) in the cortex and changes in the cell shape of TEC. In the medulla, there are also big K5– -K8–areas (asterisks), rounded TEC and K5+K8+MTS10+ immature cells (white). Scale bar: 50μm.
D) To confirm the relevance of misbalanced Eph/ephrinB signalling for the thymus phenotype, in vivo thymocyte maturation in SCID thymi receiving EphB3-deficient Lin- bone marrow cells results in profound changes in the proportions of distinct CD4/CD8 cell subsets, as compared to those found in both WT (A) and EphB-deficient mice (B, C). In these experimental conditions, there is a new balance of EphB2/B3/ephrinB signalling: thymocytes receive EphB2, but not EphB3 forward signals and reverse signals activated by both EphB2 and EphB3 expressed on SCID TEC. However, this epithelium receives forward signals mediated by EphB2 and EphB3 but reverse signals through ephrinB activated by EphB2 but not EphB3. In these chimaeras, there is a decreased proportion of DP cells, but the reduction is lower than that observed in chimaeras established with EphB2+ bone marrow cells (data not shown), with high values of both DN and SP thymocytes. Although, the models are not totally similar, the histological analysis of chimeric thymi showed alterations that remind those of EphB-deficient thymus (B, C). The chimaeras exhibited disorganized cortical epithelial network and medulla consisting of small groups of cells, which included K5+K8–-MTS10+ cells (blue) but also K5+K8+MTS10– cells (yellow) and KS+K8–MTS10–/lo (green, green/blue). On the other hand, these results support a cell-autonomous role of EphB2 and EphB3 expressed on thymic lymphoid progenitors in thymocyte development as well as a non-autonomous involvement in TEC maturation. Scale bar: 50μm.
E) A cell-autonomous role of EphB2 and EphB3 expressed on TEC in the thymic epithelium maturation was demonstrated by analyzing the maturation of WT thymic cell progenitors in 2-deoxiguanosine treated EphB-deficient FTOC grafted under the kidney capsule of WT mice. Again, changes in Eph/ephrinB signalling determine a new thymus phenotype. In this case, WT developing thymocytes receive forward signals through EphB2 and EphB3 and reverse ones through ephrinB stimulated by EphB2 but not EphB3. On the contrary, ephrinB expressed on TEC transmit reverse signals mediated by EphB2 and EphB3 but only EphB2-mediated forward signals. In this model, WT lymphoid progenitors mature normally (as compared to those developing in WT grafted alymphoid FTOC -data not shown-) but they cannot recover the alterations observed in the epithelium, including increased cortical K5+K8+ cells (yellow) and disorganization of thymic medulla, due to the absence of EphB. Scale bar: 50μm.
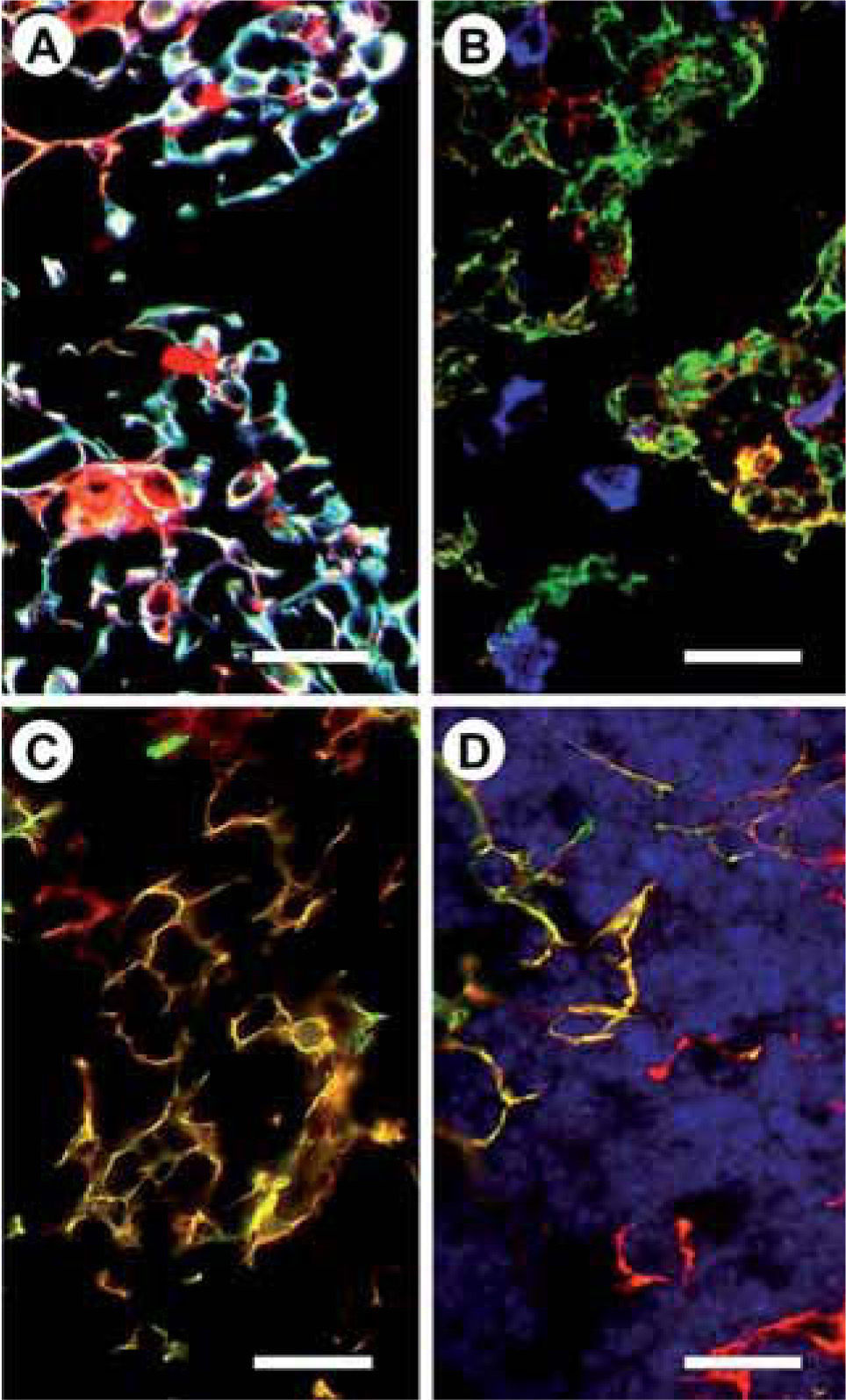
In the EphA4−/− thymi, changes in T-cell maturation coursed with profound alterations in the organization of cortical region. By light and electron microscopy, the EphA4−/− thymus showed an extremely thin cortex that consisted of a few layers of developing thymocytes in a meshwork of TEC arranged in parallel to the capsule. The immunofluorescence study with specific epithelial markers confirmed the heavy package of cortical TEC layers, although expression of ephrinA1 and A3, the highest affinity ligands for EphA4, and MHC molecules were normal(15). EphB2 and/or EphB3-deficient mice also showed profound alterations (Figure 1B, C). The three deficient animals studied contained: K5+K8+MTS10+ immature medullary epithelial cells, high numbers of K5-K8-MTS20+ cells, and abundant K5+K8+ cells and K5–K8– areas(16), but also other specific features: for example, small, scattered groups of medullary TEC in EphB2−/− thymi, or parallel columns of TEC with long cell processes in the cortex of EphB3−/− mice (Fig. 2). Increased numbers of both K5+K8+ and K5–K8– areas in the mutant thymi are