El presente estudio pretende determinar si existe o no una mayor reactividad emocional en el Trastorno Límite de la Personalidad (TLP) en un contexto de laboratorio. Se realizó una inducción emocional negativa (presentación de imágenes estandarizadas con contenido negativo) a cincuenta participantes (35 pacientes con TLP y 15 controles sanos). Para evaluar la respuesta emocional subjetiva se utilizaron cuestionarios auto-informados; los niveles de cortisol (sCORT) y alfa-amilasa (sAA) salivares se utilizaron para medir la reactividad biológica al procedimiento. En el grupo de TLP, se observaron niveles de sCORT más bajos y niveles de sAA más elevados en comparación al grupo control. No se observaron diferencias significativas en relación a la reactividad emocional auto-informada, pero los pacientes con TLP reportaron mayor intensidad de emociones negativas a nivel basal así como también durante todo el procedimiento. Los resultados no apoyan la hipótesis de hiperreactividad emocional en el TLP. Sin embargo, los pacientes con TLP presentaron mayor intensidad de emociones negativas a nivel basal, característica que debería ser considerada como esencial en el trastorno. Futuros estudios deberán incorporar otros paradigmas de inducción emocional más específicos para TLP con el fin de confirmar las tendencias observadas en el presente estudio.
The aim of this study was to determine if patients with Borderline Personality Disorder (BPD) present higher emotional response than healthy controls in a laboratory setting. Fifty participants (35 patients with BPD and 15 healthy controls) underwent a negative emotion induction procedure (presentation of standardized unpleasant images). Subjective emotional responses were assessed by means of self-reported questionnaires while biological reactivity during the procedure was measured through levels of salivary cortisol (sCORT) and alpha-amylase (sAA). Patients with BPD exhibited significant lower cortisol levels and higher sAA levels compared to controls. Self-reported emotional reactivity did not give rise to differences between groups but participants with BPD did present higher levels of negative emotional intensity at baseline and during the entire procedure. The findings do not give support to the emotional hyperreactivity hypothesis in BPD. However, BPD patients presented heightened negative mood intensity at baseline, which should be considered a hallmark of the disorder. Further studies using more BPD-specific emotion inductions are needed to confirm the trends observed in this study.
Emotional dysregulation is considered a core characteristic of Borderline Personality Disorder (BPD) and it is commonly reported by patients with BPD (Leichsenring, Leibing, Kruse, New, & Leweke, 2011; Rosenthal et al., 2008). Since many of the impulsive behaviors that are typically over-expressed in patients with BPD (i.e. self-mutilation, drug abuse, binge eating, suicide attempts) can be triggered by emotional dysregulation (Lynch, Chapman, Rosenthal, Kuo, & Linehan, 2006), it appears to play a crucial role in the severity of the disorder.
According to the biosocial model of Dialectical Behavior Therapy (Linehan, 1993), emotional dysregulation involves overall elevated negative emotional arousal, heightened emotional reactivity to emotional stimuli, and delayed recovery to emotional baseline following a negative emotional cue (Koenigsberg et al., 2009; Kuo & Linehan, 2009; Linehan, 1993). Many studies have found that BPD patients present heightened negative emotional intensity at baseline but there are conflicting results regarding emotional reactivity (especially on physiological variables; Rosenthal et al., 2008). Thus, some studies (Ebner-Priemer et al., 2005; Limberg, Barnow, Freyberger, & Hamm, 2011) have reported emotional hyperreactivity in BPD patients vs. healthy controls (HC), whereas other authors (Herpertz, Kunert, Schwenger, & Sass, 1999; Herpertz et al., 2000) have observed hyporreactivity (lower skin conductance response) in BPD patients. Similar results were also reported by Nater et al., (2010), who evaluated a sample of BPD patients with a standardized psychosocial stress protocol (Trier Social Stress Test); these authors found less reactivity of the hypothalamus-pituitary-adrenal axis (HPAA) and Sympathetic Nervous System (SNS) compared to a HC group. In addition to these conflicting results, other authors (Kuo & Linehan, 2009) have found no significant differences in reactivity between BPD and HC groups.
Given these conflicting results in the literature, we decided to perform a study to determine whether or not BPD patients exhibit greater emotional reactivity, using a standardized negative emotional induction to provoke changes in self-reported emotional variables and salivary stress markers. Additionally, higher scores of negative emotions in the BPD group are expected to be found.
