Research into the binding properties and anatomical distribution of the Mannose Receptor (MR), and the phenotypic characterisation of MR deficient animals have provided highly valuable information regarding the role of the MR in health and disease. In this review, the biology of MR is considered in three different contexts: At the molecular level as a member of the mannose receptor family of proteins; as a macrophage and dendritic cell receptor; and as marker of lymphatic endothelia. Novel observations in these three areas highlight the unique properties of MR and the need to keep an open mind when assessing its physiological role.
El estudio de la especificidad y distribución anatómica del Receptor de la Manosa (MR) y la caracterización fenotípica de ratones deficientes en el MR han permitido discernir la función de este receptor en condiciones fisiológicas normales y patológicas. En esta revisión se considera la biología del MR en tres contextos diferentes: A nivel molecular como miembro de la familia de receptores de manosa; como receptor de macrófagos y células dendríticas; y como un marcador de vasos linfáticos. Resultados recientes en estas tres áreas resaltan las propiedades únicas de este receptor y la necesidad de mantener la mente abierta a la hora de establecer su función fisiológica.
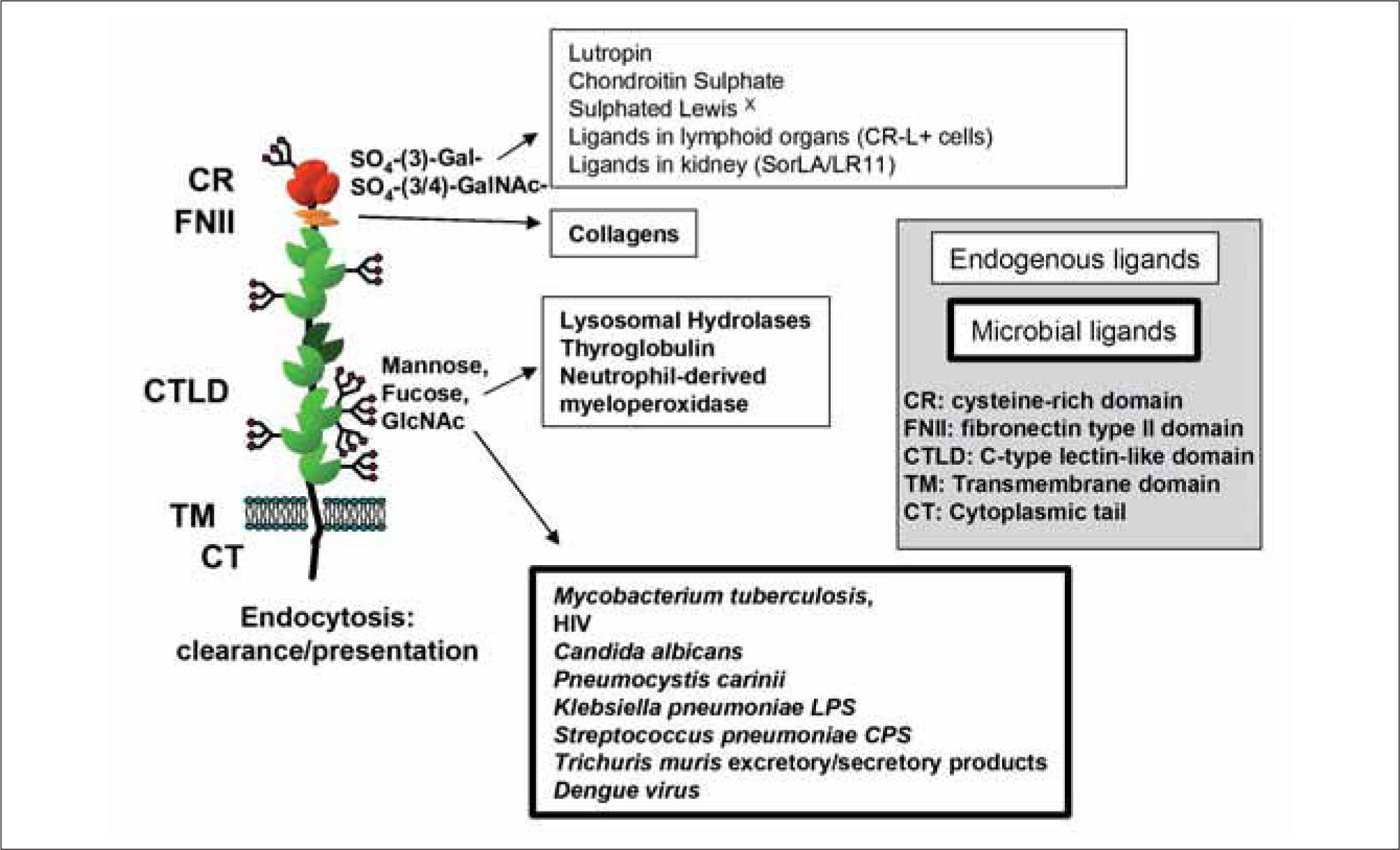
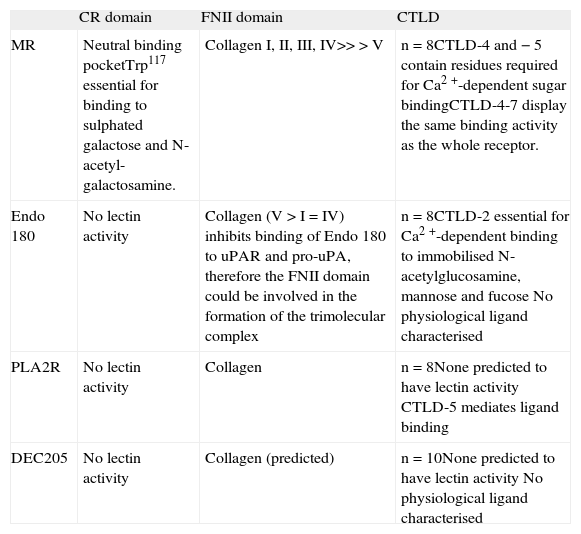
The domain structure of the members of the mannose receptor family is unique among the C-type lectin family of receptors because of the presence of several C-type lectinlike domain (CTLD) arranged in tandem within a single polypeptide. The MR (CD206), Endo 180 (CD280), and the phospholipase A2 receptor (PLA2R) contain eight CTLD whilst the antigen capture receptor, DEC205 (CD205), contains ten(1). Although all members share the presence of several CTLD, only MR and Endo 180 are able to bind carbohydrates(2, 3). In MR the core formed by CTLD-4 and CTLD-5 -the only true C-type lectin domains present in MR because of their ability to coordinate Ca2+- is essential for effective sugar binding. In Endo 180 the CTLD-2 is responsible for the lectin ability of the molecule(2, 3). Intriguingly, while there is an ever expanding list of natural glyco-conjugates for the MR CTLD (Figure 1), no natural glycosylated ligands have been described for Endo 180 to this date.