particularly remarkable. The high numbers of K5+K8+ cells did not correlate with the increased numbers of MTS20+ progenitor cells, but instead were more related to K5+K8+MTS20– cells; the capacity of which to differentiate mature TEC is controversial(32,33). They could also be the result of an up-regulated expression of both K5 and K8 in the MTS20+ epithelial progenitor cells. K5–K8– areas correlated well with the morphological evidence of degenerated epithelial cells and increased numbers of apoptotic TEC found in these mutants. Nevertheless, they could also be the consequence of a down-regulated expression of K8 or K5 cytokeratins in the cortical or medullary epithelium, respectively. On the other hand, these changes occurring in the TEC network of EphB-deficient mice are quite similar to those observed in mice with defects in molecules such as FoxNl, Kremen 1 or Stat 3, known to be involved in the maturation of TEC(34-36). Remarkably, some of these molecules are indirectly related with the activity of different Eph or ephrinB(16).
Altered TEC phenotypes found in EphB-deficient mice.
A) Presence of numerous K5+K8+MTS10+ immature cells (white) in the medulla of an EphB2/B3 double deficient thymus. Note also the presence of typical K5+K8-MTS10+ medullary epithelial cells (blue) and a few big K5-K8+MTS10– cells (red). Scale bar: 25 Ím.
B) Disorganized epithelial network in an EphB2−/−thymus. Note the presence of several rare K5-K8-MTS20+ cells (blue) in a network largely consisting of K5+K8– cells (green) with a few K5-K8+cells (red) and K5+K8+MTS20–cells (yellow). Scale bar: 25μm.
C) Numerous K5+K8+ cells (yellow) in the epithelial meshwork of an EphB3−/−thymus. Scale bar: 25μm.
D) Large K5-K8–areas occur in the thymus of EphB-deficient mice (A, B). These areas do not represent, however, empty thymic areas devoid of cells but regions that do not express keratins K5 and K8 but contain numerous cells, as demonstrated after Hoechst staining (blue). K5+K8+ cells (yellow). Scale bar: 25μm.
These results, together with the fact that the lack of EphB2 is not obviously compensated by the presence of EphB3 and vice versa, demonstrate the specificity of the observed phenotypes. It is also evident that the described phenotype for EphA4−/− thymi is profoundly different from that of EphB deficient mice. It is important to highlight this fact because some authors have justified the lack of a thymus phenotype in mice deficient in Eph or ephrin by the expression of other molecules of the same family.
Despite these conclusive results, other authors have described normal cellularity in both EphB6−/− and EphB2−/− thymi(13,23,24) and no changes in the thymi of ephrinB3-deficient mice(37), although the first ones remarkably have compromised T-cell function(23) and ephrinB1, one of the main ligands of EphB2, has been pointed out to be critical for T-cell development(12). Furthermore, EphB6 transgenic mice that overexpress the receptor under the control of either the CD2 or the lck promoter show important thymic hypocellularity; increased numbers of DN cells; modified cortex/medulla cell ratio; and altered splenic white pulp(13), a phenotype which is very similar to the one described here for EphBdeficient thymi.
THE EPH/EPHRIN SIGNAL BALANCE DETERMINES THE PATTERN OF THYMUS PHENOTYPESince most Eph and ephrin are expressed in both thymocytes and TEC, and defects observed in Eph-deficient mice also affected both components, it is important to determine whether the changes observed in T-cell maturation are due to a direct effect on the lymphoid components or are a consequence of its maturation in an altered environment. Moreover, we have studied whether the altered epithelial cell network observed was due to an autonomous role of the Eph expressed on TEC or to indirect effects mediated by altered T-cell maturation.
