A neurocognitive model of distancing has systematically identified a set of brain regions that support the control mechanisms for emotion regulation (ER). However, the temporal dynamics of these control mechanisms during ER remains unclear.
MethodTo address this issue, we recorded behavioral and electroencephalogram (EEG) data to compare proactive and reactive ER modes in an adapted ER task (N = 30 adults). In different ER modes, participants were instructed to downregulate their negative emotional experiences by applying the reappraisal tactic of distancing.
ResultsThe behavioral results showed that proactive ER, which involves preparing for the upcoming regulation, reduced the negative emotional experience more than reactive ER, which involves no preparation process, in the reappraisal-negative condition. This indicated that proactive ER was more effective than reactive ER in regulating negative emotions. Event-related potential (ERP) and multivariate pattern analysis (MVPA) results showed that ER through distancing involved two phases: First, the reappraisal cue enhanced the allocation of attention to activate the mental building blocks and constructed a new perspective in the preparation process. Second, participants who benefited from the preparation process initiated the ER earlier and adaptively re-engaged in the ER if time permitted.
ConclusionsTaken together, the control mechanisms underlying the preparation process influence the timing of ER, while the control mechanisms underlying the regulation process determine the regulatory effect.
Reappraisal is an ER strategy in which changes in the meaning of an emotional stimulus lead to changes in emotional responses (Gross, 2015). Distancing is one of the two main reappraisal tactics that simulate an alternative perspective to alter the psychological distance between the stimulus and the self (Ochsner et al., 2012). A neurocognitive model of distancing has been proposed, which systematically describes a set of brain regions that support the control mechanisms underlying distancing (Powers & LaBar, 2019). However, the temporal dynamics of these control mechanisms during ER remains unclear. We combined behavioral and neural measures to address this question.
Previous studies have applied the ERP method to investigate the cognitive processes involved in distancing. These studies have typically compared distancing with other regulation strategies (such as distraction and reinterpretation) to explore the temporal dynamics that produce different regulatory effects during the regulation process. Participants who used distancing regulated their emotions later than those who used distraction (Schönfelder et al., 2014; Thiruchselvam et al., 2011). This may be because distraction prevents affective information from being processed and directs limited attentional resources to the regulation task. However, the results regarding the regulatory effects of the two strategies were inconsistent. Distancing was less effective than reinterpretation, but the reasons for this outcome are unclear (Qi et al., 2017; Sun et al., 2022; Willroth & Hilimire, 2016). Only a few studies examined the preparation process before ER and reported that participants enhanced their orienting and preparation before engaging in distancing compared to the watch condition (Moser et al., 2009; Qi et al., 2020, 2017; Thiruchselvam et al., 2011). The specific control mechanisms underlying the preparation process are unclear. Moreover, these studies have not investigated how the preparation process interacts with the regulation process. The influence of control mechanisms underlying the preparation process on the regulation process is still unknown.
To address these limitations, we manipulated the cue preceding the emotional stimulus to accomplish two modes of ER, namely reactive ER and proactive ER (Martins-Klein et al., 2020). In reactive ER, participants received asterisks as cues accompanied by no instruction, requiring them to prepare and use the regulation strategy concurrently. In proactive ER, participants received instructional cues that enabled them to prepare for strategy use in advance. We used a within-subject design to contrast the two modes of ER directly. This design was adopted for two reasons. First, we could examine how the preparation process influenced the regulatory effect from the behavioral perspective. Second, we could investigate how the preparation process affected the timing and the control mechanisms of the regulation process; moreover, we could examine whether the preparation process altered the neural activity patterns of the regulation process.
ERP methods provide important evidence on the regulation process in which late positive potential (LPP) has been consistently reported (Hajcak & Foti, 2020). Distancing requires sustained cognitive effort, and the frontal LPP is likely related to the changes in attentional control during the regulation process (Bernat et al., 2011; Moser et al., 2014). Regarding the preparation process, P3a can be used to depict the orienting and allocation of attention to the cue (Campanella et al., 2002; Hämmerer et al., 2010). It reflects the recruitment of attentional resources following an evaluation of incoming stimuli (Polich, 2007). MVPA is a more sensitive technique than conventional ERP that investigates the temporal course of the specificity evoked by strategy preparation and use and supports examining the temporal evolution of brain activation patterns (Grootswagers et al., 2017). Therefore, ERP and MVPA methods may provide valuable information for clarifying the control mechanisms of ER through distancing.
The present study aimed to investigate how the preparation and regulation processes interact to reveal the temporal dynamics of control mechanisms during ER through distancing. We compared the regulatory effects and EEG responses of reactive and proactive ER in an adapted ER task. One hypothesis is that the preparation process may enhance the regulatory effect of ER. Accordingly, proactive ER should downregulate more negative emotional experiences than reactive ER. On the other hand, we expected that the preparation process may lead to an earlier onset of ER. Accordingly, the significant above-chance difference should occur earlier in proactive ER (Wang et al., 2022). A third possibility is that the preparation process may alter the neural activity pattern of the regulation process. Accordingly, the temporal characterization of the decoding results should differ in two modes of ER.
Materials and methodsParticipantsThe sample size required for the experiment was calculated using the G*power software (Faul et al., 2007). The power analyses (power ≥ 0.95) on within-factors designs, assuming a small-to-medium effect size of 0.25, indicated a sample size of 28. In total, we recruited 30 healthy volunteers (19 females and 11 males; mean age: 19.67 ± 1.37 years, age range: 18–23 years) through advertisements. All participants were right-handed, had normal or corrected-to-normal vision, and reported no psychiatric or neurological disorders. They all signed informed consent before the experiment. All study procedures were approved by the local Human Ethics Committee for Human Research (H22094).
