Contingency management (CM) is one of the most effective interventions for smokers with substance use disorder (SUD), and no empirical assessment of its long-term efficacy has been conducted so far in a real-world context. The objectives were: (1) examine the additive effectiveness of CM on cognitive-behavioral treatment (CBT) for smoking cessation, and (2) examine the relationship between smoking cessation and substance use abstinence.
MethodA total of 80 participants (75.8% males; Mage = 45.31; SD = 9.64) were assigned to two smoking cessation treatments: CBT or CBT+CM. A set of generalized estimating equations were conducted to examine the effect of treatment condition on smoking outcomes, as well as the effect of smoking status on substance abstinence.
ResultsAdding CM to CBT for smoking cessation improved tobacco abstinence rates at the end-of-treatment (p = .049). Tobacco abstinence rates declined over time (p = .012), but no significant effects of treatment condition were observed across follow-ups (p = .260). Smoking cessation was not significantly related to substance abstinence (p ≥ .488).
ConclusionsCM facilitates early abstinence in smokers with SUD, although effects subside after treatment termination. The lack of association between smoking abstinence and substance use suggests no jeopardizing effects as a result of quitting smoking.
Tobacco use rates have declined in recent years in general population (Wang et al., 2018), however, the smoking prevalence remains notably high among specific vulnerable populations (Drope et al., 2018). In particular, individuals with substance use disorders (SUD) are between two and four times more likely to report using tobacco compared with non-SUD populations (Fine et al., 2019; Guydish et al., 2020; Hayhurst et al., 2020). This behavior places this population at a highly vulnerable situation, where lower quality of life and impaired mental health are evinced (Lien et al., 2021). In comparison with non-smokers with SUD, smokers have a four-time higher premature mortality rate due to tobacco use (Hser et al., 1994), and are more likely to die from tobacco related diseases (Baca & Yahne, 2009; Hurt et al., 1996).
Voucher-based contingency management (CM) is a well-established intervention for smoking cessation (e.g., Hand et al., 2017; Notley et al., 2019). It consists of providing incentives contingently upon biochemically verified substance use abstinence (Ginley et al., 2021), but also in relation to other therapeutic goals (i.e., adherence to therapy tasks or attendance) (Pfund et al., 2021). A recent meta-analysis among smokers with SUD (Secades-Villa et al., 2020) has shown that CM produces a 36% of tobacco abstinence (compared to 7.8% in comparison groups) at the end of treatment. Although no long-term additive effect was observed, abstinence rates declined at 7.8% (compared to 1.7% in control groups) at six-month follow-up. A common criticism of CM is that its effect may not endure after the discontinuation of rewards. Relatedly, research has informed of larger effects when the CM protocol includes bonus for consecutive good performance (Businelle et al., 2009) and when incentives are sustained after treatment termination (Secades-Villa et al., 2019), but there is no evidence available in this field, since few studies include incentives in the follow-ups (see Cooney et al., 2017; Rohsenow et al., 2017). On the other hand, very few studies have looked at long-term effects of CM in SUD population, and these present several methodological limitations related to low sample sizes (Beckham et al., 2018), or CM is combined with pharmacological treatments (Beckham et al., 2018; Rohsenow et al., 2017; Shoptaw et al., 2002).
Furthermore, there are few studies implemented in real world settings (see Higgins et al., 2019). One of the most widespread myths is the belief that quitting smoking jeopardizes abstinence from substances other than nicotine (Gentry et al., 2017; González-Roz et al., 2019a). While smoking appears to increase the risk of substance use relapse in some studies (Fu et al., 2008; Weinberger et al., 2017), others indicate protective effects of smoking abstinence over substance relapse (Berg et al., 2015; Magee & Winhusen, 2016). Regarding studies using CM only, some found a positive effect (Orr et al., 2018), whereas others reported a null effect (Cooney et al., 2017; Rohsenow et al., 2015, 2017). It is worthy of note that these studies included a low treatment duration (19 - 21 days), and tobacco abstinence rates were too low to establish a significant relationship between tobacco and substance abstinence (ranged from 3% to 12%).
