The role of the different arms of the Immune System in the control and elimination of tumors has been the subject of increasing interest in recent years. Here, some recent findings adding support to the role of NK cells in the elimination of spontaneous tumors in transgenic models of tumorigenesis (Guerra et al, 2008), or the role of adaptive immunity in the maintenance of chemically induced tumors as a chronic disease (Koebel et al, 2007) will be discussed within the frame of the “immunoediting” theory.
El papel de los distintos brazos del Sistema Inmune en el control y la eliminación de tumores ha sido objeto de un interés creciente. En este trabajo se discuten, en el marco de la teoría de la “inmunoedición”, una serie de datos recientes acerca del papel de la inmunidad en el control de los tumores. En particular, se comentan los datos de Guerra y cols acerca del papel de las células NK en la eliminación de tumores espontáneos en modelos transgénicos de tumorigénesis (Guerra y cols, 2008), y el notable trabajo de Koebel y cols (2007) en el que se muestra el papel de la inmunidad adaptativa en el mantenimiento como una enfermedad crónica de los tumores inducidos por agentes químicos.
Cáncer is a disease initiated by a series of cumulative genetic and epigenetic changes that occur in a normal cell. However, in addition to the malignant cell itself, cancer is a disease of microenvironment and immunity. Multiple signals delivered within the tumor microenvironment by stromal and endothelial cells, and immune cells are critical factors in determining the progression versus dormancy or destruction of an initiated lesion and also whether metastasis may occur(1).
Here, some recent findings on the role of the immune system in elimination and/or contention of tumor growth will be reviewed. Particularly, recent data concerning the role of NK cells in the elimination of spontaneous tumors(2,3), or the role of adaptive immunity in the maintenance of tumors as a chronic disease(4) will be discussed.
The concept that the Immune System protects the host against cancer was first proposed by Erlich in 1909 and modified in the 1950s by Burnet and Thomas(5) The immunosurveillance theory of Burnet and Thomas proposed that adaptive lymphocytes could respond to and reduce tumor growth by recognizing tumor antigens. However, the concept fell out of favor when studies in the 1980s indicated that tumors failed to develop more rapidly in nude mice (which lack T cells and B cells, but not NK cells) than in wild-type mice. It was resurrected in the 1990s, when a body of evidence emerged indicating that immunodeficient mice were at greater risk for spontaneus tumor development(5).
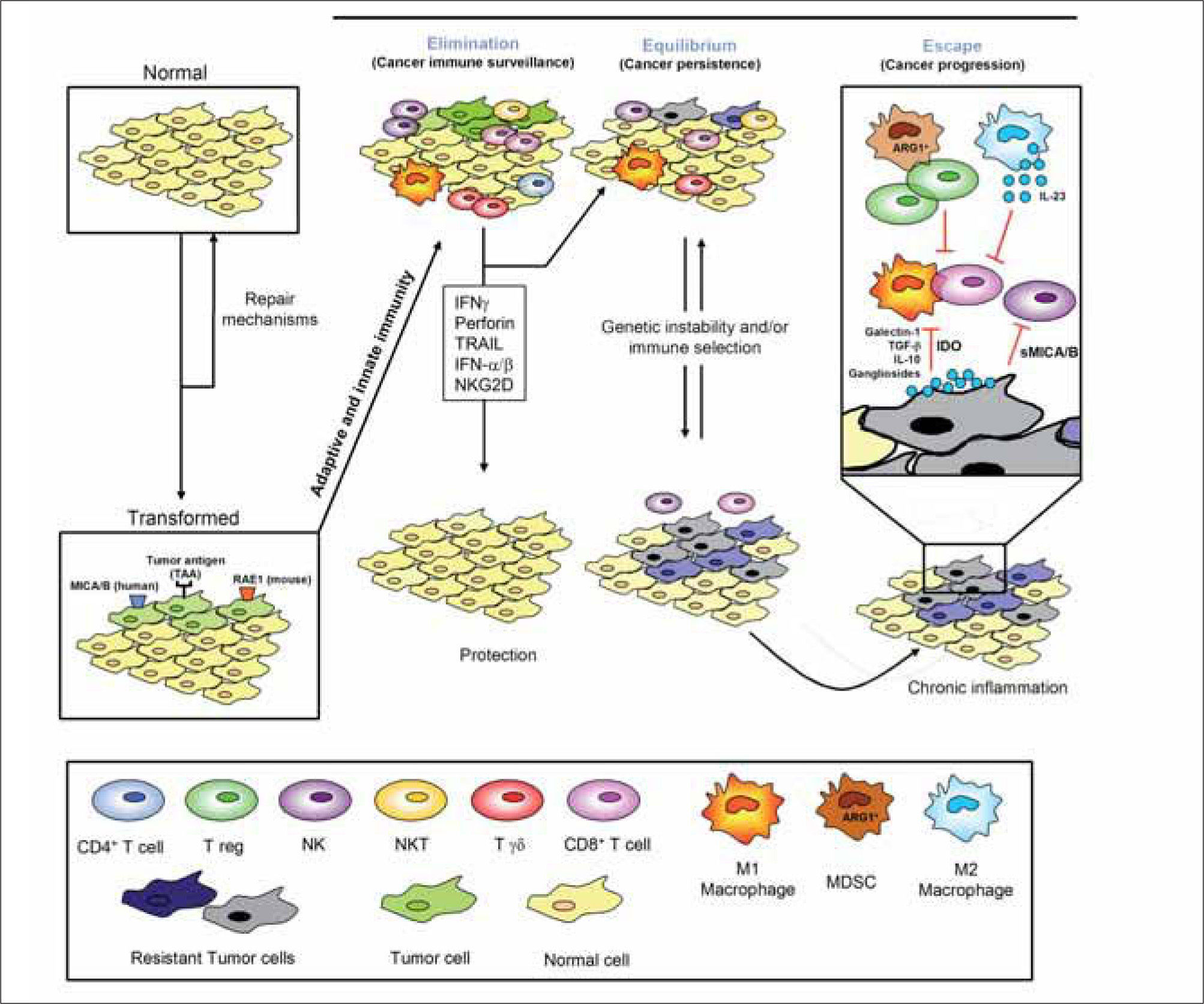