StimuliOne hundred forty-four negative images (valence = 2.50, arousal = 5.08) and 72 neutral images (valence = 5.23, arousal = 4.40) were chosen from the Chinese Affective Picture System (Bai et al., 2005). We randomly assigned all images to two stimulus sets for reactive ER and proactive ER. Both sets were similar in valence (both Fs < 0.02, p > 0.91) and arousal (both Fs < 2.10, p > 0.16); the difference in valence/arousal between negative and neutral images was significant (valence: both Fs > 1433.99, p < 0.001; arousal: both Fs > 32.95, p < 0.001). An additional set of 4 negative images and 2 neutral images was used during a practice procedure. Each image was presented only once during the ER task.
ProcedureAfter receiving a description of the task, participants had a practice session. In this session, participants learned and practiced how to implement different types of instructions. After the practice session, participants were required to report on their implementation of each type of trial to verify their comprehension of the instructions. Subsequently, they worked on the ER task with an EEG recording. We used a block design with a balanced order. The task comprised two modes of ER blocks: reactive ER block and proactive ER block. Each mode of ER comprised three conditions: watch-neutral, watch-negative, and reappraisal-negative. At the end of the first ER block, participants had to take a break. After completing the task, participants were asked to report whether they followed the regulation strategy we provided and how effective it was in regulating emotions. Finally, each participant received ¥75 (∼$10) as compensation.
Participants performed the experiment in a soundproof room. We based our study design on a previously validated ER task (Wang et al., 2022). The experimental task was performed using a program designed with E-Prime 2.0 (Psychology Software Tools). The experimental stimuli were 374 × 280 pixels in size and presented on a 19-inch color monitor with a resolution of 1024×768.
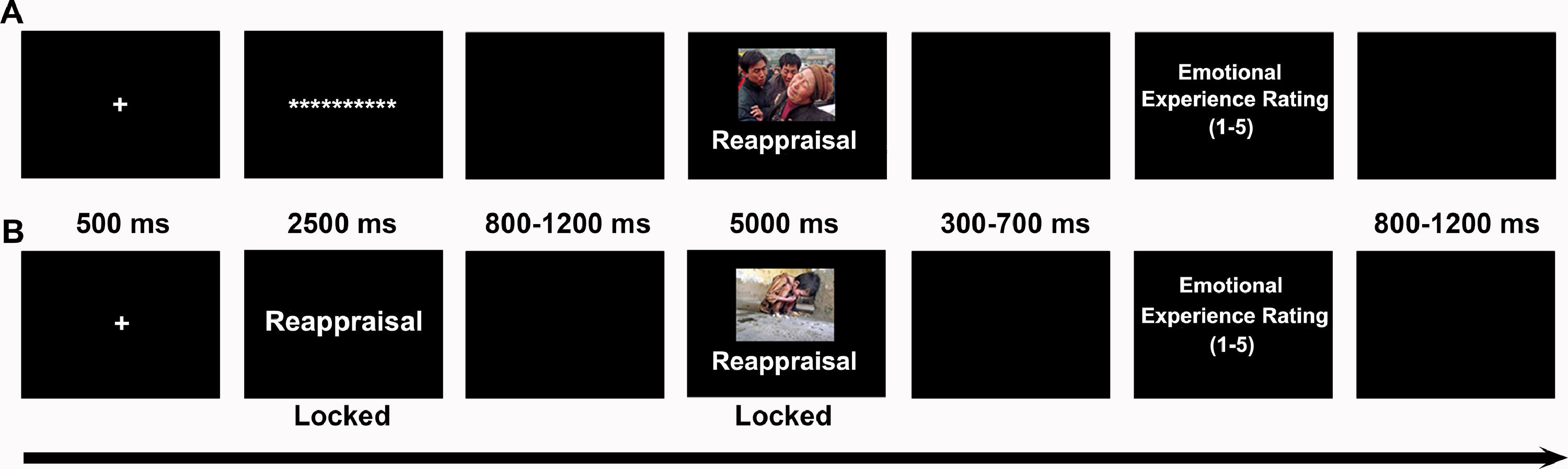
Each trial (Fig. 1) began with a fixation cross (500 ms) followed by a 2500 ms cue screen. In reactive ER, the cue was the asterisk; in proactive ER, the instructional cue was “watch” or “reappraisal”. After a jittered 800–1200 ms black screen, a picture with an instructional cue was presented for 5000 ms, during which participants implemented the required test. In watching tests, participants were instructed to allow natural thoughts and feelings to arise while looking at pictures. In reappraisal tests, participants were instructed to“increase your sense of objective distance, viewing the pictured events from a detached, third-person perspective.” After a jittered 300–700 ms black screen, participants were asked to rate the degree of their negative emotional experiences on a 5-point Likert scale, with 1 and 5 corresponding to “not at all ”and “extremely negative” respectively. Finally, a jittered 800–1200 black screen indicated that one trial was completed. Each mode of ER contained 108 trials that were divided equally into three blocks. For each block, the pictures were randomly assigned to three conditions, and the order of the conditions was randomized.