The maturation of EphA4−/− bone marrow cell precursors in a SCID thymus evaluated 6 weeks after i.v. injection was similar to that of normal, WT progenitors. On the contrary, alymphoid 15 day-old fetal thymic lobes from EphA4−/− mice grafted under the kidney capsule of SCID mice that received WT bone marrow cell precursors failed to normally support T-cell development, as compared to grafted lobes from WT mice(15). These results suggest that altered T-cell differentiation observed in EphA4 deficient mice was largely a consequence of its occurrence in an altered thymic microenvironment. The lack of EphA4 in TEC could directly affect T-cell maturation through reverse signals mediated by ephrinA expressed on developing thymocytes. However, this possibility is unlikely because ephrinA, the main ligands of EphA4, are bound to the cell surface by GPI molecules and GPI-deficient thymocytes develop normally(38). More realistically, it may be that the altered organization of TEC could indirectly contribute to the changes found in the proportions of T-cell subsets. The lack of a well organized stroma could affect the cell-to-cell interactions known to be essential for the correct differentiation of thymocytes. The lack of EphA4 signalling in TEC of mutant thymi could also affect the activation in the epithelium of genes involved in the proper control of T-cell maturation(15).
We have recently evaluated the possible autonomous role of EphB2 and/or EphB3 as well as the EphB signals involved in thymocyte development by using a chimeric model in which the maturation of EphB2 and/or EphB3 or WT bone marrow cell progenitors was analyzed in SCID thymi (Figure 1D) (Alfaro et al submitted, 2009). EphB2−/− SCID chimeras show massive accumulation of DN thymocytes, largely CD44+CD25+ (DN2) and DN3 cells, resulting in decreased numbers and percentages of CD44–CD25– (DN4) cells, and an almost total disappearance of DP cells. Increased proportions of apoptotic cells, principally affecting the DP compartment, also contribute to the severe reduction of DP thymocytes. Remarkably, when EphB2lacZ/lacZ (EphB2LacZ) progenitor cells, that transmit reverse signals to the neighbouring ephrinB-expressing cells, were provided to SCID mice, the phenotype was less severe than that of EphB2−/− SCID mice. They showed a certain recovery of the numbers - not of the proportions- of DP cells, but they were still reduced with respect to the values found in WT SCID mice. In addition, the proportion of apoptotic DP cells in these chimeras was unchanged. Accordingly, the reverse signals provided by EphB2LacZ progenitors could be involved in the control of DN-DP cell progression and provide survival signals from the ephrinB-expressing cells to developing thymocytes, thereby impeding their apoptosis; an issue that has already been discussed in this review.
EphB3 seems to also be involved in the maturation of DN to DP cells. EphB3−/− chimeras support the maturation of all T-cell subsets, although there is an important decrease in the proportions of DP cells (Fig. 1D) that, in this case, correlates well with the increase of apoptotic DP thymocytes. This remarkable and different role of EphB2 and EphB3 in the T-cell development is supported by the similarities found in the phenotype of chimeras established with bone marrow cell progenitors deficient in both EphB2 and EphB3, and that described in EphB2−/− SCID mice. Apparently, the lack of EphB2 in the double mutant progenitors causes a phenotype severe enough to mask the lack of EphB3.
Apart from its role in DN-DP cell progression, the reverse signals transmitted by EphB2 were unable to recover the normal proportion of SP thymocytes. In this respect, we studied the in vitro capacity of DP cells isolated from either EphB2 or EphB3 deficient mice to mature to SP thymocytes in reaggregates formed with fetal WT TEC. The number of SP thymocytes yielded in both reaggregates was lower than that found in those established with WT DP cells, suggesting a role for both EphB2 and EphB3 in the final maturation of DP thymocytes and, therefore, an autonomous role of these molecules in the control of the complete process of thymocyte development. On the other hand, the immunohistochemical analysis of the chimeric thymi demonstrated important alterations in the histological organization of TEC network (Fig. 1D). This also supported a non-autonomous role for these molecules expressed in developing thymocytes upon the organization of thymic stroma.
We also analyzed the opposite situation: the contribution of EphB2 and/or EphB3 deficient stroma to the observed phenotypes in EphB2 and/or EphB3 KO mice. We examined the development of both thymocytes and TEC in 2- deoxiguanosine-treated EphB deficient fetal thymus lobes grafted under the kidney capsule of WT mice (Fig. 1E) (García-Ceca et al., submitted, 2009). In these conditions, WT lymphoid progenitors colonize and mature in either WT or EphB-deficient epithelium, and the profound alterations observed in the latter one could be recovered if WT precursors send the adequate signals to the TEC. However, the immunohistochemical study of isolated grafted mutant lobes after colonization by WT cells shows a very similar phenotype to that observed in EphB-deficient thymi, i.e., they contain high numbers of K5+K8+ cells, altered distribution of medullary areas, rounded K5+K8-MTS10+ medullary epithelial cells and K5– K8– areas (Fig. 1E)(16). Therefore, these results support that TEC maturation is principally governed cell autonomously by EphB2 and EphB3 expressed on TEC. Nevertheless, since the phenotype exhibited by grafted lobes was not exactly the same as that observed in EphB-deficient thymi(16), the Eph expressed on thymocytes must also play a certain role in TEC maturation. This is also suggested by the results found in SCID chimeric mice.