In all members of the MR family the CTLD-containing region is flanked by the cysteine-rich (CR) domain and the fibronectin type II (FNII) domain at the N-terminus and the transmembrane domain and the cytoplasmic tail at the C-terminus. Again, MR is unique within the family because it is the only member for which a function has been ascribed to the CR domain. In MR this region contains a neutral binding pocket through which it recognises terminal galactose or N-acetyl-galactosamine sulphated in position 3 or 4 (Figure 1)(4, 5). On the contrary, the ability of the FNII domain to bind collagens appears to be shared by all the family members. Sequence analysis predicted that MR, Endo 180, PLA2R and DEC205 would interact with collagens through this domain and this property has now been established in the case of PLA2R, Endo 180 and MR(1, 6-8). If the ability of the FNII domain of DEC205 to bind collagen is confirmed, this would be the first description of a natural ligand for this important antigen-delivery receptor (See Table I for an overview of the binding properties of MR, Endo 180, PLA2R and DEC205).
The MR family members are highly effective recycling endocytic receptors located largely in the endosomal compartment with only a fraction of the cellular protein (20- 30%) being located at the cell surface at any time. While MR and Endo 180 target to and recycle back from the early endosomal compartment, DEC205 targets the lysosomal compartment. The sequences responsible for intracellular targeting have been extensively discussed in an earlier review(1).
Soluble MR and antigen deliveryBoth PLA2R and MR can be found as cell-associated and soluble molecules bearing the complete extracellular region of the receptor. These soluble forms are produced by different mechanisms. Soluble PLA2R is produced by the translation of a shorter mRNA generated through the use of an alternative polyadenylation signal in the PLA2R-encoding mRNA(1). Soluble MR (sMR) is generated through proteolytic processing of the mature receptor by a metalloproteinase(9, 10). The biological role of these soluble molecules is unclear.