Electrophysiological recording and preprocessingEEG data were collected using standard 64 in-cap Ag/AgCl electrodes following the extended international 10–20 system (Brain Products), and two additional electrodes were placed over both mastoids. Vertical and horizontal electrooculograms (EOGs) were recorded from below the right eye and the outer canthus of the left eye, respectively. During data acquisition, all electrodes were referenced to the electrode FCz. EEG data were continuously collected at a sampling rate of 500 Hz and online filtered at 0.1–100 Hz bandpass filter. The impedance of all electrodes remained below 5 kΩ throughout the recording process.
EEG data preprocessing was performed using EEGLAB version 13.0.0 and MATLAB R2013b. Offline data were referenced to the mean of the left and right mastoids. Data were filtered with 0.1–30 Hz bandpass filter using a basic finite impulse response filter. Continuous data were segmented from −200 to 2500 ms for cue markers and from −200 to 5000 ms for stimulus markers. After baseline correction using the prestimulus interval (−200 to 0 ms), Eye movement artifacts were removed using an independent component analysis approach (Delorme & Makeig, 2004; Mennes et al., 2010). Automated rejection of epochs was performed whenever the voltage exceeded 100 μV. The results indicated at least 22 trials per condition for the P3a and 25 trials per condition for the LPP. As P300 amplitude stabilizes with approximately 20 trials, and LPP amplitude stabilizes with approximately 8 trials (Cohen & Polich, 1997; Moran et al., 2013). Our standard was suitable and allowed for the inclusion of more participants in the statistical analysis.
Data analysisBehavioral analysisThe emotional experiences were computed for each condition and analyzed using repeated-measure ANOVA with Greenhouse-Geisser correction if needed. Mode (reactive, proactive) and condition (watch-neutral, watch-negative, reappraisal-negative) were all within-subject factors. Adjustments for multiple comparisons were realized using Bonferroni correction.
ERP analysisConsidering previous studies and grand mean mapping (Barry et al., 2020; Masson & Bidet-Caulet, 2019; Squires et al., 1975), cue-locked P3a was calculated using a time window of 100 ms (200 to 300 ms) at fronto-central electrodes (Fz, FCz, Cz). According to a previous study (Wang et al., 2022), stimulus-locked LPP was calculated using a time window of 500 ms (1800–2300 ms) at Fz.