On the other hand, normal T-cell differentiation occurs in the grafted EphB-deficient lobes colonized by WT lymphoid progenitors, although they yield lower numbers of cells than WT lobes. This remarkable, quite normal lymphoid maturation in a profoundly altered thymic environment has been also described in mutants with defects in other molecules involved in TEC development(34,35,39). Although there is no conclusive explanation for these results, it has been pointed out that a few unaltered stromal areas could be sufficient to support a normal T-cell maturation(34,39).
To better define the relevant Eph-ephrinB interactions in each cellular compartment, as well as those thymocyte-TEC interactions important for the biology of thymus gland, we are currently analyzing the effects of the lack of ephrinB1 and/or ephrinB2, ligands of EphB2 and EphB3 receptors, in either thymocytes or TEC (Cejalvo et al. 2009, in preparation). For this purpose, we have used a Cre-LoxP recombination system to specifically delete ephrinB1 or ephrinB2 genes from either thymocytes or TEC. Mice containing ephrinBdeficient thymocytes showed thymic hypocellularity and altered T-cell development, the severity of which depends on the background of the mouse strain used, but few changes in the epithelial microenvironment. On the contrary, mice with conditioned deletions in TEC, especially those deficient in both ephrinB1 and ephrinB2, showed important epithelial alterations resulting in very small thymi containing a compacted epithelial network in both the cortex and the medulla. In the latter mice, T-cell development was also partially compromised, suffering some blockade at the DN cell stage.
Taken together, these data confirm that EphB1 and EphB2 and their ligands, ephrinB1 and ephrinB2, exercise autonomous and non-autonomous effects on the development of both thymocytes and TEC. Furthermore, they reveal that it is the final balance of Eph/ephrin signals rather than the presence or absence of these molecules, as summarized in Figure 1, which determines the TEC-thymocyte crosstalk and, thus, the final thymus phenotype.
OBSERVED PHENOTYPES IN THE ADULT THYMI OF EPH MUTANTS APPEAR EARLY IN ONTOGENY: ROLE OF Eph/EPHRIN IN THYMUS ORGANOGENESISAnalysis of the fetal thymi of Eph mutant mice studied by our research group has confirmed that alterations observed in both thymocytes and TEC appear early in ontogeny, suggesting that Eph/ephrin are involved in thymus organogenesis.
An altered histological organization was already evident in E17 EphA4−/− thymi, whereas in the neonatal thymus densely packed areas appeared in some areas and no-keratin cells occurred in others. In addition, higher numbers of K5+K8+ epithelial cells were found in the mutant thymi than in the control ones. On the contrary, at the neonatal stage, the proportions of different CD4/CD8 cell subsets were normal or close to normal in most animals(15).
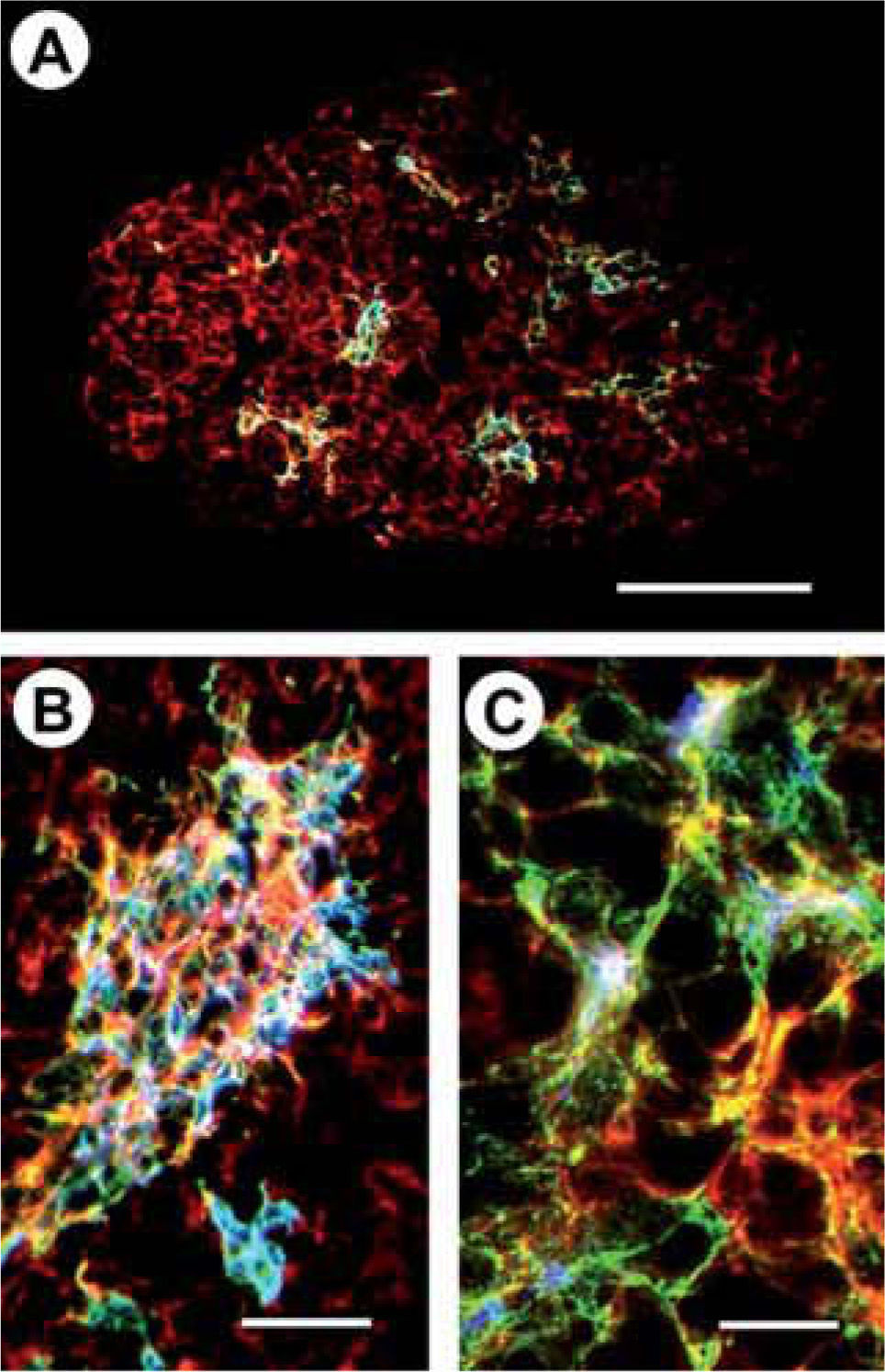