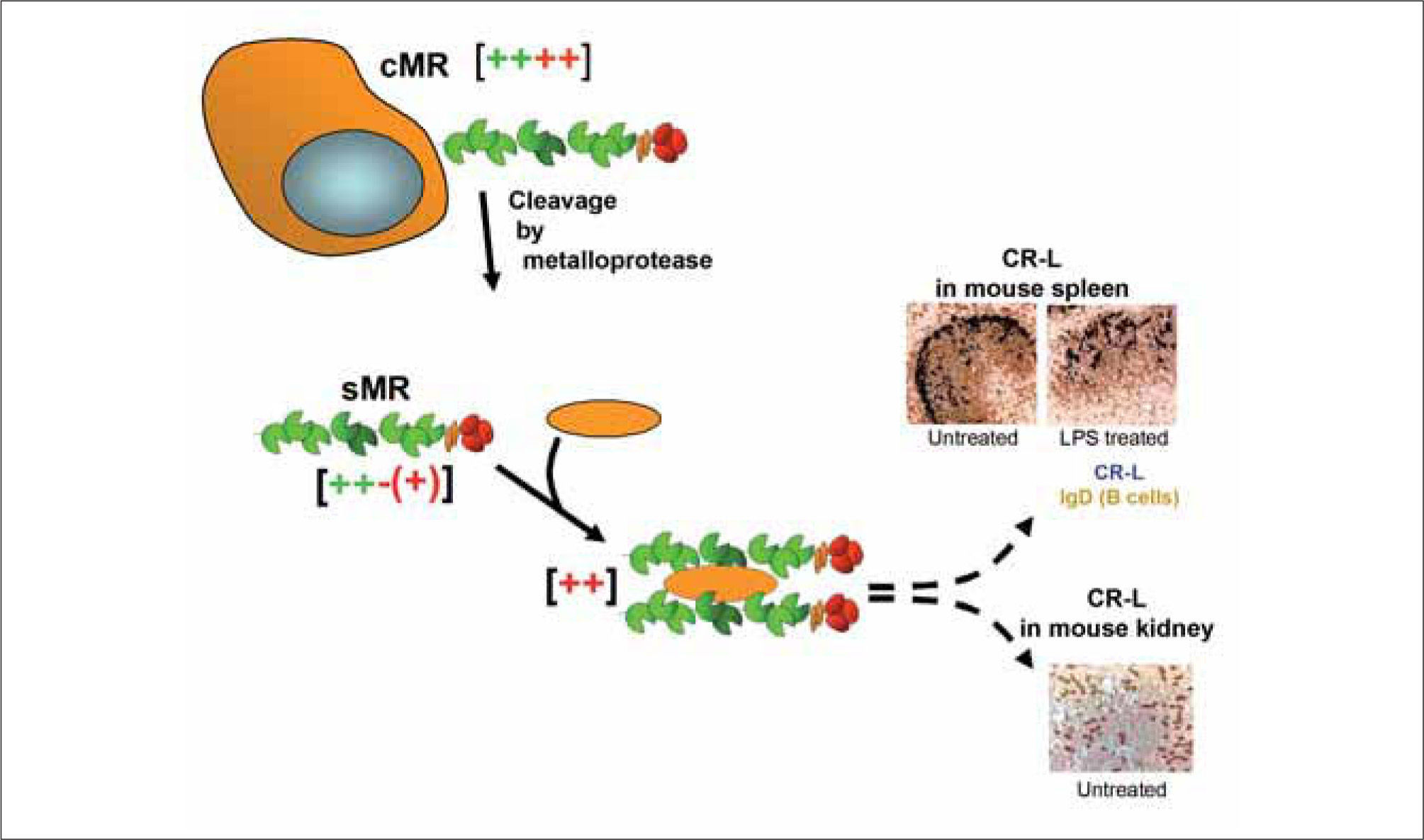
Structure-function studies suggest a role for sMR in the regulation of the immunogenicity of soluble MR ligands (Figure 2). sMR bearing N-linked complex carbohydrates terminated in sialic acid is largely monomeric and capable of binding to mannosylated ligands but not to sulphated carbohydrates or collagens(6, 11). Aggregation promoted through the interaction with multimeric ligands for the CTLD region, such as mannan, favours recognition of sulphated ligands(12), but is not enough for enabling binding to collagens (P.R. Taylor, R. Stillion, S. Gordon and L. Martinez-Pomares, unpublished). Indeed, it appears that only the cell-associated form of MR displays the FNII domain at a level of multimerisation enough to enable binding to collagens(6). When these results are considered together with the presence and distribution of ligands for the MR CR domain (CR-L) in secondary lymphoid organs(13-15) and in kidneys(16), a role can be envisaged for sMR in the delivery of soluble CTLD ligands to these relevant sites. Under homeostatic conditions CR-L are present in selected macrophage populations deficient in MR expression adjacent to B cell follicles in the spleen (marginal zone metalophillic macrophages) and lymph nodes (subcapsular sinus macrophages) and in distal collecting tubules in the kidneys.