MVPAIn light of the higher sensitivity of multivariate analyses in decoding higher-order cognitive processes, we re-preprocessed the cue-locked data and stimulus-locked data to apply MVPA. Offline data were re-referenced to an average of both mastoids and filtered at 0.1–30 Hz. The continuous data were then segmented from −200 to 2500 ms and from −200 to 5000 ms following the baseline correction mentioned above. We did not reject any trials to maintain the data balance. For the watch-cue condition vs. reappraisal-cue condition as well as watch-negative condition vs. reappraisal-negative condition in reactive ER and proactive ER, there were 36 trials per condition included for decoding. For the no-cue condition vs. instructional cue condition, there were 72 trials per condition included for decoding.
The primary analysis involved a linear support vector machine with 60 channels (excluding HEOG, VEOG, and two reference electrodes) as features. For the preparation process decoding, we used the no-cue condition vs. instructional cue condition labels and the watch-cue condition vs. reappraisal-cue condition labels as classes. For the regulation process decoding, we used the watch-negative condition vs. reappraisal-negative condition labels as classes. The analysis tested whether the classifier could learn from distinct EEG patterns following every single trial to distinguish these pairs of conditions and characterize the process of the two modes of ER.
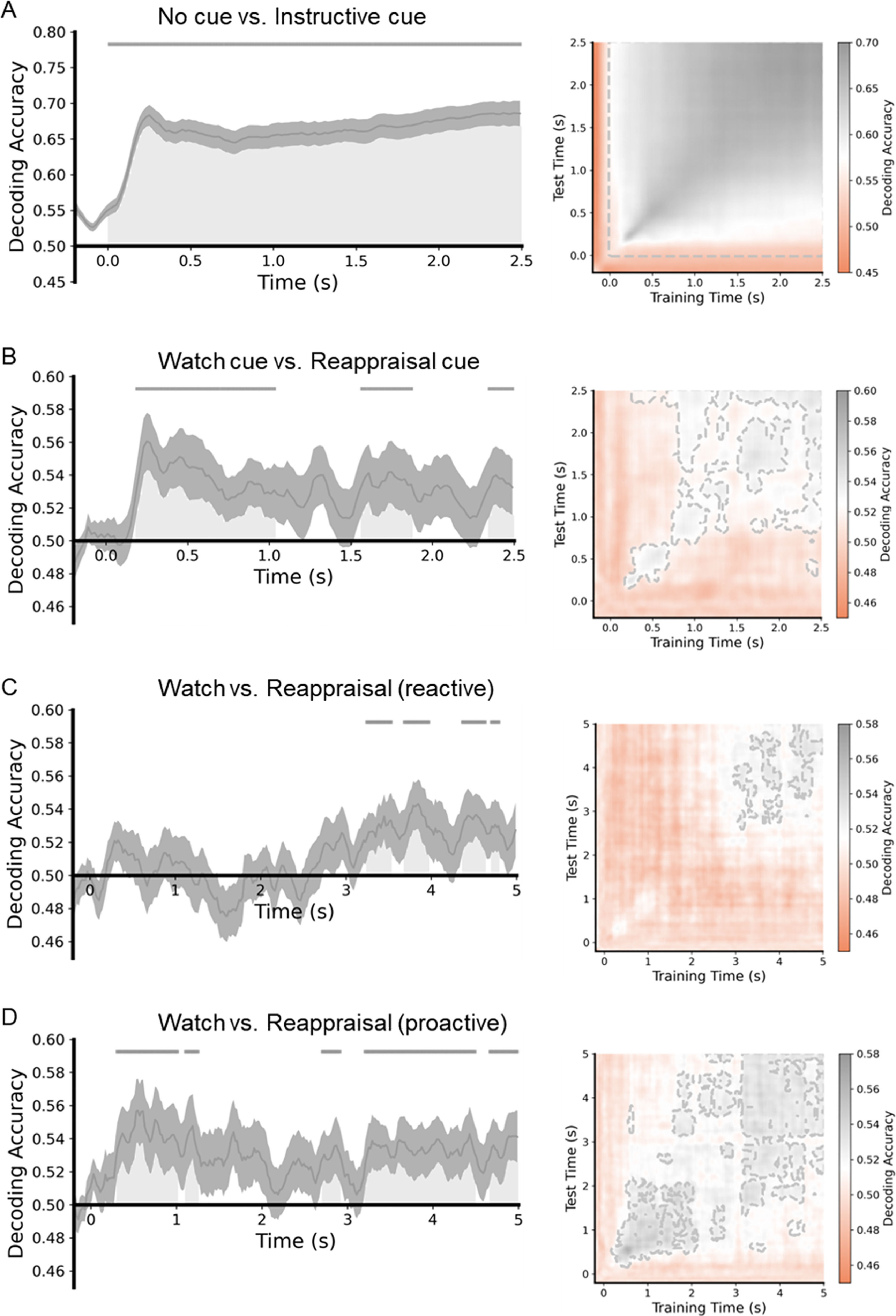
The neural representational analysis toolbox (NeuroRA; Lu & Ku, 2020) was used for the re-preprocessed EEG data. To improve the efficiency of analyses, we resampled the data at 50 Hz. According to previous studies (Bae & Luck, 2018; Foster et al., 2016), averaging trials belonging to the same exemplar before decoding can increase the general decoding performance and make signatures more pronounced. Consequently, we randomly divided trials into 9 equal-sized groups (9 groups of 4 trials for each of the conditions) or 18 equal-sized groups (18 groups of 4 trials for each of the conditions) and subsequently averaged together trials in each group for a given condition. A classifier was trained and tested at each time point by using a 3-fold cross-validation procedure; that is, data from 2/3 of the trials (selected at random) were used to train the classifier, and then the classifier performance was evaluated using data from the remaining 1/3 of the trials. This procedure was repeated 3 times until all data were tested. To minimize any bias associated with the allocation of trials to groups, we iterated the entire procedure 10 times. After completing the procedures, we smoothed the averaged decoding accuracy values across the time points in 20 ms steps to minimize noise. We performed one-tailed t-tests across subjects against a 50 % chance level to test whether the group-level decoding accuracy at each point was above the chance level. Cluster-based permutation tests (p < 0.05, 