Method
Participants
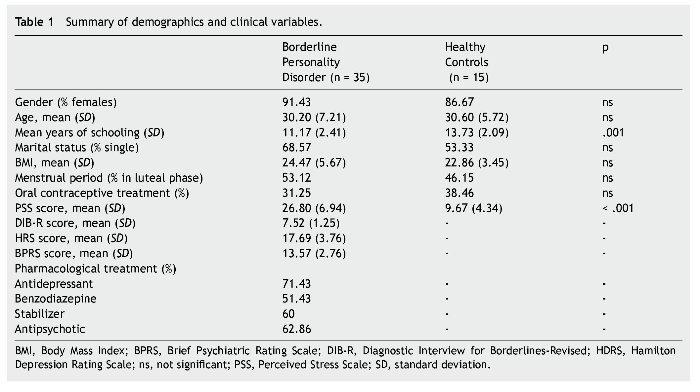
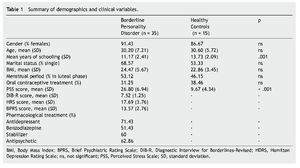
Forty-two outpatients were recruited from the BPD Unit of the Hospital de la Santa Creu i Sant Pau; of these, 7 failed to meet the exclusion criteria and therefore a total of 35 participants were included. The healthy control (HC) group included 15 volunteers matched by gender and age to the BPD group (Table 1). All HCs were recruited from employees at our hospital and agreed to voluntarily participate in the study. Since 7 participants of the BPD group and 2 from the HC group did not salivate enough, the final sample for biochemical analyses was reduced to 28 and 13 respectively.
BPD diagnosis was determined by psychiatric evaluation and two semi-structured diagnostic interviews: the SCID-II (Structured Clinical Interview for DSM-IV axis II disorders; Spanish version; Gómez-Beneyto et al., 1994) and DIB-R (Revised Diagnostic Interview for Borderline, Spanish validation; Barrachina et al., 2004). Both SCID-II and DIB-R showed good psychometric properties with an internal reliability of .89 for DIB-R (Barrachina et al., 2004) and an adequate Cronbach's alpha ranging between .71 and .94 for SCID-II (Maffei et al., 1997). Inclusion criteria for BPD patients were as follows: age between 18 and 45 years; and a score ≥4 on the Clinical Global Impression Scale for Borderline Personality Disorder (CGI-BPD; Pérez et al., 2007). All BPD patients were receiving pharmacological treatment at the time of inclusion in the study and had to have maintained their usual medications and dose levels for at least two months prior to the study. Exclusion criteria for BPD participants were as follows: a) comorbidity with schizophrenia, drug induced psychosis, organic brain syndrome, bipolar disorder, mental retardation, current major depressive episode, post-traumatic stress disorder, or current substance or alcohol abuse or dependence; b) major medical illness according to medical history and physical examination; c) current structured psychotherapy; or d) participation in any similar study or knowledge of the study's purpose. HCs were clinically interviewed to rule out the presence of axis I or II pathology and answered the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD; Zanarini et al., 2003), which specifically assess BPD symptomatology. None of the healthy controls reported previous axis I or axis II disorder (including BPD), nor any substance dependence. Like BPD participants, the HCs had no previous experience in any similar study, were unaware of the purpose and procedure of the study, and had no involvement in its development. All participants voluntarily signed the written consent form after receiving a summary of the study. The Clinical Research Ethics Committee of the Hospital de la Santa Creu i Sant Pau approved the study design, which was carried out in accordance with the Helsinki Declaration.
Instruments
Clinical scales and self-reported measures of mood:
• The Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960) is a 5-point scale (ranging from 0 for "absent" to 4 for "incapacitating symptoms") with 17 items for assessing depressive symptoms. This scale shows an adequate reliability as most of the studies indicate Cronbach's alphas >.70 (Bagby, Ryder, Schuller, & Marshall, 2004).
• The Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, 1988) is a 7-point scale (from 1: "not present" to 7: "extremely severe") with 18 items used to measure psychopathology with high reliability (Cronbach's α = .80; Nicholson, Chapman, & Neufeld, 1995).
• Self-Assessment-Manikin (SAM; Lang, 1980), a non-verbal pictorial affective rating system initially designed to assess psychological responses to visual material with emotional content (i.e. IAPS). It uses graphic figures to depict values along the dimensions of Activation (arousal), Valence (pleasure) and Dominance (perceived control). Each dimension has a 9-point rating scale ranging from 1 (the lowest rating) to 9 (the highest rating). SAM has a satisfactory internal reliability with Cronbach's alphas ranging between .63 and .98 (Backs, da Silva, & Han, 2005).
• Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1971), a 5-point scale (from 0: "not at all" to 4:"extremely") of 65 items created to assess the following six affective mood states: Anger, Depression, Tension, Fatigue, Vigor, and Friendliness. The total mood disturbance score (TMDS) is obtained from scores of the other subscales. The POMS presents an adequate reliability for all factors with Cronbach's alphas ranging between .63 and .96 (McNair et al., 1971).
• Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988), a questionnaire used to assess positive and negative emotions. The PANAS consists of 20 words (10 positive, 10 negative) that describe emotions, which are rated from 1 ("very slightly or not at all") to 5 ("very much") on a Likert scale. Respondents are asked to rate how they feel at that moment. Alpha coefficients of the scale are excellent (between .87 and .91; Sandín et al., 1999).
• Perceived Stress Scale (PSS-10; Cohen & Williamson, 1988), a 10-item scale that uses a Likert rating (from 0: "never" to 4: "very often") to assess participants' perceived stress levels during the month prior to the study. The PSS-10 shows a good internal consistency with a Cronbach's alpha= .82 (Remor, 2006).