In all studied stages (form E13.5 onward), the thymi of EphB2 and/or EphB3 mice showed increased proportions of DN cells -mainly DN3-, as previously observed in adult thymus(11). As in adult thymus, the most important changes found in EphB-deficient mice affected the TEC compartment and appeared early at E13.5 or earlier, suggesting a key role for EphB2 and EphB3 in the TEC differentiation and histological organization of the TEC network, as previously determined in other epithelial tissues(40). Presumably, there are already differences between mutant and WT mice in the thymic organization as early as 11.5 day post-coitum but, with the cell markers used, we were unable to conclusively identify them. At E13.5, the deficient thymi already show consistent changes in their epithelial parenchyma, including altered location of medullary K5+K8+ areas -presumably related to an altered positioning of medullary TEC progenitors to the adequate regions- and appearance of incipient K5– K8– regions (Figure 3A). Changes in the extension and arrangement of TEC processes become more evident from E15.5 onward. The intermediate K5+K8+MTS10+ medullary cell population appears at E13.5 in WT fetal thymi and in lower numbers in EphB-deficient ones. This cell population disappears 2 days later except in the thymus of EphB2/B3 double mutant mice in which it undergoes a more severe delay in development (Figure 3B). In the mutant thymus there is also a delayed or slow maturation of K5+K8+MTS20+ epithelial progenitors, resulting in a high number of these cells at E13.5 and E15.5 and, accordingly, a late appearance of the K5+K8-MTS10+ mature medullary TEC (Fig. 3C). Thus, the early changes observed in the deficient thymi remain and gradually increase throughout ontogeny, becoming more severe at the end of fetal life and in the neonatal thymus. On the other hand, as mentioned above for the adult thymus, most changes found in the fetal mutant thymus have been also reported in other mice with defects in TEC differentiation due to the lack of molecules involved in this process(34,36).
Alterations in the thymus epithelium appears early in ontogeny.
A) 13 day-old fetal EphB3-deficient thymus. Note the incipient disorganization of the thymic parenchyma and the small groups of medullary cells containing K5+K8-MTS10- cells (green), a few K5+K8-MTS10+ cells (blue) and K5+K8+MTS10+ cells (white) in a K5– K8+MTS10– (red) thymic parenchyma. Scale bar: 100μm.
B) Increased numbers of K5+K8+MTS10+ primitive cells (white) still appear in the 15 day-old fetal thymus of EphB2/B3 double deficient mice. Scale bar: 35μm.
C) The slow maturation of TEC in the double mutant thymus delayed the appearance of K5+K8-MTS10+ mature medullary cells which resulted in a thymic primordium in which most K5+K8– cells (green) do not express MTS10 (small blue spots in some cells), however, there are both K5+K8+MTS10– cells (yellow) and K5+K8+MTS10+ cells (white). Scale bar: 15μm.