The sMR and its potential targets in vivo.Monomeric sMR has reduced ability to bind CR-ligands [−(+) in red] but it can interact with CTLD ligands [++ in green]. Complex formation with multimeric CTLD ligands (yellow shape) will facilitate binding to cell-associated CR-ligands [++ in red] in secondary lymphoid organs and/or kidney. This hypothesis is supported by evidence demonstrating the requirement for CR multimerisation for binding to CR-L and the ability of CR-containing molecules to target to CR-L+cells in vivo.
Counter receptors for the CR domain have been identified as sialoadhesin and CD45 in secondary lymphoid organs(17) and as the endocytic receptor SorLA/LR11 in the kidneys(16). Recombinant proteins bearing the MR CR domain target CR-L+macrophages in secondary lymphoid organs. Upon binding, these proteins appear to be cleared with no phenotypical changes being observed in the targeted cells upon engagement of the CR-L, in spite of one of them being the protein phosphatase CD45(12). No information is available regarding the in vivo accessibility of kidney CR-ligands for interaction with CR domain-bearing molecules. However, it is possible that targeting of sMR-ligand complexes to this anatomical location could contribute to clearance of soluble MR ligands. Interestingly, innate stimulation modifies the distribution of CR-L in secondary lymphoid organs such that CR-L can now be observed within B cell follicles and on follicular dendritic cells(13-15). This altered pattern of CRL distribution is suggestive of an alternative fate for sMR ligands upon infection likely leading to increased immunogenicity due to improved presentation to B cells. The role of sMR in antigen (Ag) delivery has not been formally proven, but it appears that the presence of CR-L in spleen and lymph nodes does indeed highlight cells involved in Ag presentation. Recently three manuscripts have demonstrated the involvement of subcapsular sinus macrophages in the translocation of particulate native Ag from the subcapsular sinus into B follicles and its presentation to B cells. Intriguingly, the unique glycosylation machinery of these cells also generates ligands for another lectin involved in immunity, the galactose-type macrophage lectin(18).
The role of N-linked glycosylation and conformational changes in the regulation of MR binding propertiesLack of terminal sialic acid renders MR unable to bind or internalise mannosylated ligands but does not affect the recognition and internalisation of sulphated glycans or anti- MR mAb(11). The biological relevance of these findings are highlighted by the fact that MR is differentially glycosylated in vivo. MR from mouse spleen bears α2-3 linked terminal sialic acid while MR obtained from lung tissue displays terminal α2-6-linked sialic acid and mannose(11). Another consequence of the lack of sialic acid is an increased tendency of MR to multimerise, leading to increased binding to CR-L. Further comparison of MR properties at different anatomical positions will provide an insight into the relevance of these observations. Indeed, it is possible that the differing requirements for Pneumocystis carinii recognition between human and mouse alveolar macrophages might be caused by the differential glycosylation of MR in these cells between both species(19-21).