1000 iterations) were then used to perform multiple-comparison correction for these t-tests over time. Furthermore, a temporal generalization analysis using classification across time was performed to evaluate the stability of neural activity patterns by testing the classifier at the same time point on which it was trained (King & Dehaene, 2014). As a result, the classification accuracy outside the diagonal line was at the above-chance level, suggesting stable neural activity.
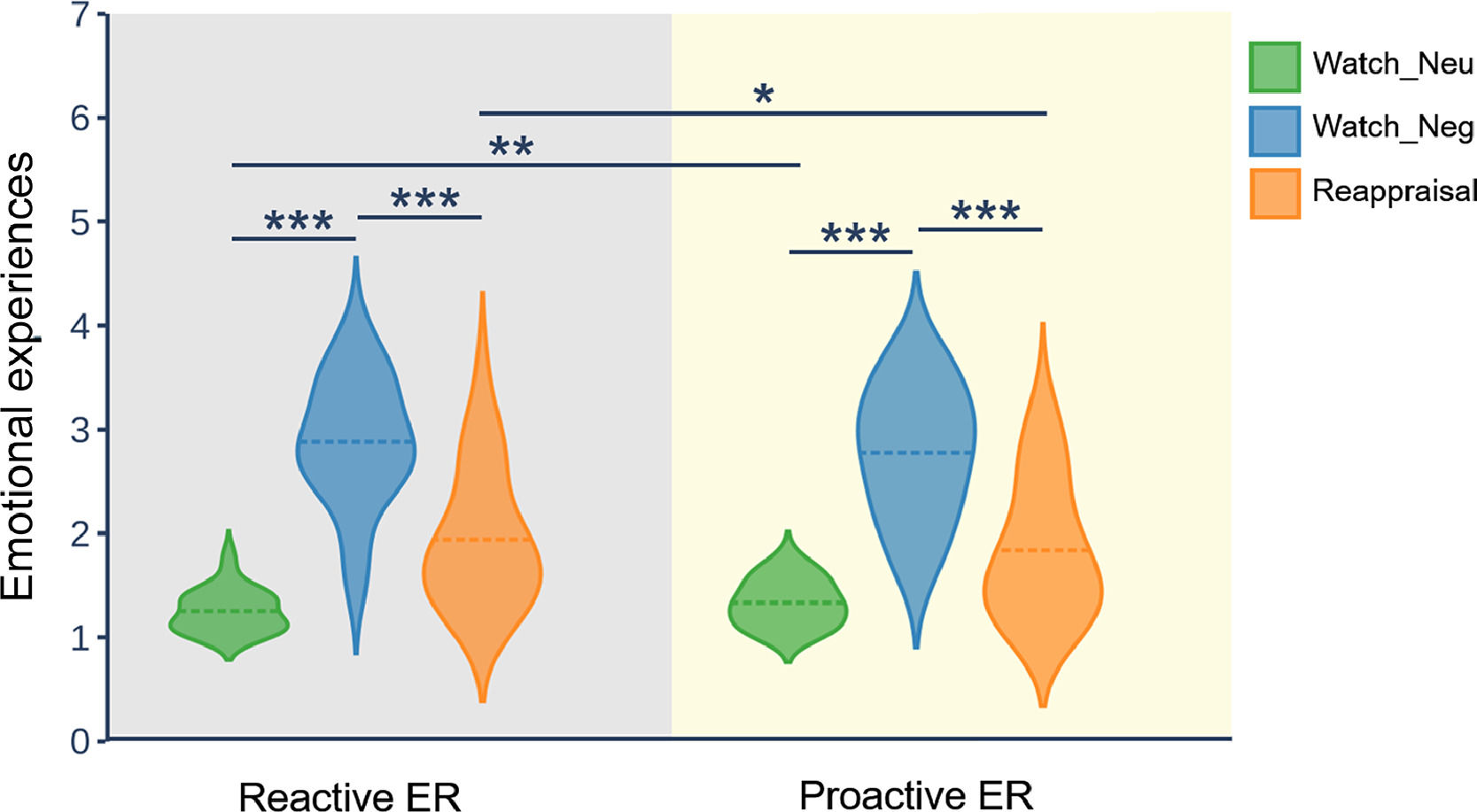
ResultsBehavioral resultsFig. 2 displays the distribution of emotional experience ratings in reactive ER and proactive ER. To test the efficacy of reappraisal, as reflected in emotional experience ratings, we employed a repeated measures ANOVA with the mode and condition as two within-subject factors. The ANOVA showed that the main effect of condition (F(2, 58) = 95.41, p < 0.001, ηp2 = 0.77) and interaction of mode × condition (F(2, 58) = 4.23, p = 0.034, ηp2 = 0.13) was significant. However, the main effect of mode (F(1, 29) = 2.18, p = 0.15) did not reach significance. Follow-up analysis of the two-way interaction indicated that, in both modes, the emotional experience for the watch-negative condition was more negative than that for the watch-neutral condition (p < 0.001) and reappraisal condition (p < 0.001). Moreover, for the watch-negative condition, the emotional experience did not differ between the reactive mode and the proactive mode. In contrast, for the watch-neutral condition, the emotional experience in the proactive mode was more negative than that in the reactive mode (p = 0.004). For the reappraisal-negative condition, the emotional experience in the reactive mode was more negative than that in the proactive mode (p = 0.01). Taken together, these results suggest that both modes of ER can successfully downregulate negative emotions. Importantly, compared with reactive mode, proactive mode downregulated more negative emotions.
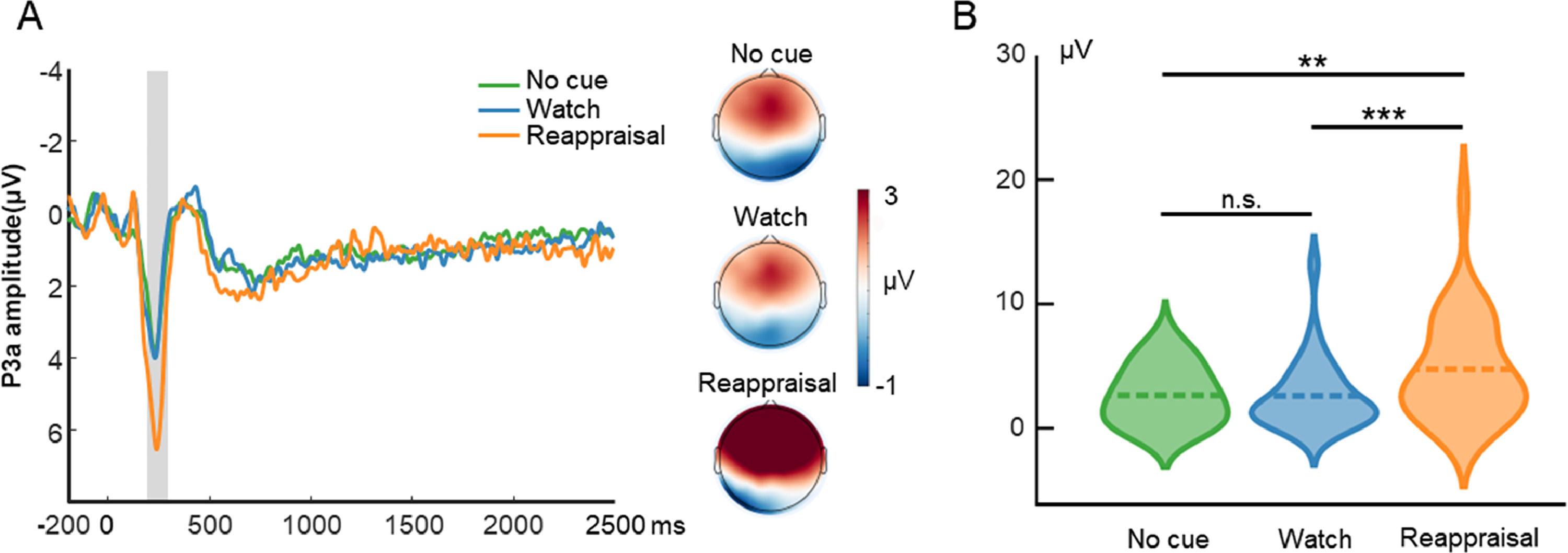
ERP resultsP3aFig. 3 displays the waveform, topography, and distribution of amplitude for the cue-locked P3a. One-way repeated measures ANOVA with the cue type (no cue, watch cue, and reappraisal cue) as the within-subject factor was performed. The ANOVA showed that the main effect of cue type (F(2, 58) = 15.15, p < 0.001, ηp2 = 0.34) was significant. Post hoc tests using Bonferroni correction showed that the P3a amplitude evoked by reappraisal cue trials (4.77, SE = 0.74) was greater than that evoked by no-cue trials (2.66, SE = 0.43; p = 0.001) and watch-cue trials (2.54, SE = 0.51; p < 0.001). On the other hand, the P3a amplitude did not differ between no-cue trials and watch-cue trials (p = 0.99).
