The “danger theory” is a set of postulates formally proposed by Polly Matzinger fifteen years ago. As a theory, it explains how it is possible that immune responses take place without infection. It provokingly proposed that the immune systems have not evolved for self/non-self discrimination, but to respond against what is causing tissue damage. The proposal of the “danger model” coincided in time with the discovery of the immune potentiating effects of microbial molecular patterns. Charles Janeway et al. proposed that infection would be detected by innate receptors for microbiological biomolecules that are either absent or different in mammals. These agents were found to stimulate antigen presenting cells in such a way that would provide T lymphocytes with appropriate costimulatory molecules that critically complement the signals raised by antigen recognition. This was considered absolutely critical to ignite and sustain immune responses. The danger theory predicted the existence of endogenous molecules released or modified by danger that would act in a similar fashion to the microbial molecular patterns on dendritic cell costimulatory functions. Recent evidence points to various molecules capable of sounding the alarm in aseptic conditions. These include: the nuclear protein HBGM-1, uric acid, Interferon-α, chaperones of the heat shock protein family, alternatively spliced domains of fibronectin, and self nucleic acids. Some of these agents act through the same Toll like receptors involved in microbial pattern recognition. Identification of these mechanisms provides molecular support for the danger theory and has an extraordinary importance for tumor and transplantation immunology.
La teoría de la “alarma” (danger) es un conjunto de postulados formalmente propuestos por Polly Matzinger hace quince años. Como teoría surgió para explicar la aparición de respuesta inmunitaria en ausencia de infección. Ha sido una teoría provocadora al proponer que el sistema inmunitario no ha evolucionado para discernir entre lo propio y lo extraño, sino para responder frente a aquello que está causando daño tisular. La propuesta del modelo coincidió en el tiempo con el descubrimiento de la potenciación de la respuesta inmunitaria por patrones moleculares presentes en micro-organismos. Charles Janeway y sus colaboradores propusieron que la situación de infección se detecta mediante receptores innatos para patrones estructurales de biomoléculas microbiológicas que están ausentes en organismos superiores sanos. Se descubrió que estos agentes estimulan a las células presentadoras de antígeno para proporcionar a los linfocitos T señales coestimuladoras complementarias para las que reciben al reconocer el antígeno. La coestimulación sería crítica para que la respuesta inmunitaria se ponga en marcha y se sostenga en el tiempo. La teoría del “danger” postula la existencia de moléculas endógenas liberadas o modificadas por la destrucción o daño tisular que pueden hacer sonar la alarma en condiciones de asepsia. Hay varios hallazgos experimentales recientes que apuntan que una serie de moléculas cumplen estas condiciones: La proteína nuclear HMGB-1, el ácido úrico, el interferon-α, el ATP extracelular, chaperonas de la familia de proteínas de stress térmico, dominios alternativos de la fibronectina y ácidos nucleicos endógenos. Algunos de estos agentes son detectados por los mismos receptores tipo Toll que detectan los patrones de biomoléculas de microbios. La identificación de estos mecanismos moleculares presta apoyo experimental a la teoría del “danger”, y sus implicaciones tienen extraordinaria importancia en inmunología tumoral y en transplante.
To explain why presentation of antigen sometimes leads to clonal expansion of antigen-specific lymphocytes and sometimes to their deletion or anergy, Melvin Cohn(1,2) proposed a model according to which a T cell needs two distinct sorts of signals to respond: Signal 1 from antigen receptors and Signal 2 from costimulatory molecules. More recently, a third type of signal (the so-called Signal 3) has been involved in the acquisition of effector function by CD8+ T cells, and is mainly mediated by cytokines(3).
Originally, dendritic cells (DC) were thought to mediate T cell stimulation in a constitutive fashion(4,5). However, this was not the case, since under steady state conditions DC do not offer sufficient costimulation(6) and they are tolerogenic(5,7,8). The factors controlling the transition between immunogenic and tolerogenic DC in vivo have been and still is a very active subject of investigation, as it is at the core of immunoregulation(5,7-10).