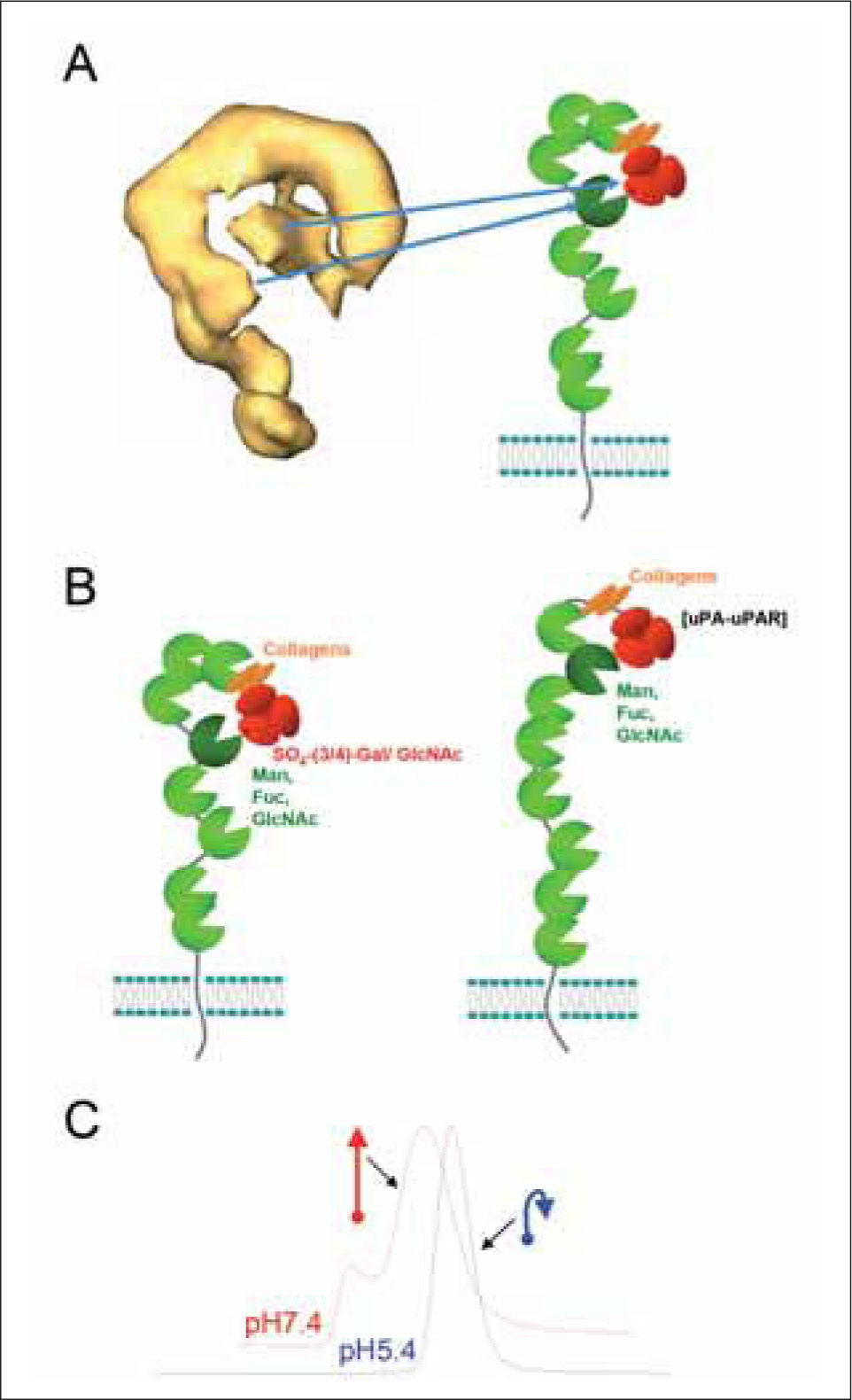
Two approaches have been used for investigating the conformation of the extracellular region of MR, ultracentrifugation and single particle electron microscopy. While the first approach led to the proposal of an extended conformation for MR(22), later microscopy studies revealed that MR can adopt a bent conformation in which the CR- domain and CTLD-4 contact each other (Figure 3A)(23). CTLD- 4 is the CTLD responsible for the selective binding of MR to D-mannose and is the only CTLD able to bind to carbohydrates in isolation. Intriguingly, in Endo 180 a similar interaction has been observed between the CR-domain and CTLD-2, again the CTLD responsible of sugar recognition (Table I, Figure 3B)(24). The conditions employed for those studies involved the use of neutral pH and presence of Ca2+. Further analysis revealed that Endo 180 adopts a more open conformation in response to pH acidification, which could be related to conformational changes required for ligand release in the endosome(23). MR can not be routinely visualised using electron microscopy due to its tendency to aggregate in solution in a Ca2+ and mannose-dependent manner(23). In this case, the evidence for conformational changes in response to acidification is provided by the altered mobility of sMR in gel-filtration chromatography observed under acidic conditions (Figure 3C)(23). The original ultracentrifugation studies were performed in the absence of Ca2+ due to problems with protein aggregation(22), therefore it is probable that the bent conformation of MR could be controlled by the presence of Ca2+ in addition to pH. Finally, in view of these results it is tempting to speculate that CTLD-4 might have a natural tendency to dimerise, as suggested by the identification of dimeric domain-swapped CTLD-4 during the investigation of its crystal structure(25).
MR conformation.A. 3D structure of the MR as determined by single particle microscopy. B. Schematic representation of the structural organisation of the MR and Endo 180. [uPA-uPAR] indicates the ability of Endo 180 to bind the complex of urokinase-type plasminogen activator (uPA) and its receptor (uPAR). This binding is inhibited by collagen. C. pHdependent conformational changes in MR. Occurrence of conformation changes in MR has been shown by gel filtration chromatography performed at neutral and acidic pH.