Cue-locked P3a results in the ER task. A is the waveform and topography for P3a. The shaded region represents the defined window. B is the distribution of amplitude for P3a in each condition. Dashed lines indicate the mean of each condition. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
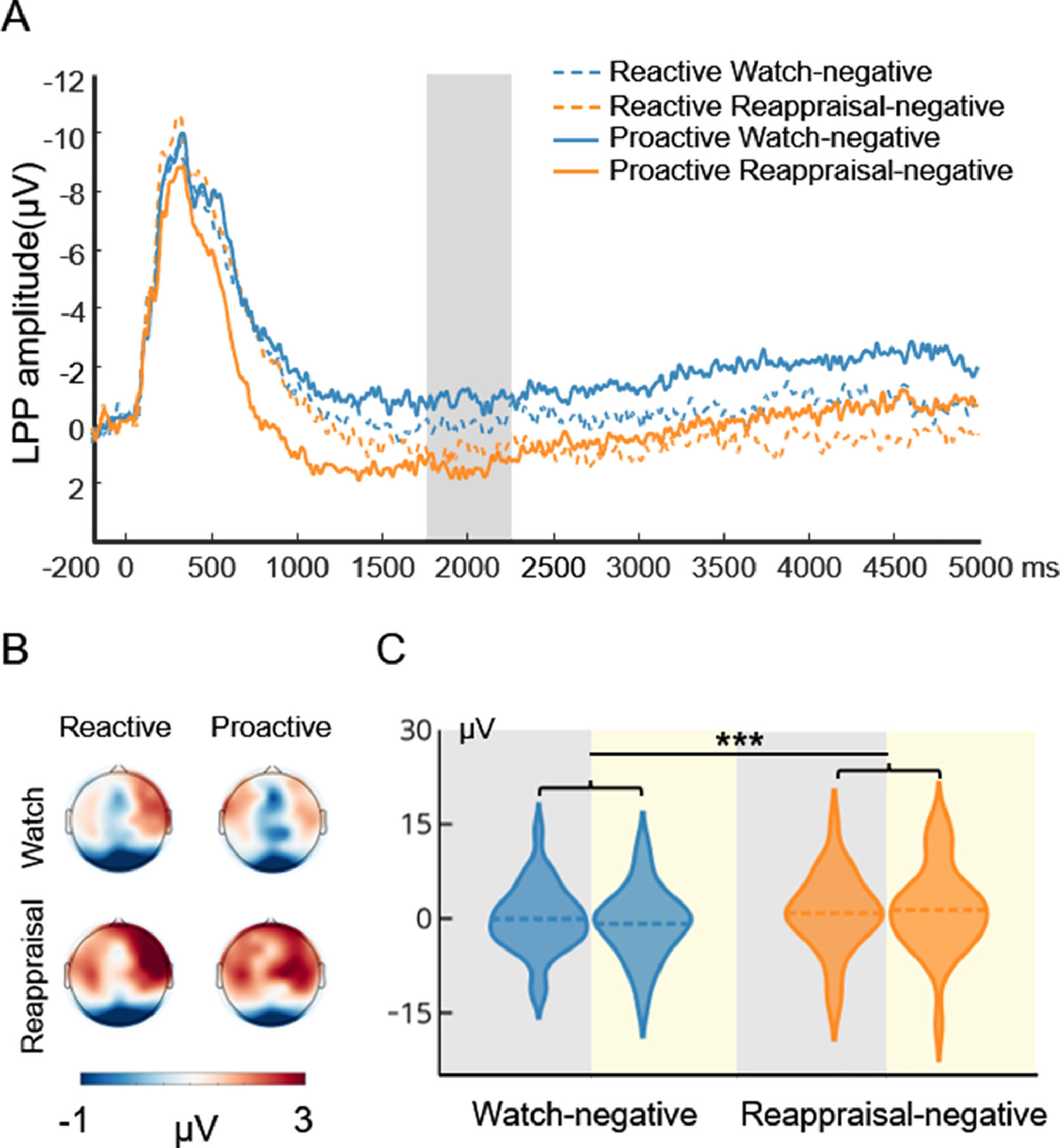
Fig. 4 displays the waveform, topography, and distribution of amplitude for the stimulus-locked LPP in the watch-negative condition and reappraisal-negative condition. To compare the cognitive effort associated with reactive and proactive ER, a repeated measures ANOVA with the mode (reactive, proactive) and condition (watch-negative, reappraisal-negative) as the two within-subject factors were performed. The ANOVA showed that the main effect of condition (F(1, 29) = 13.04, p < 0.001, ηp2 = 0.31) was significant. This indicated that compared with the watch condition, the reappraisal condition required more effort. However, the main effect of mode (F(1, 29) = 0.043, p = 0.84) and interaction of mode × condition (F(1, 29) = 2.17, p = 0.15) did not reach significance. To examine whether there was a difference between the cognitive effort that supports the two modes of ER, we added a paired sample t-test to compare the reactive reappraisal- and proactive reappraisal-negative conditions. This indicated that both modes of ER required the same effort to decrease the negative emotions, t(29) = 0.60, p = 0.55.