Biochemical measures:
• Salivary cortisol (sCORT) is a marker of HPAA activation and is used to measure the free fraction (i.e. the bioavailable fraction) of blood cortisol. Alterations in cortisol levels have been associated with negative effects of stress on cognitive processes (Portella, Harmer, Flint, Cowen, & Goodwin, 2005). Salivary alpha-amylase (sAA) is a digestive enzyme used as an indirect indicator of SNS activity (Granger, Kivlighan, el-Sheikh, Gordis, & Stroud, 2007).
The Salimetrics Oral Swabs (Salimetrics®) was used to collect saliva samples. The validity of method for concurrent assessment of sCORT and sAA has been previously demonstrated (Gröschl, 2008). Following the manufacturer's recommendations, participants placed the swab under the tongue for two and a half minutes. Unstimulated absorption was used because saliva induction can alter sAA concentration. Saliva samples were frozen at -20 ºC until laboratory analysis. Levels of sCORT were analyzed with a commercial enzyme-linked immunosorbent assay (ELISA), with intra-assay coefficients of variation less than 4% and inter-assay coefficient no higher than 6.50% (Salimetrics®). Levels of sAA were determined by an ELISA that uses a substrate that changes color in response to amylase activity; intra-assay coefficients of variation were less than 8% with inter-assay coefficients no higher than 6% (Salimetrics®). Values are expressed in μg/dL for sCORT and units of enzyme activity per millilitre for sAA.
Procedure
The laboratory sessions were conducted from January 2009 to January 2010 and took place in a hospital room conditioned for this purpose, with consistent temperature and lighting for all sessions. The sessions were conducted between 3 p.m. and 6 p.m. to minimize the effects of circadian rhythm and time of day on physiological variables. To limit possible confounding variables, the following instructions were given for the day of the experiment: wake up before 8 a.m.; not brush their teeth after dinner (to avoid gingival bleeding); not take any medications or caffeine on the day of the study; not smoke, eat or drink anything except water in the hour prior to starting the study. Participants were also instructed not to perform strenuous physical exercise or consume alcohol or illegal drugs in the 24 hours preceding the study (Granger et al., 2007; Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004).
We collected and recorded the following variables that may have an effect on sCORT and sAA levels (Hellhammer, Wüst, & Kudielka, 2009; Kudielka et al., 2004): gender; menstrual phase; use of oral contraceptives, beta-blockers, glucocorticoids and hormones; prescribed and non-prescribed drug use in the last 24 hours; alcohol use in the previous 24 hours; smoking in the last 2 hours; caffeine consumption in the last hour; intense physical exercise in the last 24 hours; time since last meal; stressful events and awakening hour on the day of the study.
For emotion induction, participants were individually shown 24 pictures taken from the International Affective Picture System (IAPS) (Lang, Ohman, & Vaitl, 1988). Images were chosen for negative Valence, high Activation and low Dominance in SAM scale scores, attributes deemed appropriate to induce a significant plasmatic cortisol response, as previously described by Codispoti, Gerra et al., (2003) -i.e. images from the IAPS have been widely used in psychophysiological (Herpertz et al., 1999, 2000) and neuroimaging research with BPD patients (Koenigsberg et al., 2009) and have been shown capable of inducing changes also in sAA levels (van Stegeren, Wolf, & Kindt, 2008). The 17-inch monitor was located at a distance of 1 meter from the participants, who were seated in a comfortable chair. All participants rinsed out their mouths prior to starting the procedure to reduce possible contaminants in the saliva samples.
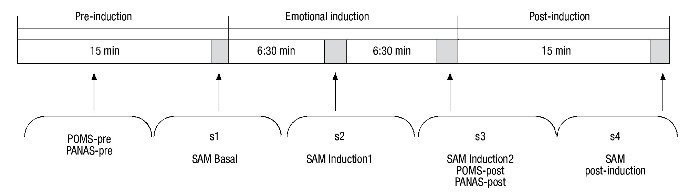
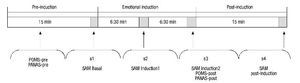
The procedure lasted 45 minutes and was divided into 3 separate phases (Fig. 1). The baseline phase (15 min) was designed to allow participants to adapt to the setting. During this time, instructions were given on how to self-collect the saliva and participants completed the computerized questionnaires which were used to gather sociodemographic data and the self-report questionnaires (POMS-pre, PANAS-pre). At the end of this phase, participants completed the self-reported affective rating scale (SAM baseline) while the initial saliva sample (s1) was collected. In the second phase (emotional induction), participants viewed the 24 IAPS images (30 sec per picture for viewing, with a 3-sec recess between pictures). Participants were told to view each picture for a full 30 seconds. During this second phase, participants completed the self-report affective scales and collected two saliva samples: first after viewing the first 12 images (SAM Induction1, s2), and again after viewing the final 12 images (SAM Induction2, POMS-post, PANAS-post, s3). Because sAA and sCORT have different latencies of response (Dickerson & Kemeny, 2004; van Stegeren et al., 2008), two saliva samples were taken during this second phase. The last phase (15 min) began after completion of emotional induction. During this time, participants finished answering the questionnaires and final emotional and biological measures were taken (SAM Post-induction, s4).
Figure 1 Schematic description of the procedure. PANAS, Positive and Negative Affect Schedule; POMS, Profile of Mood States; SAM, Self-Assessment-Manikin.