Four classes of cells have been established to have key roles in the immune response against tumors, and consequently the Immune System is totally involved in this action. These cells are: Natural Killer (NK) cells, that provide innate immune response; CD8+ T lymphocytes, that represent the adaptive immune response; NKT cells, that connect the two classical type of immune response and so are usually regarded as "transitional" immuneresponses; Tregs, that recognise an antitumor immune response as an autoimmunity response and manage to inhibit it (Figure 1).
THE ROLE OF NK CELLSSince their discovery, a large number of studies have demonstrated natural killer (NK) cell-mediated lysis of different types of tumor cells in vitro and in vivo, but, for a long time, it was unknown how NK cells recognized tumor cells, as well as other aberrant cells. Over the last 15 years, however, a large number of germline-encoded NK cell-activation and -inhibitory receptors have been discovered.
Among the activating receptors, natural killer group 2 member D (NKG2D) is one of the best characterized: It is a type II transmembrane-anchored glycoprotein expressed as a disulfide-linked homodimer on the surface of almost all NK cells as well as some CD8+ αβ+T cells, γδ+T cells, NKT cells, and a small subset of CD4+ αβ+T cells. NKG2D ligands are frequently expressed on primary tumor cells, tumor cell lines and some cells infected by pathogens, which become sensitive to NK killing. A DNA-damage pathway regulates NKG2D ligand expression. Indeed, ligandtransfected tumor cells have been shown to be rejected in vivo in an NKG2D-dependent fashion. Therefore, immunological recognition of developing tumors in the host may result not only from the recognition of specific antigens presented by MHC class I and class II molecules, but also by cellular-stress-induced ligands expressed on transformed cells but not on normal cells. Guerra and colleagues state that NKG2D deficiency promotes the development of spontaneous tumors(2). Notably, however, genetic NKG2D deficiency does not seem to affect the incidence of carcinogen-induced sarcomas(2). Whereas these findings are consistent with a role of NKG2D in tumor surveillance, they also show that some tumors may evade it, for example by shedding high amounts of soluble NKG2D ligands, which are believed to cause downregulation of NKG2D on the surface of lymphocytes. In the future it is not unlikely that more cancers will be treated with drugs or other immunomodulatory agents that affect the function of NK cells (and other immune cells), either directly or indirectly. Furthermore, certain forms of cancer may be subject to treatment with adoptively transferred NK cells, and cancer therapy may also benefit from novel strategies aimed at inducing the expression of ligands for activating immune receptors in tumors(2,3).