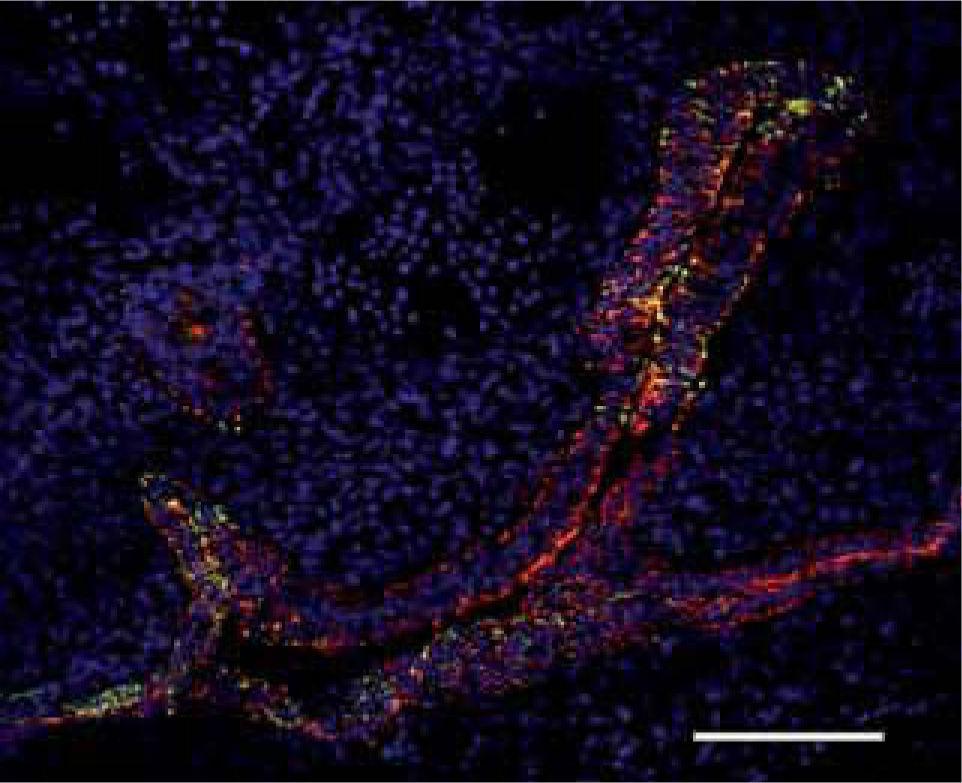
On this basis, we have recently begun to analyze the relevance of Eph/ephrin in the earliest stages of thymus development. Our preliminary results demonstrated that the branching of thymic epithelium, which will further fold repeatedly to histologically organize the gland, begins at 11.5 days post-coitum, when the thymic primordium is still joined to the pharyngeal cavity and most cells express keratins 5 and 8 (Fig. 4). One day later, it is already possible to distinguish a branching pattern that seems to determine the topological distribution of committed epithelial stem cells. An incipient thymic medulla consisting of K5+K8-/lo cells appears in the central area, K8+ cells occupy the outer zone and, between both regions, there is an enlarged area containing numerous presumptive K5+K8+ cell progenitors. The thymic epithelial branching in EphB2-deficient mice seems to follow a general pattern similar to that of WT thymus, but the epithelial cells appear more compact, almost collapsed, suggesting a new role for EphB2 in the biology of thymus gland(28).
Thymic primordium in an 11.5 day-old WT thymus. The thymus outgrowth develops from the pharyngeal cavity as a branching structure consisting of a K5-K8+bi– or pseudostratified epithelium with some K5+K8– cells (red) and K5+K8+ staining (yellow) on the lumen of growing organ. Hoechst positive nuclei appear in blue. Scale bar: 100μm.
Another key issue for thymus organogenesis is the lymphoid colonization of thymic primordium (E12.5) and the establishment of a 3D network in the organ on the basis of an intimate association between TEC and developing thymocytes. In both processes, EphB seems to be deeply involved.
We have demonstrated that murine EphB2-deficient Lin- bone marrow progenitors colonized fetal thymus lobes less efficiently than WT and EphB2LacZ expressing cells, that transmitted reverse signals to the stromal cells of lobes recovering the normal migratory capacity(28). In these experiments the migratory capacity of bone marrow cells into the thymus lobes was more severely affected when EphB-deficient lobes were used. These results emphasize once again the relevance of available niches for lymphoid progenitor recruitment and how factors, whose nature is currently unknown, presumptively released by TEC after ephrinB reverse signalling, could be involved in this process. In addition, EphB2 activation on Lin- bone marrow precursors or thymocytes with immobilized ephrinB1-Fc fusion proteins also induces reduced migration to laminin, fibronectin or chemokines (CXCL12, CCL21 or CCL25), a result previously reported by other authors(4,41). Together, these results suggest that EphB2 forward signalling reduces cell migration, whereas ephrinB reverse signals increase it.
On the other hand, Eph-mediated thymocyte-TEC interactions, are presumably, involved in the establishment of a 3D meshwork of thymic parenchyma. Supporting this idea, we demonstrated that ephrinB1-Fc fusion proteins are able to disorganize the three dimensional epithelial network resulting in a rounding of TEC, disappearance of cell processes and disorganization of cellular cytoskeleton(20).
THE PERIPHERAL LYMPHOID ORGANS OF EPH-DEFICIENT MICEAlthough a systematic study on the immune capacities of the described Eph/ephrin deficient mice has not been performed some preliminary results suggest that the observed changes in the thymus and T-cell maturation have no remarkable effect on the immune capacities of mutant mice. Firstly, the anatomical examination of sacrificed animals does not provide visual evidence of malignity or major pathological alterations in lymphoid and non-lymphoid organs.
In the EphA4−/− thymus, in correlation with the decreased proportions of DP thymocytes there are also decreased numbers of both DP TCRαβ hi cells and DP CD69+ cells, suggesting that an inefficient αβ selection could be occurring in these mice(15). Also, the blockade of Eph/ephrinB signalling prevents the formation of DP thymocyte-TEC conjugates and alters TCR signalling, two central issues for thymocyte selection. Both EphB2 and ephrinB1 mainly locate at the interaction surface between the two cells that establish the conjugate, and in vitro EphB2-Fc or ephrinB1-Fc treatment decreased the formation of cell conjugates established by DP thymocytes and TEC(20). The numbers of conjugates also diminished when they were established with EphB2 and/or EphB3 deficient DP cells, but increased with EphB2LacZ ones (Alfaro et al., submitted, 2009). The EphB2 extracellular region expressed by the latter cells could generate attraction signals more efficiently than the whole EphB2 molecule, whose cytoplasmic domain could then induce cell repulsion.