This randomized controlled trial sought: (1) to examine the additive effectiveness of voucher-based CM to a CBT for smoking cessation among smokers with SUD at the end of treatment and follow-ups (i.e., 3, 6, and 12 months), and (2) to analyze the relationship between tobacco and substance use abstinence at long-term.
MethodParticipantsParticipants were recruited from SUD treatment facilities by means of therapists’ referral. Inclusion criteria were self-reporting 10 cigarettes per day within the last year, receiving outpatient SUD treatment at the time of the study entry, and being able to attend the full smoking cessation treatment. Exclusion criteria were self-reported diagnosis of severe mental disorder (i.e., active psychotic disorder and/or suicidal ideation); current cannabis use; and current use of pharmacotherapy or behavioral treatment for smoking cessation.
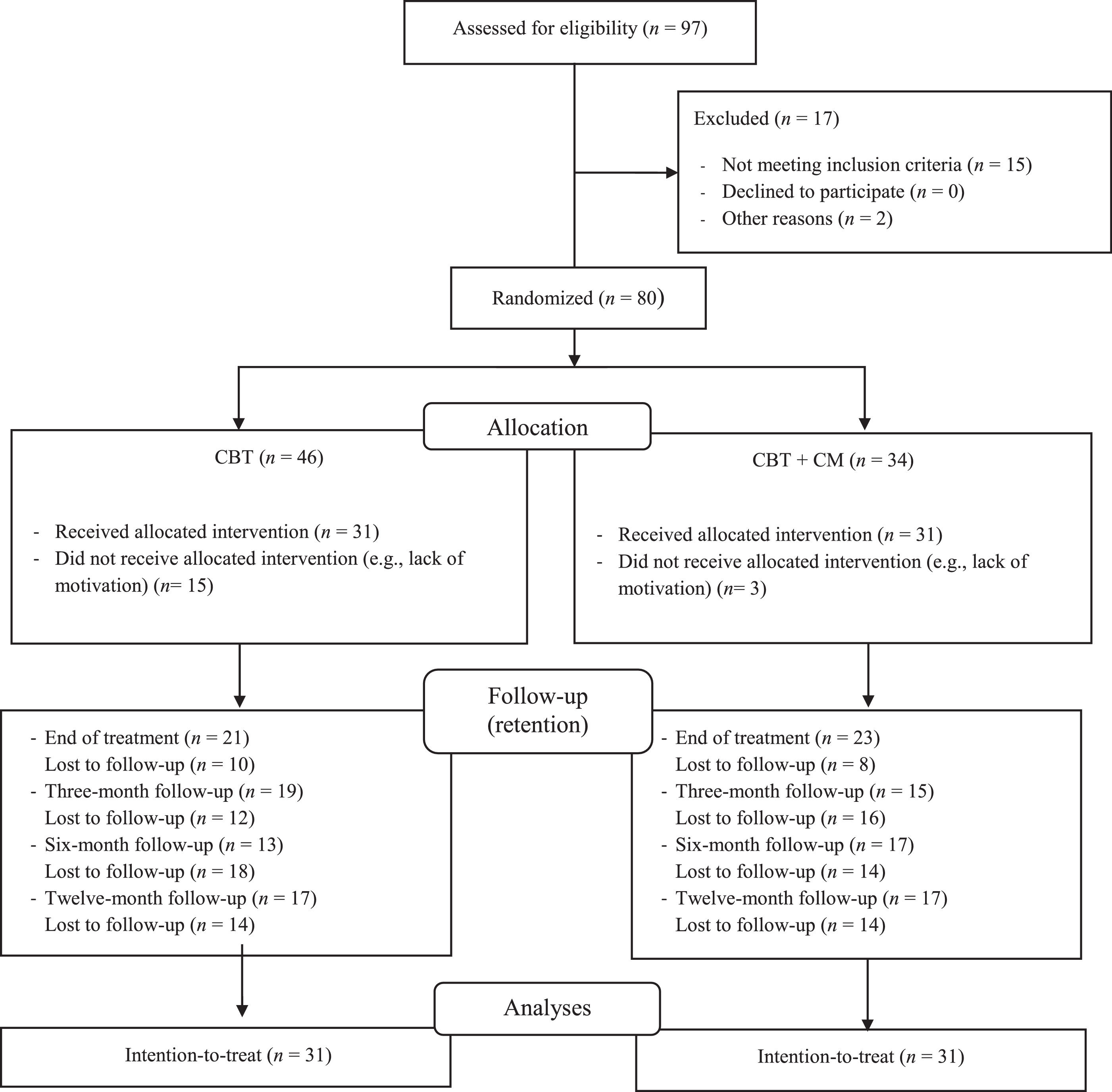
A total of 97 participants were recruited for this randomized control trial, of which 17 were excluded due to cannabis use (n = 8), severe mental disorder (n = 2), not being in receipt of SUD treatment (n = 2), lack of motivation to quit tobacco (n = 2), self-quitting prior to treatment onset (n = 2), and electronic cigarette use (n = 1) (see Fig. 1). A total of 80 participants were randomly assigned to two treatment conditions: CBT (n = 46) or CBT+CM (n = 34). Fifteen participants in CBT and three in CBT+CM refused to participate after the baseline assessment, leaving a total of thirty-one participants in each treatment condition. There were not significant differences in baseline characteristics between treatment conditions (all p-values ≥ 0.115) (Table 1).
Baseline participants’ characteristics.
| CBT(n = 31) | CBT + CM(n = 31) | p | |