THE ROLE OF NKT CELLSNKT cells have begun to yield increasingly interesting results concerning tumor immunity. NKT cells and γδ++ T cells may "sit" between innate and adaptive immunity. Like adaptive cells, they bear receptors encoded by somatically rearranged genes, yet like innate cells, they generally lack distinct potential for establishing antigen-specific clonal memory. The type of response mounted by such cells has been referred to as "transitional immunity", and its importance in tumor surveillance is evident in the antitumor activity of the NKT cell agonist α-galactosylceramide, or the observation that mice deficient in γδ T cells are much more susceptible to several protocols of skin carcinogenesis induction(6).
THE ROLE OF CD8+ T CELLSCD8+ T cells are key to our ability to control intracellular infections and cancer. They act as cytolytic T lymphocytes (CTLs) that not only eliminate in a cytotoxic manner the infected/tumor cells but also carry out regulatory functions, being capable of either suppressing or supporting immune responses. Whereas the mechanism of the suppressive activity of CD8+ T cells is far from clear, it has been shown that perforin- and granzyme-dependent elimination of antigen-carrying dendritic cells (DCs) by antigen-specific CD8+ T cells can act as a suppressive mechanism, providing self-limiting character to CTL responses and restricting efficacy of vaccinaton. On the other hand, CD8+ T cells can also activate DCs, this "helper" function depending on their ability to produce IFN-y and to promote production of IL- 12p70 by DCs(7).
TUMOR ESCAPE: TREG AND MYELOID-DERIVED SUPPRESSOR CELLS (MDSC)T cell tolerance plays an important role in tumor escape and is one major obstacle, limiting the effectiveness of cancer vaccines. Tumors express antigens that should induce immune-mediated rejection, yet spontaneous rejection of established tumors is rare because tumors can actively fight and defeat host immunity. The active mechanisms involved in the suppression of host immunity include altering APC function, fostering dysfunctional T cell co-signaling, or generating an immune-subversive cytokine milieu. Given these premises, reducing tumor-driven immune suppression should be clinically beneficial.
The current paradigm around which most cancer immunotherapies have been developed arose from observations made in infectious diseases. So, many of these vaccines have been developed against tumor associated antigens (TAA) shown to elicit antigen-specific antitumor CD8+ CTLs. Unfortunately, although immunity against tumors and pathogens share some characteristics, they also have substantial differences. According to the current paradigm, tumor express TAA that can be captured by professional APCs, notably DCs, which then prime naive T cells (through the expression of co-signaling molecules, the production of soluble factors, or other mechanisms) to become antigenspecific CD8+ CTLs. These cells, when found in appropriate numbers and directed against the appropriate antigens, are then able to eradicate the tumor. According to this paradigm, simply supplying more of the missing elements should result in immune rejection of the tumor. Numerous anticancer immunotherapeutic strategies have been developed based upon these premises, including infusing additional tumor antigen or antigen-pulsed APCs, supplying T cells generated from tumor-infiltrating lymphocytes, T cells together with a soluble growth factor, T cells activated ex vivo with cytokines, or T cells engineered to express receptors for specific TAA and boosting the effect of co-signaling molecules or activating cytokines. The fundamental difference between infection and cancer is that the first has an extrinsic origin while the second is intrinsic (self) to the individual. Because tumors are intrinsic, generating effective antitumor immune responses requires mounting a substantial autoimmune attack, which involves breaking self-tolerance. Indeed, for example, tumors actively fight back by producing immunosuppressive factors such as IL-10, TGF-β and VEGF.
APCs are primary responsible for the induction of tumorinduced T-cell tolerance. A group of Gr-1+CD11b+MDSCs have been identified as primarily responsible for tumorassociated CD8+ T-cell tolerance. These MDSC are immature cells, comprising precursors of macrophages, granulocytes, dendritic cells (DCs) and myeloid cells at earlier stages of differentiation, and they can suppress immune response in vitro through direct cell-cell contact, and antigen-specific MHC class I-restricted tolerance of CD8+ T cells in vivo. Accumulation of these cells has been described in individuals with cancer, and this pathway may explain the difficulties in maintaining the antigen-specific immune response after vaccination in cancer patients. There are two basic mechanisms to induce T-cell tolerance, namely deletion and anergy, yet MDSCs seem to act through another, largely unknown mechanism. Nagaraj and colleagues, using an experimental in vivo model, had shown that MDSCs, by generating reactive oxygen species (ROS) and peroxynitrite, induce modification of TCR and CD8 molecules, resulting in the loss of ability of CD8+ T cells to bind pMHC. MDSCs do not affect the expression of TCR or CD8 molecules on the surface of T cells. This third mechanism may be acting in many pathological conditions in addition to cancer, including infection, inflammation and trauma, all of them associated with the accumulation of MDSCs overproducing peroxynitrite(8).