Picture-locked LPP results of the ER task. A is the waveform for LPP. The shaded region represents the defining window. B is the topography for LPP. C is the distribution of amplitude for LPP in the watch-negative and reappraisal-negative conditions. The gray background indicates the distribution of LPP in reactive ER, and the yellow background indicates the distribution of LPP in proactive ER. Dashed lines indicate the mean of each condition. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To determine the temporally extended pattern of neural activity during the process of ER, support vector machines were trained as classifiers at each time point to distinguish between the no-cue and instructional cue conditions (including watch-cue and reappraisal-cue conditions; Fig. 5A), watch-cue and reappraisal-cue conditions (Fig. 5B), and watch-negative and reappraisal-negative conditions in reactive ER and proactive ER, respectively (Fig. 5C and 5D).
MVPA results of the ER task. The left is the classification accuracy for the four datasets. Each gray area shows a cluster of time points for which the decoding was significantly greater than chance-level after correction for multiple comparisons. The gray lines indicate clusters of time points in which the decoding was significant. The right is the time generalization matrix of classifier performance. After correction for multiple comparisons, areas with p < 0.05 are surrounded by gray contours.
MVPA revealed a significant above-chance difference between classes of the no-cue condition and instructional cue condition from 0 to 2500 ms after the cue was displayed (p < 0.05, cluster-corrected). Regarding the watch-cue and reappraisal-cue conditions, MVPA showed a significant above-chance difference between the two classes from 180 ms to 1040 ms, 1560 ms to 1880 ms, and 2340 ms to 2500 ms after the cue was displayed (p < 0.05, cluster-corrected). As for the watch-negative and reappraisal-negative conditions in reactive ER, MVPA revealed a significant above-chance difference between classes of the two conditions from 3239 to 3540 ms, from 3679 to 3979 ms, from 4360 to 4640 ms, and 4700 to 4800 ms after the image was displayed (p < 0.05, cluster-corrected). In proactive ER, MVPA revealed a significant above-chance difference between classes of the watch-negative and reappraisal-negative conditions from 300 to 1020 ms, from 1100 to 1260 ms, from 2699 to 2920 ms, from 3199 to 4500 ms, and from 4660 to 5000 ms after the image was displayed (p < 0.05, cluster-corrected). In reactive ER, the earliest significant above-chance difference between the two conditions occurred at around 3239 ms. On the other hand, in proactive ER, the earliest significant above-chance difference between the two conditions occurred at around 300 ms. These results suggest that proactive ER started earlier than reactive ER.
Because successful classification was observed in all four data-sets, in the next step, the temporal generalization matrices were calculated to test the stability of neural activity patterns with underlying significant classification performance. The time-by-time generalization results are presented in Fig. 5. The time generalization matrices showed that significant above-chance activity was observed during the significant time windows acquired from the MVPA, suggesting that the differences between pairs of conditions were stable over time.
DiscussionThis study investigated the temporal dynamics of control mechanisms during ER through distancing by exploring behavioral and neural effects in reactive and proactive ER. Behavioral results showed that both reactive and proactive ER reduced negative emotional experiences successfully, but proactive ER was more effective. For the ERP results, cue-locked P3a did not differ between no-cue and watch-cue conditions but was larger in the reappraisal-cue condition than in the other two. The reappraisal cue enhanced the recruitment of attention resources for the ensuing ER. For picture-locked LPP, watch-negative and reappraisal-negative conditions followed the same pattern in both reactive and proactive ER. The reappraisal condition required more effort than the watch condition, but the two reappraisal conditions did not differ in LPP. Regarding MVPA, we found that the cue's instructive information could be decoded from the scalp distribution of sustained ERP responses. Decoding between watch-cue and reappraisal-cue was significantly above chance-level in two stages. We also decoded watch-negative and reappraisal-negative conditions for reactive and proactive ER. Proactive ER showed an earlier distinction between neural representations than reactive ER did. Reactive ER had significant above-chance decoding only at late time points, while proactive ER had it at both early and late time points.