|---|---|---|---|
| Age | 44.77 (10.70) | 45.84 (8.59) | .667 |
| Sex (male)a | 24 (77.42%) | 23 (74.19%) | .767 |
| Marital status (married)a | 8 (25.80%) | 9 (29.03%) | .776 |
| Working status (employed)a | 10 (31.25%) | 12 (38.71%) | .596 |
| Educational level (< High School)a | 15 (48.38%) | 13 (41.94%) | .610 |
| Monthly income (€) | 1397.71 (1,527.73) | 1450.45 (1,214.36) | .881 |
| Tobacco use related variables | |||
| CPD | 22.55 (10.34) | 20.58 (8.31) | .412 |
| Years of regular use | 27.05 (10.73) | 27.50 (10.48) | .870 |
| Previous 24 h quit attempts | 1.35 (1.66) | 1.55 (1.36) | .618 |
| CO (ppm) | 22.32 (15.64) | 25.71 (15.96) | .402 |
| FTCD | 6.48 (2.2) | 5.70 (1.82) | .136 |
| SCID-5 – Tobacco use disorder | 5.71 (1.93) | 4.87 (2.19) | .115 |
| Stages of change | .610 | ||
| Pre-contemplation | 1 (3.23%) | 0 (0%) | |
| Contemplation | 20 (64.51%) | 20 (64.52%) | |
| Preparation | 10 (32.25%) | 10 (32.26%) | |
| Substance use related variables | |||
| Days on substance use treatment | 490.26 (918.06) | 232.23 (250.60) | .136 |
| Primary substancea | .836 | ||
| Cocaine | 13 (41.93%) | 10 (32.26%) | |
| Alcohol | 12 (38.71%) | 13 (41.94%) | |
| Opioids | 5 (16.13%) | 6 (19.35%) | |
| Otherb | 1 (3.22%) | 2 (6.45%) | |
| Secondary substancea | .612 | ||
| Cocaine | 2 (6.45%) | 3 (9.68%) | |
| Alcohol | 6 (19.35%) | 3 (9.68%) | |
| Cannabis | 4 (12.90%) | 3 (9.68%) | |
| Opioids | 0 (0%) | 1 (3.23%) | |
| Benzodiazepines | 1 (3.22%) | 0 (0%) | |
| Abstinence (days) from primary substance of use | 296.65 (541.85) | 295.71 (427.87) | .993 |
| Abstinence (days) from secondary substance of use | 468.83 (810.33) | 950.60 (1621.24) | .376 |
Note.
includes cannabis, ketamine, GHB, and benzodiazepines.