CD4+CD25+ regulatory T lymphocytes (Treg) are the second important actors of tumor-driven immune evasion providing prototypical targets to test new anticancer treatment strategies. Although suppressor T cells were discovered about 40 years ago, it was Sakaguchi the first to describe phenotypical characteristics to identify these cells, in 1995. He demonstrated the active suppressive potential of a population of CD4+ cells expressing high levels of CD25, now called Tregs. Tregs suppress autoreactive T cells by a not yet clearly understood contact-dependent mechanism.
Interestingly, suppression of tumor immunity by Tregs was first described in the early 1980s but was largely ignored. However, the recent demonstration that depletion of Tregs in mouse improves endogenous immune-mediated tumor rejection and tumor antigen-specific immunity has boosted the interest in the role of Tregs in tumor immunity. It was soon demonstrated that Treg depletion augments tumor immunotherapy, including vaccination and CTLA4 blockade. It is currently thought that Tregs suppress CD4+ and CD8+ T cell responses by cell-cell contact mechanisms and/or by the production of immunosuppressive soluble factors like IL-10 or TGF-β. It has been shown that the number of Tregs is increased in the blood of patients with different kinds of tumors, and these cells populate the tumor mass and the draining lymph nodes. Furthermore, in some cases there seems to be an inverse correlation between the presence of intra-tumor Treg and patient survival, and Treg depletion has been useful to improve the results of anti-cancer immunotherapy. The increased number of Tregs might be due to either tumor immunity being considered as an autoreactive response, or as a response to an inflammatory condition, or to other as yet undetermined factors. To be therapeutically useful, it is important to study these cells in the appropriate anatomical compartment, and to deplete them locally, i.e., within the tumor or the draining lymph nodes. In this regard, it is important to note that there are two phenotypically identical populations of CD4+CD25+ Tregs, i.e. adaptive and natural. Natural Treg arise in the thymus as a safeguard against autoimmunity, while adaptive arise during inflammatory processes including infections or cancer. Therefore, Tregs that infiltrate the tumor microenvironment are probably adaptive and they are induced to differentiate in situ into several subpopulations by tumor cells or by other cells, such as intratumor DCs. It should be also mentioned that Tregs can also inhibit the function of NK and B cells(9).
THE THEORY OF THE "IMMUNOEDITING"The complex interactions between different cells of the Immune System and between these cells and the microenvironment in the course of antitumor responses are well described by the term "immunoediting" (Figure 1). Stromal cells in the solid tumor microenvironment nourish and often outnumber the tumor cells themselves: Endothelial cells, fibroblasts, inflammatory cells and T regulatory immune cells are all generally abundant. Immunoediting starts with recognition and destruction of transformed cells that have acquired genetic damage (immunosurveillance). The 3 stages of immunoediting lead to control, stasis or outgrowth of a tumor and are usually named as follows: 1. Elimination: The immune system recognizes tumor cells and destroys them. 2. Equilibrium: The immune system is unable to completely destroy the tumor but converts it in a quiet mass of unproliferating cells. 3. Escape: The tumor evades the effector mechanisms of the Immune System and proliferates without control (Figure 1)(1,5).
So, one interesting assert of this theory is that, in addition to the Immune System's capacity to destroy and shape cancer, immunity can also have cancer under control for long periods of time by a process called equilibrium. Equilibrium is a component of cancer immunoediting because cells in equilibrium are highly immunogenic, whereas those cells exiting equilibrium and becoming growing tumors have attenuated immunogenicity. These results then place this process temporally between elimination and escape. Whereas elimination requires elements from both the innate and the adaptive immune response, equilibrium is solely maintained by adaptive immunity. Immune mechanisms can influence cancer growth both quantitatively and qualitatively, the quality and quantity of the immune reaction being reliable prognostic indicators of cancer patient survival(10). Extensive experimental support exists nowadays about the elimination and escape processes because immunodeficient mice develop more carcinogen-induced and spontaneous cancers than wild-type mice, and tumor cells from immunodeficient mice are more immunogenic than those from immunocompetent mice. In contrast, the equilibrium process has been inferred largely from clinical observations, including reports of transplantation of undetected (occult) cancers from organ donor into immuno-suppressed recipients.