Polly Matzinger thought that the immune system might have evolved to defend us against what is dangerous instead of what is foreign(11). She departed from conventional wisdom of the time: "The assertion that the immune system does not discriminate between self and non-self is a strong one. It is in absolute opposition to the fundamental belief, held by most immunologists, that the immune system is designed to attack anything foreign while remaining tolerant of self”. Subsequently she made a deep criticism on the flaws of self-non self discrimination as the paradigm to understand immune function in terms of both response and tolerance(11,12). The proposal in its simplest form went then as follows: “…If we move away from the idea that self-non-self discrimination is the immune system's primary goal, and consider instead that it might simply be the best mechanism that the immune system could find to distinguish dangerous from non dangerous structures…"(11).
In her system, danger, mainly defined as tissue injury, should be the main driver to dictate if the immune response must take place or not(13,14). According to the new hypotheses, some kind of message should tell the DC that tissue cells are injured or dying in a violent, stressful or unexpected fashion(11,13,14). It was assumed that natural apoptosis of cells as the result of normal turnover of tissues should pass unnoticed to the immune system. Indeed, experimental reports have provided evidence that apoptosis as related to the normal cellular turn-over in tissues tended to be immunosuppressive or at least immunologically silent.
WHAT DOES IT TAKE TO ACTIVATE A DC?With the intuition that DC costimulatory activities were not constitutive but induced, Charles Janeway proposed his infectious non self notion for immunity(7,15). These ideas postulated that the immune system had evolved to defend us against infection and that innate immune mechanisms were there to tell that a pathogenic infection was taking place. Indeed, the immune system had to be endowed with means to know when it was dealing with microbes. Pathogenic microbes would be unmasked when molecular patterns that are critical for their structure become detected by receptors expressed on immune system cells in a constitutive fashion(16,17).
An incredible array of these receptors has been identified theresince including surface and endosomal Toll-like receptors(16,18) as well cytoplasmic sensors for viral nucleic acids and bacterial components(18,19).
DC would then detect these microbial components and become costimulatory(7). It was also found that inflammatory cytokines play a role in modulating DC activation and, more importantly, that activated T helper cells could extend the activation state to the DCs whereupon their cognate antigen is detected(20,21). This is critical to license DCs to prime cytotoxic T lymphocytes(22,23).
However, how can one explain the initiation or abortion of immune responses in the absence of "bugs" or in the absence of inflammation? The Danger theory needed bridges connecting DC activation to stressful cell death or tissue injury. Polly Matzinger suggested this possibility as early as in 1994(11), and later postulated that exposed hydrophobicity could be a major feature in danger-denoting molecules(24).
DISCOVERY OF ENDOGENOUS MOLECULES THAT DENOTE DANGERThe search for molecular messages used by the immune system to decipher danger is far from being over, and new moieties may join the list. A common theme seems to be the usage of molecules with very diverse functions that conceal their activity to spell danger from the immune system under healthy steady state conditions. One of the main hurdles in this discovery quest is the fact that minute bacterial contaminants (i.e. endotoxin or bacterial DNA) are strongly sensed by the pathogen-associated molecular pattern (PAMP) receptors with an overlapping read-out of effects with the putative genuine endogenous danger signals. Accordingly, once an endogenous danger signal candidate is detected, a lot of effort is needed to rule out artefacts caused by microbial contamination.
The following molecules fulfil the features as endogenous danger molecules:
Heat shock proteinsChaperones are an early idea selected by evolution(25). In essence, they are scaffold proteins that bind, fold, and protect other proteins and peptides inside cell compartments. Many of them are overexpressed under stress induced by different means including high temperatures(25). With regard to DCs, heat shock proteins mediate two functions. On the one hand they can induce maturation and on the other they may efficiently transfer antigenic peptides from the cell that is succumbing. These peptides enter the DC in a fashion that greatly facilitates delivery to antigen presentation pathways(26,27). In viable cells these chaperones remain in the cell interior and become exposed under necrosis or programmed cell death. HSPs such as HSP70 or gp96 have been tested for cancer vaccination(28) exploiting their properties for antigen transfer(29) and DC maturation. In the case of gp96 an interaction with CD91 has been shown to mediate internalization into DC(30). The nature of the receptors involved in the proinflammatory effects remains undefined with a controversial involvement of TLRs(31,32).