CBT = cognitive-behavioral treatment; CM = contingency management; CPD = cigarettes per day; CO (ppm) = carbon monoxide in parts per million; FTCD = Fagerström test for cigarette dependence; SCID = Structured Clinical Interview for DSM-5 disorders.
All participants were interviewed in an individual single assessment which gathered data about sociodemographic characteristics (e.g., age, sex, monthly income, marital status), as well as tobacco- and substance-related variables. Variables related to tobacco were number of cigarettes per day, years of regular use, motivation to quit (i.e., pre-contemplation, contemplation, and preparation stages) and previous quit attempts. Nicotine dependence was evaluated through the Fagerström Test for Cigarette Dependence (FTCD; Fagerström, 2012), which consist of 6 items. FTCD scores yield five levels of cigarette dependence: very low (0–2), low (3–4), medium (5), high (6–7), and very high (8–10) (Fagerström & Kozlowski, 1990).
The Structured Clinical Interview for the DSM-5 (SCID-5) was used to assess past year tobacco use disorder. The SCID-5 covers the 11 DSM criteria in a dichotomized scale (i.e., yes/no). SUD severity was interpreted based on the DSM-5 guidelines: absence (0–1), minimal (2–3), moderate (4–5), and severe (6–11). Substance-related variables were assessed as well, including primary and secondary substance of use, substance use abstinence (in days), and substance use treatment length (in days).
Both tobacco and substance use were biochemically verified. Carbon monoxide (CO) was used to assess tobacco use exposure at the baseline assessment and follow-ups, and urine cotinine was used to confirm smoking abstinence status from the sixth session, at the end of treatment, and each of the follow-up assessments. The cut-off for determining smoking abstinence were CO ≤ 4 ppm and urine cotinine ≤ 80 ng/ml. At the baseline assessment and each of the study visits, substance use (cannabis, cocaine, opioids, amphetamines, and methamphetamines) was assessed through test cassettes, and alcohol use was monitored through air expired. Worthy of note is that due to the COVID-19 lockdown (between March and June 2020), abstinence, both from tobacco and other substances, was not verified biochemically.
ProcedureThe treatment protocols and study procedures were approved by the Local Research Ethics Committee (No. 144/16) and registered in the ClinicalTrials-gov database (ref. NCT03551704). All participants provided a written informed consent prior to the baseline assessment.
Treatment interventionsBoth treatment conditions (i.e., CBT and CBT+CM) included an eight-week smoking cessation treatment. Patients had to attend the clinic twice a week, one for the therapy session, with a duration of one hour and half, and the control session, whose objective is to collect biochemical samples.
Cognitive-Behavioral Therapy (CBT). The CBT protocol included several components previously described in standard cognitive-behavioral smoking cessation treatments (Secades-Villa et al., 2014) such as: psychoeducation, biochemical feedback, stimulus control, and training in strategies for reducing impulsivity, dealing with nicotine withdrawal symptoms, relapse prevention, and problem solving, among others. The CBT included a nicotine fading component that consisted of decreasing 20% of nicotine each week. Patients were asked to gradually reduce the number of cigarettes and switch their brand to lower-nicotine-content cigarettes each week. Patients were trained in EFT (the capability to pre-experience and project oneself into specific future events) (Morris et al., 2020). Further details on the EFT procedure may be consulted elsewhere (Aonso-Diego et al., 2021). In brief, participants were trained in the visualization of a total of five situations (one situation in one week, two in two weeks, one in a month, and one in three months), and practice visualizing them at each of the therapy sessions and at home.
Cognitive-Behavioral therapy (CBT) + Contingency Management (CM). This condition included the CBT, as described above, and voucher-based CM component for reinforcing tobacco abstinence. It consisted of providing contingent points (incentives) in exchange for biochemically verified tobacco abstinence (CO ≤ 4 ppm and urine cotinine ≤ 80 ng/ml). Incentives started at 20 points (€20) in the sixth session and increased by 5 points (€5) for each negative sample. Additionally, after each two consecutives negatives samples, patients received an additional 10 points. The reinforcement was continued through follow-ups: 45, 50, and 55 points were given to abstinent patients at one-, two-, and three-month follow-ups, respectively. The total maximum amount of vouchers a patient could earn if abstinent throughout the entire treatment and follow-ups was 340 points.