Data analyses
SPSS v.18.0 (SPSS Inc., Chicago, Illinois) was used to perform the statistical analysis. All hypotheses were tested with a two-tailed significance level of .05. Sociodemographic variables were compared using the chi-square test for categorical variables and the Student's t-test for continuous variables. PSS scores were compared by means of a t-test analysis.
Hierarchical Linear Modelling (HLM) was used to investigate group (BPD vs. control), time (phase), and interaction effects for SAM and biological variables (corrected df reported). We determined the appropriate covariance structure using Akaike's and Schwarz's information criteria. We used the restricted maximum likelihood method, while the distribution for residuals was tested using a Kolmogorov-Smirnov test. For all analyses, participants were only included if they had a baseline measure and at least one induction or post-induction measure. For scales with 2 measurements (i.e. baseline and the post-induction phases: POMS and PANAS), repeated measures MANOVA were performed. To assess between-group differences in recovery, the deltas between post-induction and induction1 phases for SAM variables were calculated by subtracting the post-induction values from Induction2, and a MANOVA analysis was performed. Depressive symptomatology on biological response, correlation analyses within the BPD group between HRDS scores and sCORT and sAA levels were performed.
Results
Patient demographics and clinical characteristics
There were no significant differences between groups regarding gender, age and marital status. However, significant statistical differences were observed in years of schooling and PSS scores (see Table 1).
Biological variables
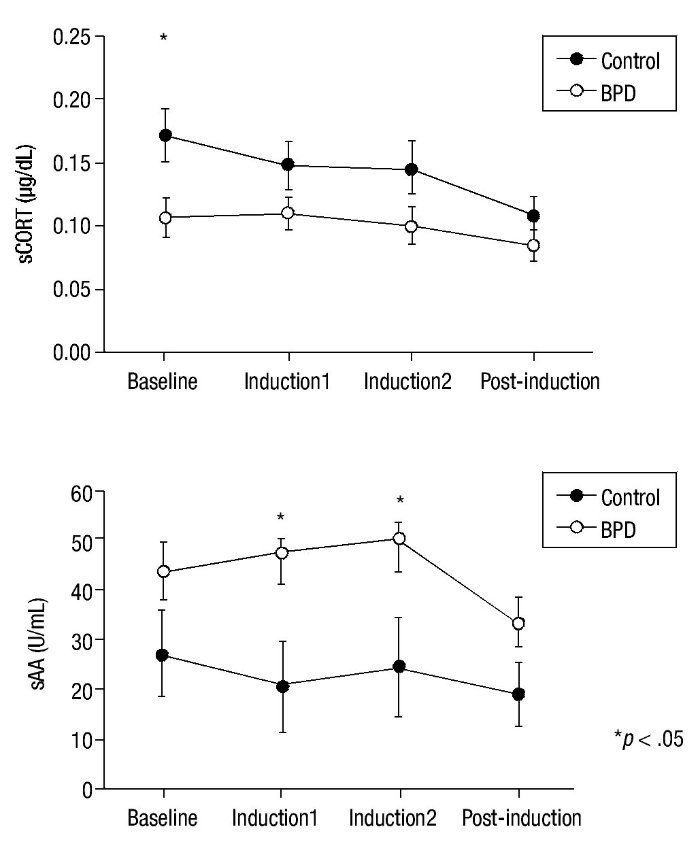
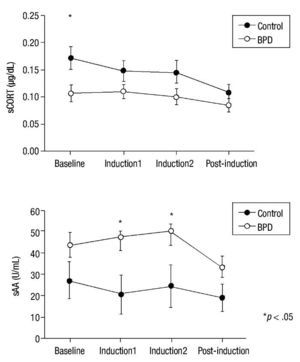
Emotion induction failed to induce an increase in cortisol levels in either group. Nevertheless, significant main effect group x time differences in HLM analysis were found for sCORT (F(3,58.40) = 2.90; p = .040). Group differences in cortisol levels showed a tendency for significance (F(1,37.36) = 3.86; p = .057), with lower cortisol levels in the BPD group. Univariate analysis revealed significant differences between groups in sCORT at baseline (p = .015) (see Fig. 2 for details).
Figure 2 Mean and SEM values for salivary cortisol (sCORT) and alpha-amylase (sAA) during the procedure. BPD, Borderline Personality Disorder.
Significant between-group differences were found for sAA levels (F(1,35.83) = 4.54; p = .040), with higher levels of amylase activity in the BPD group but without any group x time effects (p = .149). Univariate analyses for sAA revealed significant differences in the Induction1 and Induction2 phases (p = .024 and p = .048, respectively) but not at baseline (Fig. 2). A significant time effect was also found (F(3,36.40) = 4.63; p = .008). No significant effect emerged from correlation analyses of HRDS scores and biological variables (p> .108).
Self-reported variables
At baseline, all PANAS and POMS subscales showed significantly higher scores in the BPD group vs. HC (p< .001).
The MANOVA repeated measures analysis showed significant between-group differences for POMS and PANAS variables (F(1,9) = 8.02; p< .001), with the BPD group tending to show a more negative mood. A time effect was also observed in the MANOVA, indicating that emotion induction was effective (F(1,9) = 2.97; p = .009). However, no group x time effect was observed (F(1,9) = .73; p = .681).