Characteristics and binding properties of the members of the MR family of receptors
| CR domain | FNII domain | CTLD | |
| MR | Neutral binding pocketTrp117 essential for binding to sulphated galactose and N-acetyl-galactosamine. | Collagen I, II, III, IV>>>V | n=8CTLD-4 and −5 contain residues required for Ca2+-dependent sugar bindingCTLD-4-7 display the same binding activity as the whole receptor. |
| Endo 180 | No lectin activity | Collagen (V>I=IV) inhibits binding of Endo 180 to uPAR and pro-uPA, therefore the FNII domain could be involved in the formation of the trimolecular complex | n=8CTLD-2 essential for Ca2+-dependent binding to immobilised N-acetylglucosamine, mannose and fucose No physiological ligand characterised |
| PLA2R | No lectin activity | Collagen | n=8None predicted to have lectin activity CTLD-5 mediates ligand binding |
| DEC205 | No lectin activity | Collagen (predicted) | n=10None predicted to have lectin activity No physiological ligand characterised |
MR is a major homeostatic receptor responsible for the efficient removal of molecules requiring a short half-life in circulation. Clearance of synthetic glycoproteins bearing mannose, N-acetyl-glucosamine and sulphated-galactose is defective in the viable strain of MR-deficient animals(26). MR has been identified as the receptor responsible for the uptake of selected lysosomal hydrolases under inflammatory conditions(26), sulphated glycoprotein hormones produced by the anterior pituitary(27, 28), and collagens(6, 7, 29). Therefore, it is not surprising that under steady-estate conditions, MR expression in the myeloid lineage is largely restricted to tissue macrophages, which maintain tissue homeostasis by clearing potentially harmful molecules and sensing the health status of tissues. Accordingly, and probably due to the lack of signalling motifs from the cytoplasmic tail of MR, uptake of MR-ligands does not appear to trigger an activating signalling cascade which would be the desirable scenario for the silent removal of endogenous molecules.
MR and induction of immunityHow can we reconcile this immunologically inert scenario with the well-established role of MR in pathogen recognition (Figure 1) and antigen presentation?(30-35). Again, the analysis of MR expression in situ might provide some clues. A scenario similar to that previously described for the CR-L appears possible. The distribution of MR changes upon innate stimulation and while MR is relatively absent from the T cell areas of secondary lymphoid organs under steady state conditions(36), MR-expressing cells with characteristics of dendritic cells can be clearly observed at these sites in the presence of Toll-like receptor agonists such as endotoxin or flagellin(36). Intriguingly, even when innate immune stimulators were administered systemically, the presence of these MR+dendritic cells remained restricted to peripheral lymph nodes, and no MR expression in T cell areas could be detected in mesenteric lymph nodes or spleen. The in situ detection of anti-MR mAbs after subcutaneous administration associated with MR+ dendritic cells in T cell areas of draining lymph nodes and the increased immunogenicity of anti-MR mAb in the presence of innate stimulation suggest that, upon innate stimulation, MR functions as an Ag delivery system leading to enhanced Ag presentation(36). The increased presence of MR in dendritic cells is concomitant with the reduced expression of MR in macrophages; i.e., during the course of infection there might be a shift from clearance of MR ligands to their presentation. This would ensure that an effective sampling system, such as MR, can be exploited by the acquired immune system for the presentation of mannosylated ligands associated to infection. The kinetics of appearance of MR+dendritic cells is consistent with an influx of resident cells from the periphery (skin) where MR is present in cells characterised as macrophages(36). These observations suggest that upon peripheral stimulation some of the first cells to reach the draining lymph nodes could be migratory macrophages that have acquired dendritic cell characteristics upon activation.