EDA domain of fibronectinThe extracellular matrix is wrongfully considered a silent structural network of proteins. Rather, it plays an active role in inflammation and remodelling. For instance, glycosamine glycans fix the chemokine gradients that govern lymphocyte migration(33). Recently an alternative splicing product of the fibronectin gene was found to be proinflammatory/proimmunogenic(34,35). Reportedly, the exon that codifies for the extra domain A (EDA) is expressed in inflamed tissues. The EDA protein sequence has been found to stimulate Toll-like receptor-4 (TLR4)(34) and to behave as an adjuvant for vaccination(35). Would other molecules of the extracellular matrix join the list of endogenous danger signals? Protease degradation products or posttranscriptional modifications are to be watched. In either case, it makes physiological sense that fibers in the extracellular matrix can keep record of danger either by adsorbing cytokines or because of their intrinsic properties.
HMGB-1This protein is normally a non-histone complex of the nuclear proteome. It therefore ought to be confined into compartments that are not exposed(36,37). Several articles showed that it has stimulatory activity on machrophages and other leukocytes, especially enhancing TNF-α expression and secretion(37,38). In a seminal article from the group of Lawrence Zitvogel(39) it has been demonstrated that HBGM- 1 release enhances immunogenicity in tumors. The authors went on to demonstrate quite convincingly that HBGM-1 acts on DC by inducing maturation via the Toll like receptor molecule TLR4(39). Again, an endogenous protein acts on receptors believed to be deployed to detect prevalent molecular components of microorganisms. Notably, when a hyporesponsive allele of TLR4 is correlatively plotted with post-chemotherapy survival in a series of breast cancer patients, a poorer prognosis phenotype is adscribed for the hyporesponsive TLR-4 allele(39).
It is worth commenting that apoptosis and necrosis could be distinguished by this molecule that remains attached to chromatin upon apoptotic death but gets freed following necrosis and is thereby active to mediate inflammatory functions(40).
Uric acidUric acid seems to be a principal endogenous danger signal released from injured cells(41-43). Uric acid in its crystalline form stimulates DC maturation and, when coinjected with antigen in vivo, significantly enhances the generation of responses from CD8+ T cells(41) as well as the humoral response(44). Eliminating uric acid in vivo inhibits the immune response to antigens associated with injured cells, but not to antigens presented by already pre-activated DC. The identity of the receptor which detects the crystals of uric acid remains elusive. Interestingly, injured cells under metabolic stresses increase their content in uric acid(41,43). It is also interesting that aluminium salt precipitates (alum) which are the most widely used adjuvants for human vaccination seem to act at least in part via production of uric acid crystals that activate DC(45). More recently, succinate, an intermediate intracellular metabolite has been shown to mediate DC maturation acting on the surface receptor GPR91(46).
RNA and DNA from apoptotic cells.Very recently a set of interesting mechanisms has been discovered involving TLR3. TLR3 is a receptor present in DC endosomes that sense double-stranded RNA reaching this subcellular location(18). It has been found that RNA from necrotic or apoptotic neutrophils can induce the activation via TLR3(47,48). This is also the case of double stranded DNA from apoptotic bodies: when this DNA is not degraded fast(49,50) the apoptotic bodies are detected by TLR9. Indeed, it was surprising to find that eucaryotic DNA can also activate TLR9(51).
Under normal circumstances, nucleic acids are confined within the limit of membranes and are not accessible. Stressful or dangerous death can abruptly expose them by means of membrane disruption. Moreover, it is being studied that, upon activation/death, neutrophils project nets of DNA that trap microbes and may also constitute endogenous alarms(52).
NucleotidesRecent evidence also points to a role of nucleotides(53), including short lived extracellular ATP, in danger signalling. Mechanisms seem to involve purine nucleotide receptors(54), the inflammasome via NALP-3(54), and Interleukin-1 [(53) and Kroemer G. et al personal communication].