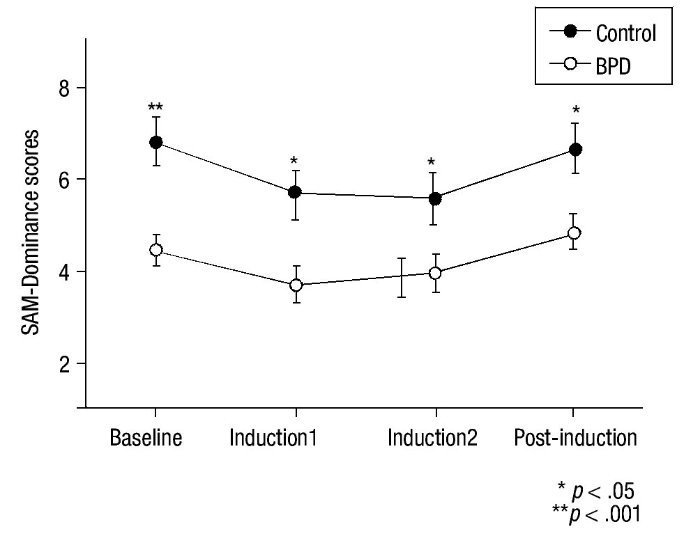
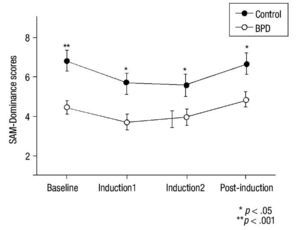
For the SAM scale, HLM analysis indicated a time effect in all SAM subscales (p< .016) and significant inter-group differences for Valence (F(3,48) = 17.40; p< .001), Activation (F(3,48) = 8.25; p = .006), and Dominance (F(3,48) = 10.66; p = .002) (Figure 3). No significant main effects group x time were observed for these variables (p> .30). Univariate analyses revealed between-group differences at baseline for SAM-Valence (p< .001) and SAM-Dominance (p< .001). Between-group scores for SAM-Activation at baseline were close to significance (p = .053). A MANOVA analysis of recovery found no significant differences in any SAM subscale (p = .570).
Figure 3 Mean and SEM values for Self-Assessment-Manikin (SAM) subscales (Valence, Activation and Dominance) during the procedure. BPD, Borderline Personality Disorder.
Discussion
The results only partially support Linehan's theory (1993). As expected, the self-reported variables show that BPD individuals have a heightened negative emotional intensity at baseline but they do not demonstrate higher emotional reactivity to negative stimuli, nor do they show a distinct pattern of recovery when compared to healthy controls. Apparently, the emotion induction procedure that we used was insufficiently specific to induce a clear response in endocrine parameters; nevertheless, sCORT levels at baseline and throughout the procedure suggest an overall altered emotional arousal in these patients.
BPD participants displayed lower levels of sCORT at baseline and throughout the experiment. Although other studies have also reported lower sCORT baseline levels in
BPD patients (Nater et al., 2010), higher cortisol levels have also been described (Lieb et al., 2004). This discrepancy could be partially explained by differences in methodology and sample characteristics (Wingenfeld, Spitzer, Rullkötter, & Löwe, 2010). Furthermore, inconsistencies among studies could also rely on the use of relatively small samples to study a disorder with a high heterogeneity (151 possible combinations resulting from the polythetic criteria set for BPD diagnosis). However, the higher PSS scores observed in our BPD group suggest a relation between diminished cortisol levels at baseline and sustained stress, as low cortisol levels have also been reported in other populations under long-term stress (Fries, Hesse, Hellhammer, & Hellhammer, 2005). It is known that most patients with BPD have a life-history of traumatic experiences (Leichsenring et al., 2011); this persistent exposure to stressors -and also to stress-related hormones- could induce changes in the HPAA structures thus reducing glucocorticoid release. Additionally, low cortisol levels have been found in other patient samples that also present behavior problems (Brewer-Smyth, Burgués, & Shults, 2004), suggesting that downregulation of HPAA could play a role in the behavioral component of the disorder.
While no differences in sAA levels were observed at baseline, significant between-group differences in sAA values during the procedure were found, indicating that the BPD group had some degree of sympathetic overactivation, a finding that is in line with that of other authors who have previously described this phenomenon in other SNS-related variables (e.g. Ebner-Priemer et al., 2005; Limberg et al., 2011). To our knowledge, only Nater et al. (2010) have examined sAA levels in patients with BPD; interestingly, they found -in contrast with our results- some evidence of SNS hyporreactivity in the BPD group. However, higher overall levels of sAA have been reported in a sample of young women with high self-reported shame and depression (Rohleder, Chen, Wolf, & Miller, 2008), both symptoms usually present in BPD (Gratz, Rosenthal, Tull, Lejuez, & Gunderson, 2010). Remarkably, in the present study an asymmetry between sCORT and sAA levels were observed in the BPD group compared to HC. Ali and Pruessner (2012) have recently reported a similar physiological pattern associated with anxiety, social stress and depressive symptomatology in a sample of participants exposed to early life adversities. Since HPAA and SNS seem interact in a complementary way to return the organism to homeostasis (Bauer, Quas, & Boyce, 2002), a persistent asymmetry between sCORT and sAA levels could indicate dysregulation of the stress response.