Work with the model Ag ovalbumin (OVA), which requires MR expression for efficient uptake by cultured dendritic cells, raised the possibility of MR being specially suited for the delivery of Ag for cross-presentation(30, 31). This process enables the presentation of exogenous Ag through the MHC-I pathway, the Holy Grail for the generation of potent cytotoxic responses against intracellular pathogens and tumours. Indeed, MR expression contributes to the activation of OVA-specific CD8 T cells in vivo in response to soluble OVA(31). This surprising role for MR as unique route for cross-presentation has now been placed in a new light by the use of endotoxin-free OVA, which has enabled the dissection of the contribution of MR and Toll-like receptor activation to the cross-presentation process(32). MR-mediated uptake does not automatically lead to crosspresentation; OVA internalisation is indeed largely mediated by MR but efficient cross-presentation requires Toll-like receptor activation. Toll-like receptor activation leads to the appearance of the peptide transporter TAP in the early endosomal compartment, which facilitates cross-presentation of the material endocytosed through MR, OVA, in the endosomal compartment. These results are in agreement with the work of Apostolopoulos et al.(37), who originally established that MR binding didn't automatically lead to cross-presentation; in this model it was the presence of aldehyde groups in the Ag, originated during the coupling reaction to mannan under oxidative conditions, which promoted cross-presentation. It would be of interest to investigate if a similar process would apply for other Ag internalisation systems.
A non-redundant role for MR in the elicitation of mannoprotein-specific T cell responses in response to pulmonary challenge with Cryptococcus neoformans has been recently described. In this model lack of T cell activation correlates with increased susceptibility of MR−/− mice to C. neoformans infection(38). These findings validate an important contribution of the Ag-presentation capacity of MR in the context of infection. Intriguingly, no major differences in susceptibility to infection between WT and MR-deficient animals have been observed in the case of C. albicans, P. carinii or Leishmania sp(39-41), all pathogens able to bind MR. These results suggest that even though MR is able to bind to pathogens, its contribution to immune defence might not to be readily perceived. For instance, enhanced resistance to oral infection with C. albicans has been recently observed in response to treatment with an agonist for the nuclear receptor peroxisome proliferator-activated receptor-Y (PPAR-y) or IL-13, both enhancers of MR expression(42, 43). In fact, IL- 13 treatment induces the expression of endogenous PPAR-y ligands through activation of cytosolic phospholipase A2. These results correlate with enhanced Candida uptake, candidastatic activity and MR expression in cultured macrophages treated with PPAR-y, and with increased expression of MR in macrophages recruited to the cecal mucosa of infected animals. Moreover, this model shows that a path of macrophage differentiation normally associated with parasitic infections and down-modulation of immune responses (see below), can be effective in protection against this opportunistic fungal pathogen in the context of mucosal immunity.
MR as a marker of alternative activated and deactivated macrophagesAn aspect of MR biology that deserves further investigation is its contribution to the functional properties of macrophages modified by the presence of the Th2 cytokines IL-4 and IL-13 (alternatively activated macrophages, Ym-1+, Fizz-1+, arginase-1+, with a highly developed endocytic compartment) and in macrophages cultured in the presence of IL-10 (deactivated macrophages, with a poorly developed endocytic compartment) as MR is up-regulated under both conditions(44, 45). Both of these types of macrophages appear to be part of an immune down-modulation program designed for the maintenance of tissue homeostasis [adipose tissue-associated macrophage(46), influenced by the lipid environment through the PPAR-y receptor(47)], or to regulate the response to sterile damage [surgery-induced macrophage(48)]. Probably, these initial responses, achieved in the absence of strong innate immune activation, later enable the induction of the Th2- driven immune responses characteristic of parasitic infections.
Indeed, in the case of schistosomiasis alternative macrophage activation has been shown to be essential for survival(49). On the other hand, when the immunomodulatory properties of the parasite are lacking, innate alternative activation could promote the development of a detrimental allergic response(50). Finally, these pathways of macrophage activation have been documented in tumour-associated macrophages and appear to correlate with their tumour promoting properties(51). In the context of MR biology, it is intriguing that in a recent work looking at the role of MR in tumour metastasis (see below), 50% of the tumour cell lines tested displayed ligands for the MR CTLD region(52).