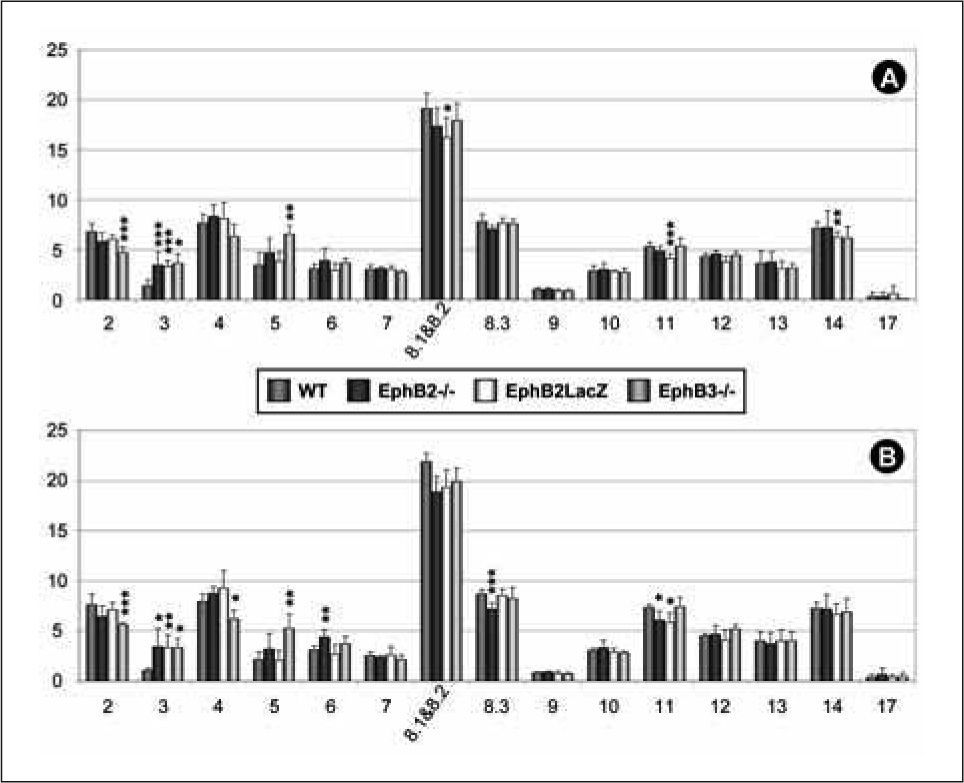
We have also used a collection of monoclonal antibodies to specifically distinguish different V, expressed segments to analyze by flow cytometry possible changes in the central and peripheral TCRαβ repertoire. The proportions of Vβ-expressing cells were evaluated comparatively within the SP-CD4 cell population of thymus and lymph nodes from EphB-deficient and WT mice (Fig. 5). The only change that affected the three studied mutants was an increase in the proportion of Vβ3+ SP-CD4 cells observed in both thymus and lymph nodes. Apart from this, EphB2-deficient mice did not show any variation in the analyzed TCRαβ repertoire within the CD4 thymocyte population although in the periphery an increase in Vβ6 CD4 T lymphocytes and decreased proportions of Vβ8.3+ and Vβ11+ cells occurred. In the EphB3-/- mice, the percentages of Vβ8.1, 8.2, Vβ11 and Vβ14 cells decreased within the SP-CD4 thymocyte population but only a significant decrease in the proportions of Vβ11+ SP-CD4 lymphocytes were found in the lymph nodes. Decreased proportions of Vβ2+ cells, together with an increase in Vβ5+ cells, occurred in the SP-CD4 thymocytes of EphB2/B3 double mutant mice, whereas in lymph nodes, a similar decrease was found in both Vβ2 and Vβ4 SPCD4 lymphocytes but the proportions of Vβ5-expressing SP-CD4 T-cells increased (Fig. 5).
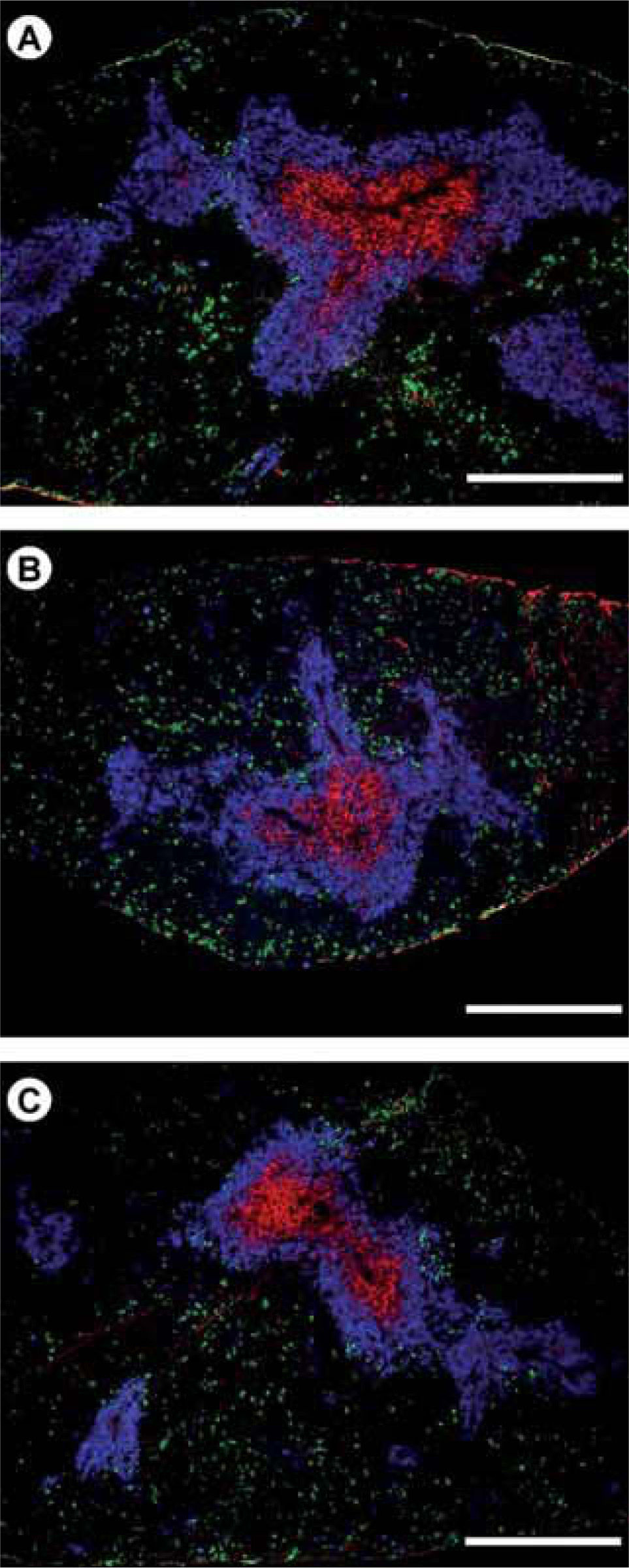
On the other hand, in agreement with the observed variations in the cell content of Eph deficient thymi, the number of T lymphocytes varied in the peripheral lymphoid organs of deficient mice. The cell numbers of total T-cells, SP-CD4 and SP-CD8 T lymphocytes undergo a significant reduction in both lymph nodes and spleen of EphA4 mutants(15). In EphB2 and/or EphB3 deficient mice, the absolute numbers of T lymphocytes was significantly lower in peripheral blood, mesenteric lymph nodes and spleen (except for EphB3−/− mice). The numerical variations, however, did not result in statistically significant differences in the proportions of peripheral CD4/CD8 defined T-cell subsets from EphBdeficient mice(11). Although exhibiting a smaller size than WT mice, both lymph nodes and spleen of EphB2 and/or EphB3 deficient mice maintain a normal histological organization. In the spleen, the periarteriolar T-cell area appeared surrounded by lymphoid follicles and a marginal zone largely containing B lymphocytes and macrophages that also predominate in the red pulp (Fig. 6). These results concerning both cell content and morphology of peripheral lymphoid organs of EphB-deficient mice reflect quite well the condition of thymi, in which an important hypocellularity courses without variations in the proportions of thymocyte subsets, except for a slight increase in DN3 cells. Apart from the colonization of mature T-cells from thymus, other factors, including in situ apoptosis and cell proliferation, and distinct specific migration to different peripheral lymphoid organs, contribute to the homeostasis of peripheral lymphocytes. Moreover, as shown by ourselves and other authors, Eph and ephrin are involved in all of them.