The data on self-reported measures reveal that mood did not worsen faster in BPD patients vs. HC following the negative emotional induction, but rather that these patients may have a more negative emotional state at baseline. Interestingly, scores in self-reported negative emotion states have been also positively related to depression and anxiety symptomatology (Watson, Clark, & Stasik, 2011), symptoms that are commonly present in patients with BPD. Similar results have recently been described by other authors such as Kuo and Linehan (2009), who found no between-group differences in emotional reactivity to negative film clips or images of a personally-relevant condition even though BPD patients had a heightened negative emotional intensity at baseline. Self-reported data on emotional states described in other stressor induction paradigms (Jacob et al., 2009; Staebler, Gebhard, Barnett, & Rennenberg, 2009) also support our findings regarding a lack of emotional hyperreactivity and heightened basal emotional intensity in BPD.
However, evidence suggesting that emotional hyperreactivity in BPD should be considered a cue-dependent feature rather than a trait of this disorder is beginning to accumulate. In this regard, Gratz et al. (2010) subjected BPD patients to two stressors, one general and the other involving negative evaluation (specifically designed to induce shame), finding that emotional hyperreactivity in BPD was cue-specific and is not present in response to a standardized stressor without evaluative content. The higher scores in sensitivity to social rejection in BPD patients vs. HC reported by Staebler, Helbing, Rosenbach, & Renneberg (2011) strengthen the findings reported by Gratz et al. (2010) and suggest that further emotional induction paradigms should include shame as a key emotion to study emotional dysregulation in BPD.
The present study has certain limitations that need to be taken into account when evaluating our findings. Primarily, the small sample size may have reduced our sensitivity to detect differences between groups. In addition, the lack of an evident response to emotion-induction in sCORT values and the wide dispersion of sAA levels make it difficult interpret the results, thus limiting the significance of the biological data. Because most BPD patients receive pharmacological treatment (Pascua et al., 2010), we elected to include these patients in the study in order to increase external validity (actually all subjects in the clinical group were on psychopharmacological treatment), so the effect of medication on biological and self-reported emotional response could not be controlled. Future studies will also need to use more appropriate interviews to assess possible Axis-I comorbidities.
To conclude, the findings presented here do not support the hypothesis that BPD patients present greater emotional reactivity. However, we did find that BPD patients have heightened negative mood intensity at baseline, which we believe should be considered a hallmark of the disorder. Further studies should incorporate various BPD-specific emotional inductions in order to a)determine if emotional dysregulation -understood as a stimulus-related feature- is actually present in BPD and, if so, b) identify the principal emotional cue(s) responsible for triggering this dysregulation. Likewise, it seems necessary that any future studying include both self-reported variables and main biological stress-markers in order to accurately describe the processes involved in the emotional response.
Funding
This project was supported by the Spanish Ministry of Health, Instituto de Salud Carlos III, CIBERSAM. A. Sanz, F. Villamarín, and X. Borràs were supported by grant SEJ2006-12418 from Dirección General de Investigación del Ministerio de Educación y Ciencia. A. Armario and J. Carrasco are funded by grants from Ministerio de Ciencia e Innovación (SAF2008-01175), Instituto de Salud Carlos III (RD06/0001/0015, Redes Temáticas de Investigación Cooperativa en Salud, Ministerio de Sanidad y Consumo), Plan Nacional sobre Drogas and Generalitat de Catalunya (SGR2009-16).
Authorship
Albert Feliu-Soler and Xavier Borràs contributed equally to this work.
*Corresponding author at:
Department of Psychiatry,
Hospital de la Santa Creu i Sant Pau, St. Antoni M.ª Claret, 167,
08025 Barcelona, Spain.
E-mail address:afelius@santpau.cat (A. Feliu-Soler).
Received June 18, 2012;
accepted October 29, 2012
References
Ali, N., & Pruessner, J. C. (2012). The salivary alpha amylase over cortisol ratio as a marker to assess dysregulations of the stress systems. Physiology & Behavior, 106, 65-72.
Backs, R. W., Da Silva, S. P., & Han, K. (2005). A comparison of younger and older adults' self-assessment manikin ratings of affective pictures. Experimental Aging Research, 31, 421-440.
Bagby, R. M., Ryder, A. G., Schuller, D. R., & Marshall, M. B. (2004). The Hamilton depression rating scale: Has the gold starndard become a lead weight? American Journal of Psychiatry, 161, 2163-2177.
Barrachina, J., Soler, J., Campins, M. J., Tejero, A., Pascual, J. C., & Pérez-Solá, V. (2004). Validation of a Spanish version of the diagnostic interview for borderlines revised (DIB-R). Actas Españolas de Psiquiatría, 32, 293-298.