Outcomes measuresThe primary outcome variable was smoking abstinence, for which two measures were considered: (1) self-reported point-prevalence abstinence (24-hour tobacco abstinence at the end of treatment and 7 days in follow-ups), and (2) days of continuous abstinence (i.e., number of consecutive days without smoking, not even a puff). Substance use abstinence was a secondary study outcome and was operationalized as 7-day point-prevalence, as well as by biochemical analysis. Worthy of note is that some follow-up visits were not collected due to COVID, and abstinence was based on self-report assessments.
Following an intent-to-treat approach, participants with missed study follow-up visits were considered as smokers. It is worth noting that the intention-to-treat approach was not considered for substance use outcomes (other than cigarette smoking). This means that missing participants were not considered as actively using substances (other than nicotine) at their corresponding follow-ups but were removed from the analyses. As participants were receiving treatment at the time of the smoking cessation trial, interpretations of the ‘true’ effects of study treatments were less straightforward and considering an intent-to-treat approach would unequivocally lead to high rates of false positives.
Data analysisBivariate analyses and descriptive statistics were performed to examine differences in baseline characteristics and abstinence rates. Differences between the two treatment conditions in continuous variables were examined with t-tests, while chi-square analyses were run for categorical variables. Risk ratios (RR) were performed to determine the risk of being a smoker in the follow-ups compared to end of treatment. The RR was estimated by dividing the incidence in the exposed group (i.e., smokers) by the cumulative incidence.
To support methodological convergence, a set of three generalized estimating equations (GEEs) were conducted to examine the efficacy of the tested interventions across time. The first one was performed to assess the main effects of treatment condition (i.e., CBT+CM vs. CBT) and time (i.e., end-of-treatment, 3-, 6-, and 12-month follow-up), as well as its interactive effect, in predicting point-prevalence smoking abstinence. The second and third GEE were aimed at examining the predictive capability of point-prevalence smoking abstinence and treatment conditions on substance abstinence at medium-term (i.e., 3 and 6 months) and long-term (i.e., 12 months). Given that both dependent variables (i.e., smoking abstinence and substance use abstinence) were dichotomous, the model was adjusted using logit link function, assuming a binomial distribution for the random component and with an unstructured working correlation.
Descriptive analyses were conducted using SPSS (version 24, Inc., Chicago, IL, USA), whereas the GEE was implemented through the PROC GENMOD procedure using SAS software version 9.4 (SAS Institute, Cary, NC). The confidence level for all the analyses was set at a 95% level.
ResultsTreatment effect on smoking abstinenceAt the end of treatment, the 24-h point-prevalence was 30.65% (19/62). Nearly twice as many of the participants in CBT+CM vs. CBT attained at least 24-h tobacco abstinence (41.94% vs. 19.35%; p = .049; φ = 0.245). Smoking abstinence rates by treatment condition at follow-ups are displayed in Table 2. Seven-day point-prevalence tobacco abstinence rates were 14.52% at 3-month follow-up (CBT: 12.90%; CBT+CM: 16.13%; p = .354), and 6.45% at 6- and 12-month follow-up (CBT: 6.45%; CBT+CM: 6.45%; p = 1).
Point-prevalence smoking abstinence and days of continuous abstinence at end of treatment and each follow-up.
| EOT | 3-month FU | 6-month FU | 12-month FU | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBT | CM | p | CBT | CM | p | CBT | CM | p | CBT | CM | p | |
| PP | 6 (19.35%) | 13 (41.94%) | .049 | 4 (12.90%) | 5 (16.13%) | .718 | 2 (6.45%) | 2 (6.45%) | 1 | 2 (6.45%) | 2 (6.45%) | 1 |
| CAa | 5.65 (12.25) | 5.52 (7.89) | .961 | 15.48 (41.10) | 14.71 (36.48) | .938 | 14.06 (53.08) | 12.77 (49.45) | .921 | 25.32 (98.96) | 24.39 (94.40) | .970 |
Note.