Splenic sections of 5 day-old postnatal WT (A), EphB2−/−(B), EphB2LacZ (C) mice. Note the similar histological organization in the three cases with a periarteriolar T-cell area (CD3+ cells) (red), IgM+ B lymphocytes (blue) surrounding the T-cell areas and in the marginal zone and the numerous MAC1+ macrophages (green) scattered throughout the red pulp. Scale bar: 200μm.
In this respect, although several studies have demonstrated the involvement of diverse Eph and ephrin in lymphocyte activation as co-stimulatory molecules(12,37) there is no evidence, until our findings, to suggest that immune deficiencies could be associated with a lack of these molecules. Only, Luo and colleagues(23) reported compromised immune responses in EphB6-deficient mice. Previously, this same group had described that isolated EphB6−/− T-cells responded poorly to anti-CD3 plus anti-CD28 stimulation(42). in vivo T-cell mediated cellular immune responses, evaluated by delayed hypersensitivity and EAE induction, were depressed in EphB6-deficient mice. On the contrary, T-cell dependent humoral responses were not defective(23). However, other authors had previously found no remarkable phenotypes in the lymphoid tissues of the same EphB6 KO mice(24).
CONCLUSIONS AND FURTHER DIRECTIONSIn summary, current evidence demonstrates a role for Eph and ephrin of families A and B in numerous processes occurring during thymus organogenesis and T-cell maturation, including lymphoid colonization, TEC development, cell survival, T-cell selection, etc. It is, therefore, necessary to incorporate these receptors and ligands to the long list of molecules implicated in governing the functioning of the thymus gland; a central part of the immune system. Three important issues remain, however, unsolved and deserve special attention in the near future: 1) The cellular and molecular mechanisms used by Eph/ephrin to modulate the thymus phenotype; 2) The relationships between these molecules and others known to be involved in similar processes related to the thymus; mainly those involved in determining organ size, epithelial maturation and the thymocyte-TEC interactions necessary for thymocyte selection, and 3) The role of these molecules in the fine-tune control of immune functions, including their possible role in autoimmunity and immunodeficiencies.
CONFLICT OF INTERESTThe authors declare no financial conflict of interest.
We would like to thank Dr. Mark Henkemeyer for providing EphB-deficient mice and the Microscopy and Cytometry Centre of the Complutense University of Madrid for the use of its facilities and technical assistance. We also thank the "Developmental Studies Hybridoma Bank" of the Iowa University for supplying the anti-K8 keratin antibody. This work was supported by grants BFU 2004–03132 and BFU 2007–65520 from the Spanish Ministry of Education and Science; grant RD06/0010/0003 from the Spanish Ministry of Health and Consumption; grants S-BIO/0204/2006 and R74/91 05552/08 from the Regional Government of Madrid and PHB2008-0002-PC from the Spanish Ministry of Science and Innovation.