We manipulated the cue to implement two modes of ER. The cue-locked P3a is believed to index attentional orienting, with large amplitude indicating more attention recruitment (Polich, 2007). The present results showed that proactive ER required cue re-evaluation and enhanced attentional allocation to prepare for the upcoming ER task, while reactive ER did not involve anticipatory preparation before applying the ER strategy. This manipulation demonstrated the distinct characteristics of the two ER modes. Fig. 5B displays a significant above-chance difference between watch-cue and reappraisal-cue conditions. In accordance with the temporal generalization matrix, the above-chance decoding of a single time window was characterized by a combination of two different temporal structures. Different temporal structures indicate the different organization of brain processes (King & Dehaene, 2014). The first temporal structure (about 200–280 ms), named chain, displayed a diagonal-like decoding performance, and the classifier trained on these time points can predict class labels for data at nearby time points along the diagonal. The decoding accuracy reached its peak and faded in this time window. The second temporal structure (about 280–2500 ms), named sustained, showed a square-like decoding performance, and the classifier trained on these time points can predict class labels for data at distant time points gradually away from the diagonal. The decoding accuracy kept stable in this time window. The first temporal structure may correspond to P3a. In this structure, the reappraisal cue prompted the participants to set a goal of reducing their negative emotions. To achieve this goal, they adaptively allocated more attentional resources to the preparation process (Hommel, 2022). Using distancing, participants are required to construct and adopt a detached, third-person perspective through a process of self-projection (Buckner & Carroll, 2007). This change in perspective prompted the individuals to distance themselves from negative emotions, thereby lowering their negative emotional experiences. In this process, the new perspective was mainly constructed from mental building blocks that stored various associations and semantic knowledge in memory (Powers & LaBar, 2019). We speculate that the preparation that begins in the second temporal structure refers to activating the mental building blocks to construct a new perspective. The new perspective is integrated with self-referential processing and stored in working memory (Powers & LaBar, 2019). This integration facilitates the participants to recruit and adopt the new perspective as a self-reference, leading them to initiate ER rapidly. Thus, earlier ER in the proactive mode is attributed to the preparation.
In addition to the P3a, the process of down-regulating the negative emotions is associated with the LPP, and its functional significance depends on the source. The frontal LPP has been linked to cognitive effort and it has been shown that compared with the watch condition, the reappraisal condition requires more effort (Bernat et al., 2011; Moser et al., 2014; Shafir et al., 2015). The results of this study are consistent with a previous study (Wang et al., 2022). However, the two reappraisal conditions did not differ in the LPP amplitude, which indicated that although both reactive and proactive ER exerted the same cognitive effort, proactive ER had a better regulatory effect than reactive ER. Fig. 5, C and D displayed significant above-chance differences between watch and reappraisal conditions in the reactive and proactive ER. The decoding results were in keeping with Wang et al. (2022). Proactive ER showed an earlier above-chance difference than reactive ER, implying that proactive ER started earlier. In accordance with the temporal generalization matrix, the above-chance decoding of the time window in reactive ER was characterized by one temporal structure named sustained, implying that the reactive ER accomplished the regulation in one temporal structure. The above-chance decoding of the time window in proactive ER was characterized by two temporal structures named reactivated. Both temporal structures showed a square-like decoding performance like the sustained activity. The second temporal structure indicated the reactivation of the brain processes (Meyers et al., 2008). The proactive ER benefited from the reappraisal cue and should need less effort when downregulating the negative-emotional experience to the same degree (Wang et al., 2022). We speculate that the proactive ER started earlier and may be accomplished in the first temporal structure. Since the regulation time has not expired, the subjects would follow the regulatory goal to recruit cognitive effort and re-engage in ER after a period of adjustment and preparation. This may be the reason why there was no significant difference in the cognitive effort, but proactive ER obtained the better regulatory effect.
The link between ER and fundamental cognitive control mechanisms is widely recognized (Martins-Klein et al., 2020; McRae & Gross, 2020; Tamir, 2021). However, little attention has been given to the control mechanisms that specifically support distancing. Following Powers and LaBar (2019), we also identified that distancing includes cognitive control processes to facilitate ER. The difference between our investigation and that of Powers and LaBar (2019) is that we depicted the control mechanisms of preparation and regulation processes involved in distancing from a temporal dynamic perspective. The MVPA results even sensitively revealed that the control mechanisms in the preparation process affect the timing of ER while the control mechanisms in the regulation process affect the regulatory effect. Theoretically, these findings could elucidate the mechanisms of successful distancing and their variability across different populations, such as older adults or patients. Future research could apply this experimental design to characterize the temporal dynamics of control mechanisms during ER through other regulation strategies. Impaired ER is a risk factor for various psychopathologies, such as (social) anxiety, eating disorders, and depression (Lincoln et al., 2022). Clinically, the neural activity patterns during the preparation and regulation processes could serve as electrophysiological biomarkers for identifying and assessing this risk factor. By addressing the impaired control mechanisms that affect ER ability, targeted interventions could be developed to enhance it, which may increase the effectiveness of prevention and treatment.
Despite the novel features of the study, some limitations and future directions warrant consideration. One point of concern is the sample size. We used a within-subject design and a relatively modest sample size. Although the analyses were adequately powered, a larger sample is needed to replicate and enhance the reliability of the results. Furthermore, the current study mainly focuses on the downregulation of negative emotions, leaving open the question of whether different regulation goals (e.g., upregulation of positive emotions) affect the control mechanisms of ER through distancing. Future studies should address this issue to enhance the generalizability of the findings.
In conclusion, the present study provides the first piece of neurophysiological evidence on the control mechanisms of ER through distancing by combining ERP methods with MVPA. The results indicated that control mechanisms of ER through distancing involved two stages: First, in the preparation process, the goal of downregulation prompted the participants to recruit more attention resources to activate mental building blocks and construct an alternative perspective. Second, in the regulation process, participants who benefited from the preparation process started the ER earlier and adaptively re-engaged in the ER if needed. Therefore, the control mechanisms in the preparation process affect the timing of ER, while the control mechanisms in the regulation process affect the regulatory effect.
This work was supported by National Natural Science Foundation of China (Grant Nos. 32171040 & 32371105, to Dr. Antao Chen).