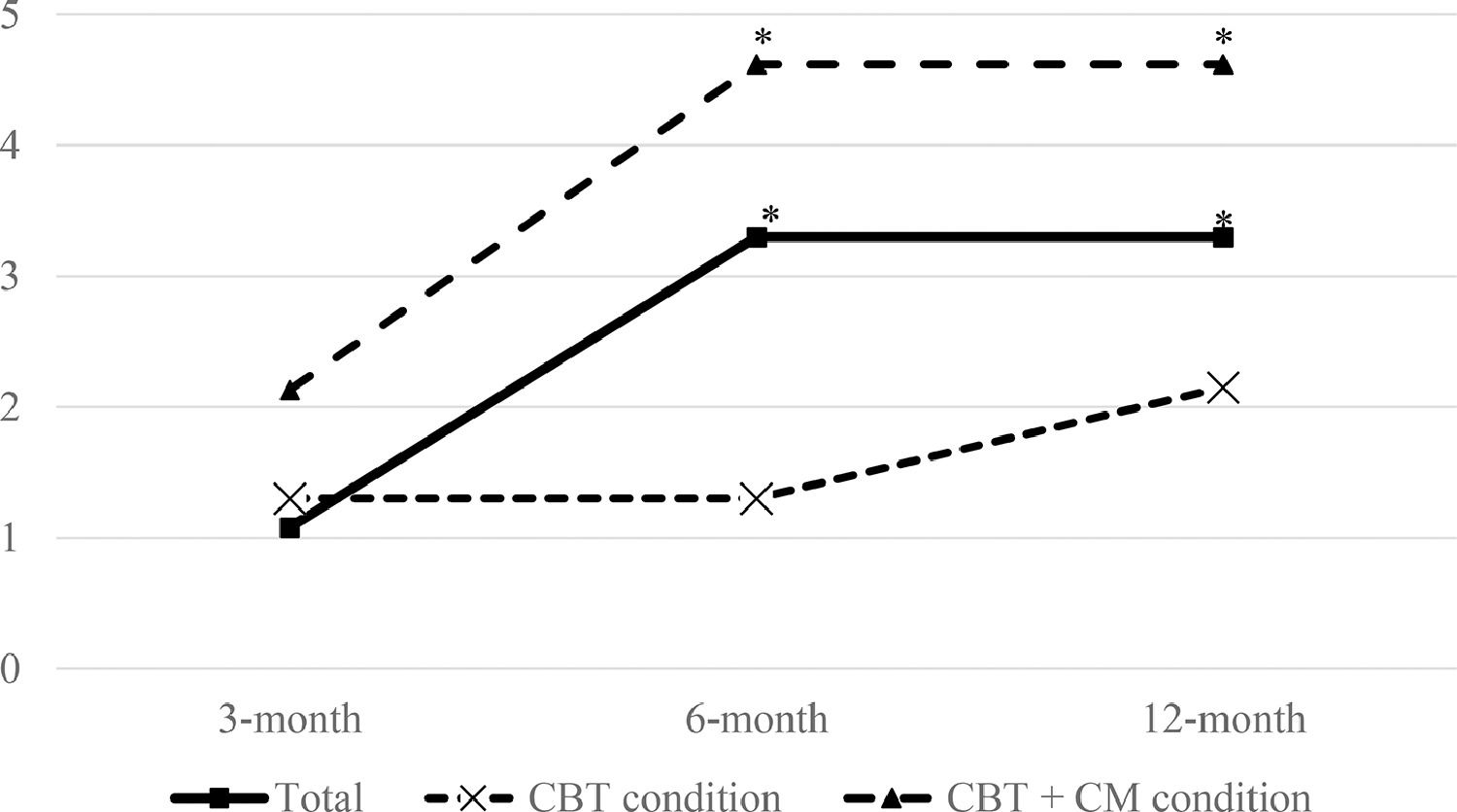
Although both smoking cessation treatments produced positive effects on smoking abstinence, the GEE revealed a non-significant effect of treatment condition over point-prevalence smoking abstinence across time (see Table 3), meaning no additive effects of CM over CBT beyond treatment termination. The main effect of time was statistically significant, meaning that the odds of abstinence progressively declined across follow-ups. Lastly, the interaction between treatment and time did not yield statistical significance (β = −0.205, p = .260). For the whole sample, the risk of being a smoker steadily increased at sixth months (RR = 3.30, 95%CI 1.33, 8.17), and remained stable at one year. An analysis by treatment condition revealed that such risk was statistically significantly higher at 6 and 12 months, only in CM condition (RR = 4.62; 95%CI 1.249, 17.146) (see Fig. 2).