Secondary danger signals extending the alarm: Type I interferons and NKG2D ligandsInnate cytokines constitute a way to amplify the local signals of danger and to communicate with the headquarters of immune cells at lymph nodes. Type I interferons (IFNs) are well known for their antiviral effects(55), but maybe they are also early mediators in response to damage as well as to viral infection/replication. The finding that TLR3 and TLR4 might detect not only viral or microbiological invasion but also endogenous danger signals points in this direction because the IFNα/β system is a major mechanism downstream stimulation of these TLRs(16,18). Type I IFNs upregulate the functions of most leukocytes including DC(56,57). In the case of type I IFNs the danger signal is not pre-stored but must be transcriptionally up-regulated in the neighbouring injured or dying cells. As such, it can be considered a secondary danger signal as opposed to primary danger signals acting directly on DC.
Some surface proteins with homology to MHC class I molecules denote genotoxic and other cellular stresses(58). They are activating ligands for NKG2D(59) that can trigger NK cells and costimulate subsets of T lymphocytes(60). Some tumors can be rejected on the basis of expression of these ligands(61,62). Hence, NKG2D ligands delineate another way to tell danger from a normal healthy situation and provide another code whereby non-immune tissues can speak to immune system cells. In this case the activation of costimulatory functions of DC would be also indirect and perhaps bridged by DC cross-talk with activated NK and T cells(63,64).
In the overall scheme of Polly Matzinger ideas all tissues are part of the immune system in an ever ongoing crosstalk with the immune system to tell when to respond or to refrain. In addition, this tissue-immune system dialogue will dictate which type of response (i.e humoral or cellular) is best suited for a given situation. The molecular vocabulary of the language used in these dialogues will be a fruitful area of research.
What does it take to be an endogenous danger signal?The common features of endogenous danger signals seem to be:
- 1.
Upregulated, released or modified in injured or stressed cells.
- 2.
Confined to compartments from which they can be passively or activelly released following injury, thereby becoming exposed to receptors expressed on the surface or the endosomes of neighbouring DCs.
- 3.
Be sensed by a receptor or detection system of DC that induces maturation.
- 4.
Induce a DC maturation program in an effective way.
- 5.
Exclusion beyond doubt of experimental artefacts due to contamination with microbial molecules.
Moieties satisfying these previous 5 conditions can be considered danger mediators, although further experimentation is advisable to catch them in action in a physiological (or pathological) range of conditions. That would be the in vivo veritas final condition.
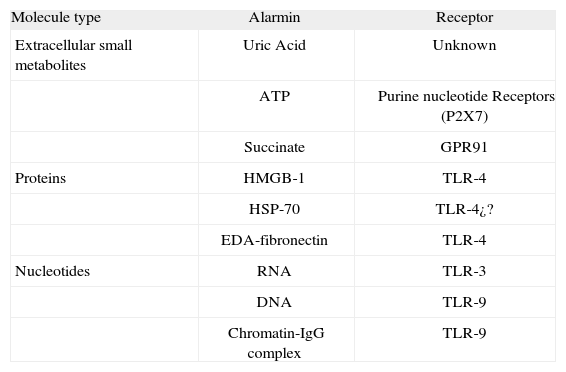
Secondary danger signals such as IFNα/β or NKG2D ligands do not fulfil these criteria. In the case of IFNα/β the molecule is not directly induced by the cell-damaging mechanism. In the case of NKG2D ligands they do not directly stimulate DC. However, functionally speaking they behave as close allies of the danger signal amplifying and spreading its effects. According to the criteria proposed above the confirmed endogenous danger signals or "alarmins" are summarized in Table I. The list is not complete, and more members may be added in the near future.