MANNOSE RECEPTOR IN THE CONTEXT OF LYMPHATIC ENDOTHELIUMMR expression is highly characteristic of human and mouse lymphatic endothelia and the macrophages located in the medullary sinuses in lymph nodes. Jalkanen and co-workers originally described a role for MR expressed in human lymphatic endothelia in lymphocyte binding(53). Recent work by the same group has confirmed these observations and, furthermore, has implicated MR in the facilitation of tumour metastasis(52). Upon subcutaneous inoculation, tumour spreading into draining lymph nodes was significantly reduced in MR-/-animals. The authors rule out a role for MR in the elicitation of an anti-tumour response and confirm the presence of MR in the afferent arm of the lymphatic system by investigating its expression in lymphatic vessels in the skin. It is remarkable that the overall growth of the tumour is maintained in WT and MR_/_animals but, while in WT animals the tumour mass is distributed between the site of the injection and the regional draining lymph nodes, in MR-/_mice most of the tumour mass remains in the site of injection. Further investigation into the expression of MR in lymphatic vessels in normal and cancerous human tissues will help to elucidate the biological relevance of these observations. The presence of MR ligands at the surface of tumour cells mentioned above might have a double role: (i) the engagement of MR in tumour associated myeloid cells with the potential effect of down modulating effector immune responses within the tumour, as suggested for DC-SIGN and the macrophage galactose-type lectin(54), or (ii) the facilitation of the interaction between tumour cells and lymphatic vessels and promotion of metastasis(55). It is worth mentioning that while in the mouse spleen MR is expressed in macrophages and non vascular endothelial cells located at the red pulp(56), in human spleen MR is restricted to the cells lining the venous sinuses(57, 58). These unique cells co-express the lymphatic endothelial marker Lyve-1 and form a physical barrier for blood cells that literally need to squeeze through the slits between the cells for successful exit from the spleen parenchyma. It is tempting to speculate that MR at this distinct anatomical location might be contributing to the unique filtering properties of this organ as suggested by its involvement in lymphocyte trafficking in the mouse model(52). Unlike the mouse system where CR-L and MR are never present in the same cells, the cells lining the venous sinuses in human spleen co-express MR and CR-L. Thus, the cellular biology of MR could be altered in these cells by the presence of molecules able to interact with the MR CR domain.
CONCLUSIONSIn this review, we have tried to provide an updated and critical (or even speculative) view of recent literature on MR. This multitalented molecule does not stop surprising us. Being first identified as a homeostatic receptor involved in clearance of self-molecules and later as an important pattern recognition receptor involved in pathogen recognition, it has returned to teach us about the fine line between homeostasis and immunity. The increasing range of studies involving the use of MR_/_animals, the availability of useful techniques to switch off MR expression in vitro which go beyond the indicative but limited approach of sugar inhibition, and a better understanding of MR molecular and binding properties and distribution are finally paying dividends.
DISCLOSURESThe author has no conflicting financial interest.
ACKNOWLEDGEMENTSThe author would like to thank the members of the Lectin Biology Group for making doing science so much fun. The University of Nottingham, Mizutani Foundation for Glycoscience and Asthma, UK, has provided funding.





![The sMR and its potential targets in vivo.Monomeric sMR has reduced ability to bind CR-ligands [−(+) in red] but it can interact with CTLD ligands [++ in green]. Complex formation with multimeric CTLD ligands (yellow shape) will facilitate binding to cell-associated CR-ligands [++ in red] in secondary lymphoid organs and/or kidney. This hypothesis is supported by evidence demonstrating the requirement for CR multimerisation for binding to CR-L and the ability of CR-containing molecules to target to CR-L+cells in vivo. The sMR and its potential targets in vivo.Monomeric sMR has reduced ability to bind CR-ligands [−(+) in red] but it can interact with CTLD ligands [++ in green]. Complex formation with multimeric CTLD ligands (yellow shape) will facilitate binding to cell-associated CR-ligands [++ in red] in secondary lymphoid organs and/or kidney. This hypothesis is supported by evidence demonstrating the requirement for CR multimerisation for binding to CR-L and the ability of CR-containing molecules to target to CR-L+cells in vivo.](https://static.elsevier.es/multimedia/02139626/0000002700000003/v1_201305141258/S0213962608700611/v1_201305141258/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![MR conformation.A. 3D structure of the MR as determined by single particle microscopy. B. Schematic representation of the structural organisation of the MR and Endo 180. [uPA-uPAR] indicates the ability of Endo 180 to bind the complex of urokinase-type plasminogen activator (uPA) and its receptor (uPAR). This binding is inhibited by collagen. C. pHdependent conformational changes in MR. Occurrence of conformation changes in MR has been shown by gel filtration chromatography performed at neutral and acidic pH. MR conformation.A. 3D structure of the MR as determined by single particle microscopy. B. Schematic representation of the structural organisation of the MR and Endo 180. [uPA-uPAR] indicates the ability of Endo 180 to bind the complex of urokinase-type plasminogen activator (uPA) and its receptor (uPAR). This binding is inhibited by collagen. C. pHdependent conformational changes in MR. Occurrence of conformation changes in MR has been shown by gel filtration chromatography performed at neutral and acidic pH.](https://static.elsevier.es/multimedia/02139626/0000002700000003/v1_201305141258/S0213962608700611/v1_201305141258/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)