Generalized Estimation Equations (GEE) predicting point-prevalence smoking abstinence.
Note. CM = contingency management; CBT = cognitive-behavioral treatment.
Risk estimates for cigarette smoking across follow-ups (3-, 6-, and 12-month) in the whole sample and by treatment arm. Note. Risk estimates (RR) are provided for each follow-up assessment in comparison to the end-of-treatment smoking status. * p ≤ 0.05. CBT = cognitive-behavioral treatment; CM = contingency management.
At the end of treatment, 79.54% (35/44) of the participants remained abstinent from their primary and secondary substance (CBT: 76.19%; CBT+CM: 82.61%; p = .668). At three-month follow-up, 88.24% (30/34) maintained substance abstinence (CBT: 84.21%; CBT+CM: 93.33%; p = .464), at six-month follow-up, 80% (24/30) (CBT: 76.92%; CBT+CM: 82.35%; p = .764), and at twelve-month follow-up 91.18% (31/34) (CBT: 94.12%; CBT+CM: 88.24%; p = .579).
A total of 63.16% (12/19) of those who quitted tobacco at end of treatment, remained abstinent from their primary and secondary substance in all follow-ups while 36.84% (7/19) of those who quitted were using substances at either follow-up. Among participants who did not successfully quit smoking, 55.17% (16/29) sustained substance abstinence across the entire study period (RR = 1.14, 95%CI 0.71, 1.84). An analysis by treatment condition suggested no significant increased risks in substance use as a result of quitting smoking [CBT: RR = 1.11, 95%CI 0.57, 2.17; CBT+CM: RR = 1.38, 95%CI 0.59, 3.23].
The GEE modeling the main effects of smoking abstinence, treatment condition, and its interaction with time (see Table 4) showed a non-significant effect of point-prevalence tobacco abstinence across time, neither medium-term (β = 0.489, p = .488) nor long-term (β = −0.456, p = .532). Similarly, the interaction between smoking abstinence and time was not significant (p ≥ .352). Neither the main effects of treatment condition (p ≥ .740) nor its interaction with time (p ≥ .548) were statistically significant.
Generalized Estimation Equations (GEE) predicting medium- and long-term substance abstinence.
Note. CBT = cognitive-behavioral treatment; CM = contingency management.
The primary aim of this study was to examine the efficacy of CBT+CM compared to CBT only for smoking cessation in individuals receiving SUD treatment. It also sought to analyze the relationship between tobacco abstinence and substance use. Two results are underlined: (1) CM improved short-term tobacco abstinence rates, but treatment effects diminished across time; and (2) no evidence of negative impact of tobacco abstinence over substance use abstinence was observed.