Endogenous danger signals (Alarmins) and their receptors
| Molecule type | Alarmin | Receptor |
| Extracellular small metabolites | Uric Acid | Unknown |
| ATP | Purine nucleotide Receptors (P2X7) | |
| Succinate | GPR91 | |
| Proteins | HMGB-1 | TLR-4 |
| HSP-70 | TLR-4¿? | |
| EDA-fibronectin | TLR-4 | |
| Nucleotides | RNA | TLR-3 |
| DNA | TLR-9 | |
| Chromatin-IgG complex | TLR-9 |
The danger model makes physiological and pathological sense. But does it make sense in terms of evolution? Polly Matzinger has speculated whether, from an evolutionary point of view, microbes evolved for some reason to have molecules to interact with TLR receptors. This idea challenges the extended view that TLRs evolutionary respond to the need of superior organisms to detect microbes. In other words, the real selection force on superior organisms would have been the need to detect endogenous (self) danger signals(24), instead of infectious microorganisms. Alternatively we favour the interpretation that coevolution may have generated a common theme of receptors to detect both infectious non self and dangerous self. The answer to these fascinating biological questions promises to be very difficult from an experimental point of view.
Polly Matzinger has proposed that, perhaps, shared chemical features can reflect in terms of evolution an ancient set of molecular mechanisms to detect when something is dangerous for the integrity of living tissues. Hydrophobicity is shared by many (albeit not all) of the molecules described to act as endogenous danger signals. It is possible that exposed hydrophobic cores of the moieties are a common theme at signalling danger(24). It would not be surprising that the ability to behave as a danger signal is not constitutive but controlled and regulated in some of these molecules that reflect danger (i.e., by conformational changes, proteolysis, oxydation, or other chemical modifications).
FITTING MANY PUZZLE PIECES IN A DIFFERENT WAYThe beauty of a theory is that it stands as an attempt to explain diverse experimental observations and makes some testable predictions. In the danger theory its simplicity deserves admiration. Still, it crashes with previous theories and psychological resistance to change. Most of us are very fond of what we learn in textbooks and therefore reluctant to abandon old paradigms. After all we all were trained with the "mantras" of self non-self discrimination.
An important step in the air at the time of proposal of the danger model was the prediction of endogenous danger signals to understand how the immune system can work in certain aseptic conditions. Theory has fuelled the search for moieties performing as danger signals and here they are now… Importantly, there may be many more in store providing new biological functions for intracellular molecules. Biomolecules are often like Swiss knives with more than one single utility. Who among biochemists could have predicted a key role in apoptosis for cytochrome C?
Possible therapeuthic applications to inhibit or exploit the functions of danger molecules are countless. In particular transplantation and tumor immunotherapy can benefit much from these paradigms. Using P. Matzinger's own words about transplantation: "Danger model suggests that healthy fetuses should not be rejected because they do not send alarm signals. Transplants, however, cannot be performed without surgical and/or ischemic damage. Thus, to induce the acceptance of transplants without lifelong immunosuppression, we should mimic the body's own way of inducing tolerance, i.e., by blocking the endogenous alarm and/or costimulatory signals"(14).
In the case of tumor immunotherapy the important corollaries that Matzinger's ideas put forward were: "tumors should not stimulate immunity, either because they are not associated with microbial stimulators, or because they are healthy growing cells that do not send alarm signals. Thus, to eradicate a tumor, we should infect it, or cause it repeated damage to alert the local APCs (as Bill Coley did in the late 1800s), or we should vaccinate repeatedly with a tumor vaccine that stimulates immunity"(14). It is difficult to say more with such a few words.
To think differently takes the intelligence, the creativity and the courage to challenge conventional wisdom. Only sometimes it pays off. This seems to be the case for Polly Matzinger's danger theory as it is accomplishing its predictions on danger-denoting molecules.
CONFLICT OF INTERESTThe authors do not have any conflict of interest regarding the opinions on this article.
Ainhoa Perez Diez is greatly acknowledged for discussing and revising the manuscript.We are grateful for support from MEC (SAF2005-03131 and SAF2008-03294), Departamento de Educación del Gobierno de Navarra, Departamento de Salud del Gobierno de Navarra (Beca Ortiz de Landázuri), Redes Temáticas de Investigación Cooperativa (RETIC) (RD06/0020/0065), Fondo de Investigación Sanitaria (PI060932), European Commission VII Framework Program (ENCITE), Fundación Mutua Madrileña, and "UTE for Project FIMA". MS-H receives a Ramon y Cajal contract from Ministerio de Educación y Ciencia, and M.C.O. a Sara Borrell contract from Instituto de Salud Carlos III.