Bauer, A. M., Quas, J. A., & Boyce, W. T. (2002). Associations between physiological reactivity and children's behavior: Advantages of a multisystem approach. Journal of Developmental & Behavioral Pediatrics, 23, 102-113.
Brewer-Smyth, K., Burgués, A. W., & Shults, J. (2004). Physical and sexual abuse, salivary cortisol, and neurologic correlates of violent criminal behaviour in female prison inmates. Biological Psychiatry, 55, 21-31.
Codispoti, M., Gerra, G., Montebarocci, O., Zaimovic, A., Raggi, M. A., & Baldaro, B. (2003). Emotional perception and neuroendocrine changes. Psychophysiology 40, 863-868.
Cohen, S., & Williamson, G. (1988). Perceived stress in a probability sample of the U.S. In S. Spacapam, & S. Oskamp (Eds.), The social psychology of health: Claremont Symposium on Applied Social Psychology(pp. 31-67). NewburyPark: Sage.
Dickerson, S. S., & Kemeny, M.E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130, 355-391.
Ebner-Priemer, U. W., Badeck, S., Beckmann, C., Wagner, A., Feige, B., Weiss, I., Lieb, K., & Bohus, M. (2005). Affective dysregulation and dissociative experience in female patients with borderline personality disorder: A startle response study. Journal of Psychiatric Research, 39, 85-92.
Fries, E., Hesse, J., Hellhammer, J., & Hellhammer, D. H. (2005). A new view on hypocortisolism. Psychoneuroendocrinology, 30, 1010-1016.
Gómez-Beneyto, M., Villar, M., Renovell, M., Pérez, F., Hernández, M., Leal, C., Cuquerella, M., Slok, C., & Asencio, A. (1994). The diagnosis of personality disorder with a modified version of the SCID-II in a Spanish clinical sample. Journal of Personality Disorders, 8, 104-110.
Granger, D. A., Kivlighan, K. T., el-Sheikh, M., Gordis, E. B., & Stroud, L. R. (2007). Salivary alpha-amylase in biobehavioral research: Recent developments and applications. Annals of the New York Academy of Sciences, 1098, 122-144.
Gratz, K. L., Rosenthal, M. Z., Tull, M. T., Lejuez, C. W., & Gunderson, J. G. (2010). An experimental investigation of emotional reactivity and delayed emotional recovery in borderline personality disorder: The role of shame. Comprehensive Psychiatry, 51, 275-285.
Gröschl, M. (2008). Current status of salivary hormone analysis. Clinical Chemistry, 54, 1759-1769.
Hamilton, M. (1960). A rating scale for depression. Journal of Neurology Neurosurgery, and Psychiatry, 23, 56-62. Hellhammer, D. H., Wüst, S., & Kudielka, B. M. (2009). Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology, 34, 163-171.
Herpertz, S. C., Kunert, H. J., Schwenger, U. B., & Sass, H. (1999). Affective responsiveness in borderline personality disorder: A psychophysiological approach. American Journal of Psychiatry, 156, 1550-1556.
Herpertz, S. C., Schwenger, U. B., Kunert, H. J., Lukas, G., Gretzer, U., Nutzmann, J., Schuerkens, A., & Sass, H. (2000).Emotional responses in patients with borderline as compared with avoidant personality disorder. Journal of Personality Disorders, 14, 339-351.
Jacob, G. A., Hellstern, K., Ower, N., Pillmann, M., Scheel, C. N., Rüsch, N., & Lieb, K. (2009). Emotional reactions to standardized stimuli in women with borderline personality disorder: Stronger negative affect, but no differences in reactivity. The Journal of Nervous and Mental Disease, 197, 808-815.
Koenigsberg, H. W., Siever, L. J., Lee, H., Pizzarello, S., New, A. S., Goodman, M., Cheng, H., Flory, J., & Prohovnik, I. (2009). Neural correlates of emotion processing in borderline personality disorder. Psychiatry Research, 172, 192-199.
Kudielka, B. M., Buske-Kirschbaum, A., Hellhammer, D. H., & Kirschbaum, C. (2004). HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology, 29, 83-98.
Kuo, J. R., & Linehan, M. M. (2009). Disentangling emotion processes in borderline personality disorder: Physiological and self-reported assessment of biological vulnerability, baseline intensity, and reactivity to emotionally evocative stimuli. Journal of Abnormal Psychology, 118, 531-544.
Lang, P. J. (1980). Behavioral treatment and bio-behavioral assessment: Computer applications. In K.B. Sidowski, J. H. Johnson, & T.A. Williams (Eds.), Technology in mental health care delivery systems(pp. 119-137). Norwood: Ablex.
Lang, P., Ohman, A., & Vaitl, D. (1988).The international affective picture system. Gainesville: University of Florida, Centre for Research in Psychophysiology.
Leichsenring, F., Leibing, E., Kruse, J., New, A. S., & Leweke, F. (2011). Borderline personality disorder. Lancet, 377, 74-84.
Lieb, K., Rexhausen, J. E., Kahl, K. G., Schweiger, U., Philipsen, A., Hellhammer, D. H., & Bohus, M. (2004). Increased diurnal salivary cortisol in women with borderline personality disorder. Journal of Psychiatric Research, 38, 559-565.