Adding CM into a CBT protocol produced positive effects on smoking abstinence (41.94 % vs. 19.35%), suggesting CM facilitates end-of-treatment smoking abstinence, but its effect was diminished across follow-ups. Smoking abstinence outcomes were superior compared to other CM studies, which informed on 2.22% - 4.12% of tobacco abstinence rates at 12-month follow-up (Rohsenow et al., 2015, 2017; Shoptaw et al., 2002). CM effects are attributable to incentives that facilitate early abstinence in difficult to treat populations, such as those with mental health conditions (Secades-Villa et al., 2019). Incentives (e.g., cash, vouchers that are exchangeable for free time activities) act as competing reinforcers to nicotine use, thus making smoking less desirable (i.e., opportunity cost). Other explanatory mechanisms have been related to ‘nudge’ effects occurring with the provision of vouchers that facilitate increased involvement in non-substance use activities (González-Roz et al., 2019b). The fact that CM effects subsided beyond treatment termination are well described in the prior literature (Notley et al., 2019; Secades-Villa et al., 2020), and can be explained by the parameters considered in the CM treatment protocol. Both the immediacy (between targeted behavior and provision of the voucher) and frequency of incentives provision, are associated to improved abstinence (Pfund et al., 2021). In the present study, incentives were provided twice a week and a bonus for continuous smoking abstinence (i.e., two consecutive abstinence samples) during the smoking cessation treatment was considered. After treatment termination, frequency of incentives delivery was reduced, which could arguably explain the observed effects.
Long-term tobacco abstinence rates were similar to other studies conducted with SUD smokers, which reported 6% of tobacco quitters at 6- or 12-month follow-up (Apollonio et al., 2016; Prochaska et al., 2004). Worthy of note is that regardless of treatment condition, tobacco abstinence rates are notably low, even when CBT is considered one of the most effective interventions for smoking cessation. Findings suggest a meaningful decline in tobacco abstinence rates, which could be explained by the emotion regulation difficulties in this population (Garke et al., 2021; Johnson & McLeish, 2016), increased withdrawal symptoms (Johnson et al., 2020), as well as COVID impact on smoking behavior (Chen, 2020). In this sense, including emotion regulation strategies, recall sessions after end of treatment, and implementing mobile telephone-delivered CM (e.g., DeFulio et al., 2021; Hammond et al., 2021; Zastepa, Sun, Clune, & Mathew, 2020), could be effective options for this purpose.
A second and important finding of the current study is that quitting smoking had no negative impact on substance use abstinence. This result is consistent with previous studies (McKelvey et al., 2017; Piercy et al., 2021) and may be explained by two rationales: (1) the skills learned during the smoking cessation treatment that can be extrapolated to other substances (e.g., problem solving, relapse prevention strategies, stimulus control), and (2) tobacco abstinence effects which relate to involvement in a healthy lifestyle incompatible with substance use (Sohlberg & Bergmark, 2020). This finding emphasizes the need of considering specific smoking cessation interventions as part of any comprehensive addiction treatment approach given the high prevalence of tobacco use among individuals with SUD.
This study should be interpreted under several limitations. The relatively small sample size might have impacted on the lack of significance of several results. Hence, future large-scale research should be conducted to elucidate the impact of CM on quitting rates. Second, the majority of sample was comprised of men, which precluded us from conducting analyses by sex. Third, the lockdown imposed by the Spanish government to prevent the spread of COVID-19 prevented us from carrying out face-to-face follow-ups, and therefore, collecting biochemical samples, which had a probable impact on tobacco use. Because some participants did not attend the follow-ups, we could not elucidate whether missing participants maintained abstinence from their primary and secondary substance, and were thus excluded from the proposed analyses, resulting in an overly conservative approach.
ConclusionThe study findings supported the effectiveness of CBT+CM for facilitating early abstinence in smokers with SUD. CM effects steadily diminish beyond the end of treatment and further research looking at effective procedures to sustaining abstinence is needed. The fact that smoking cessation did not impact on substance abstinence adds support to the convenience of providing simultaneous treatment for tobacco and other substances. Given the high rates of cigarette smoking in SUD populations and the negative impact it causes both physically and mentally, health professionals should provide smoking cessation treatments as a standard practice, as well as encouraging smoking cessation by increasing their motivation to change.
CRediT authorship contribution statementRoberto Secades-Villa: conceptualization, resources, writing – review and editing, supervision, project administration, funding acquisition. Gema Aonso-Diego: conceptualization, methodology, software, formal analysis, data curation, writing – original draft. Alba González-Roz: conceptualization, data curation, writing – original draft, supervision.