THE ROLE OF ADAPTIVE IMMUNITY IN IMMUNOEDITINGIn December 2007, Koebel and colleagues published interesting results that extend our knowledge of the role of the Immune System in the equilibrium state, providing experimental support to this process and opening the possibility that tumors could be treated like a chronic diseasé(4). They demonstrated that the equilibrium estate is mechanistically distinguishable from elimination and escape and that neoplastic cells in equilibrium are transformed but proliferate poorly in vivo. The aim of these authors was also to find molecular markers to characterize the equilibrium state and the other two phases of the immunoediting process. Indeed, in recent years, immunotherapeutic approaches to treat cancer, with either adoptive transfer of immunity or stimulation of the endogenous immune system, have shown increasing promise. Clearly, the goal of cancer therapy is to kill residual tumors that cannot be excised surgically. Being of host origin, cancer cells share features of the host that make effective treatments difficult, due to side effects that limit the therapeutic window. Moreover, the plastic nature of tumors make them remarkably resilient in rebounding from clinical radiotherapy and chemotherapy treatments that are traditionally used. The tumor cells develop resistance under the selective pressures posed by cytotoxic agents. Genetic plasticity is a key characteristic of cancer cells, so that successful targeting requires the application of multiple agents that target different survival mechanisms(11). A solution may be to redirect the focus of the attack from the tumor cells to the environment that sustains their growth and survival (i.e., stromal cells in the tumor environment are not genetically plastic), or to engage the immune system.
It is in this point where the importance of the work of Koeble and colleagues resides: Even treatments that did not cure cancer but rather converted it to a long-term subclinical or at least manageable condition, would represent a resounding success. The key question is how can a tumor outrun an activated Immune System, so that the balance might be tipped back in favor of the Immune System. Some cancer immunologists are using the term "immune checkpoint" to refer to the negative-acting suppression pathways that prevent activation of the immune system.
The strategy of Koebel and colleagues was to use C57BL/6 and 129/SvEv mouse colonies established in two different laboratories to inject age- and sex-matched groups with 3'-methylcholanthrene (MCA) to induce sarcoma formation in the mice (primary tumorigenesis model). The interest of the authors then focused on the mice that did not develop progressively growing tumors, so that they eliminated all mice that had expanding tumors by 200 days. The animals that had only small stable masses at the injection site (supposed to be in an equilibrium state) were then treated with either control immunoglobulins or a mixture of monoclonal antibodies to deplete CD4+ and CD8+ T cells and to neutralize IFNγ or IL-12p70. They found that sixty per cent of these mice developed progressively growing tumors, whereas no growth was observed in control mice. Thus, the treatment facilitated the expansion of pre-formed occult cancer cells. Importantly, suppression of NK cell function did not induce tumor outgrowth. This finding indicated that equilibrium involves only adaptive immunity mechanisms. To examine this process more carefully, the authors then used mice deficient in recombinant-activating genes (Rag1−/− or Rag2−/− mice) which have an innate, but not an adaptive immune response. The results showed that sarcoma formation in Rag−/− mice was essentially complete within 200 days of MCA exposure. In addition, the mean time to tumor formation in MCA-treated Rag−/− mice differed strickingly from that found in MCA-treated wild-type mice, rendered immunodeficient at day 200 using the anti-CD4/-CD8/IFNγ mixture (105±5 days versus 25±6 days). Taken together, these data argue strongly against continuous de novo transformation as the mechanism underlying the late tumor outgrowth in wild-type mice after immunodepletion. Rather, they raise the possibility that at least some of the proposed tumor-promoting actions of chronic inflammation may be a result of interfering with adaptive immunity's capacity to hold unnoticed cancers in equilibrium. Furthermore, the immunohistochemical staining of the tumor stable masses in MCA-treated wild-type mice detected a population of atypical fibroblast-like cells that formed tumors when injected into Rag2−/− mice. This fact demonstrates the existence of occult tumor cells and explains how an occult cancer can be transplanted from a donor organ to a recipient(12), because tumor cells held in equilibrium in the donor may grow in a recipient that is at the same time naïve to the antigens of the transplanted tumor cells and immunosuppressed.
DISCLOSURESThe author declare no financial conflicts of interest.