Limberg, A., Barnow, S., Freyberger, H. J., & Hamm, A. O. (2011). Emotional vulnerability in borderline personality disorder is cue specific and modulated by traumatization. Biological Psychiatry, 69, 574-582.
Linehan, M. M. (1993). Cognitive-behavioral treatment for borderline personality disorder; the dialectics of effective treatment. New York: Guilford.
Lynch, T. R., Chapman, A. L., Rosenthal, M. Z., Kuo, J. K., & Linehan, M. M. (2006). Mechanisms of change in dialectical behavior therapy: Theoretical and empirical observations. Journal of Clinical Psychology, 62, 459-480.
Maffei, C., Fossati, A., Agostoni, I., Barraco, A., Bagnato, M., Deborah, D., Namia, C., Novella, L., & Petrachi, M. (1997). Interrater reliability and internal consistency of the structured clinical interview for DSM-IV axis II personality disorders (SCIDII), version 2.0. Journal of Personality Disorders, 11, 279-284.
McNair, D. M., Lorr, M., & Droppleman, L. F. (1971). Manual for the profile of mood states. San Diego: Educational and Industrial Testing Services.
Nater, U. M., Bohus, M., Abbruzzese, E., Ditzen, B., Gaab, J., Kleindienst, N., Ebner-Priemer, U., Mauchnik, J., & Ehlert, U. (2010). Increased psychological and attenuated cortisol and alpha-amylase responses to acute psychosocial stress in female patients with borderline personality disorder. Psychoneuroendocrinology, 35, 1565-1572.
Nicholson, I. R., Chapman, J. E., & Neufeld, R. W. (1995). Variability in BPRS definitions of positive and negative symptoms. Schizophrenia Research, 17, 177-185.
Overall, J. E., & Gorham, D.R. (1988). The Brief Psychiatric Rating Scale (BPRS): Recent developments in ascertainment and scaling. Psychopharmacology Bulletin, 24, 97-99.
Pascual, J. C., Martín-Blanco, A., Soler, J., Ferrer, A., Tiana, T., Alvarez, E., & Pérez, V. (2010). A naturalistic study of changes in pharmacological prescription for borderline personality disorder in clinical practice: From APA to NICE guidelines. International Clinical Psychopharmacology, 25, 349-355.
Pérez, V., Barrachina, J., Soler, J., Pascual, J. C., Campins, M. J., Puigdemont, D., & Álvarez, E. (2007). The clinical global impression scale for borderline personality disorder patients (CGI-BPD): A scale sensible to detectchanges. Actas Españolas de Psiquiatría, 35, 229-235.
Portella, M. J., Harmer, C. J., Flint, J., Cowen, P., & Goodwin, G. M. (2005). Enhanced Early Morning Salivary Cortisol in Neuroticism. American Journal of Psychiatry, 162, 807-809.
Remor, E. (2006). Psychometric properties of a European Spanish version of the Perceived Stress Scale (PSS). The Spanish Journal of Psychology, 9, 86-93.
Rohleder, N., Chen, E., Wolf, J. M., & Miller, G. E. (2008). The psychobiology of trait shame in young women: Extending the social self preservation theory. Health Psychology, 27, 523-532.
Rosenthal, M. Z., Gratz, K. L., Kosson, D. S., Cheavens, J. S., Lejuez, C. W., & Lynch, T.R. (2008). Borderline personality disorder and emotional responding: A review of theresearchliterature. Clinical Psychology Review, 28, 75-91.
Sandín, B., Chorot, R., Lostao, L., Joiner, T. E., Santed, M. A., & Valiente, R. M. (1999). Escalas PANAS de afecto positivo y negativo: validación factorial y convergencia transcultural. Psicothema, 11, 37-51.
Staebler, K., Gebhard, R., Barnett, W., & Renneberg, B. (2009). Emotional responses in borderline personality disorder and depression: Assessment during an acute crisis and 8 months later. Journal of Behavior Therapy and Experimental Psychiatry, 40, 85-97.
Staebler, K., Helbing, E., Rosenbach, C., & Renneberg, B. (2011). Rejection sensitivity and borderline personality disorder. Clinical Psychology & Psychotherapy, 18, 275-283.
Van Stegeren, A. H., Wolf, O. T., & Kindt, M. (2008). Salivary alpha amylase and cortisol responses to different stress tasks: Impact of sex. International Journal of Psychophysiology, 69, 33-40.
Watson, D., Clark, L. A., & Stasik, S. M. (2011). Emotions and the emotional disorders: A quantitative hierarchical perspective. International Journal of Clinical and Health Psychology, 11, 429-442.
Watson, D., Clark, L. A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063-1107.
Wingenfeld, K. Spitzer, C. Rullkötter N., & Löwe, B. (2010). Borderline personality disorder: Hypothalamus pituitary adrenal axis and findings from neuroimaging studies. Psychoneuroendocrinology, 35, 154-170.
Zanarini, M. C., Vujanovic, A. A., Parachini, E. A., Boulanger, J. L., Frankenburg, F. R., & Hennen, J. (2003). A screening measure for BPD: The McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). Journal of Personality Disorders, 17, 568-573.