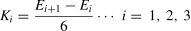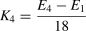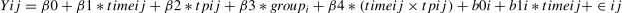Diminished capacity for maintaining positive affect (PA) has been identified in subthreshold depression (StD). While recent studies have explored affective dynamics among StD, the relationship between early emotional processing impairments and the capacity to prolong PA remains uncertain. Furthermore, it is unclear how brain connectivity patterns observed in StD are associated with PA maintenance.
MethodsThe experimental procedure comprised a baseline rs-fMRI scan, followed by a PA-inducing movie viewing task, and three further rs-fMRI sessions. Participants provided PA ratings following each session. PA maintenance was quantified through the slope of mood change between each session after movie viewing. We performed a dynamic functional connectivity analysis on movie viewing data, as well as a series of static functional connectivity (FC), analyses on data of all rs-fMRI sessions from 25 StD and 25 healthy controls (HC). Correlations between brain-related measures and slope of mood change were calculated.
ResultsIndividuals with StD exhibited reduced capacity in sustaining PA, reflected in a decrease in PA in the early maintenance stage. StD also had a lower number of transitions between four brain states during movie viewing, which was related to subsequent impairment in sustaining PA. In addition, StD had weaker static FC between left inferior frontal gyrus and right middle occipital gyrus during the first resting-state session following movie viewing, which in turn was related to a steeper decline in PA.
ConclusionsThese results highlight the brain features driving PA dysregulation in StD and provide a potential avenue for the development of future interventions.
“Do you feel happy now?” Most of the time, individuals experiencing the symptom of anhedonia would answer “no”. Anhedonia is defined as loss of interest or pleasure in previously enjoyable activities (Rado, 1956). It is a prominent feature of many mental disorders, especially major depressive disorder (MDD), and associated with numerous undesirable outcomes, such as increased suicide risk and a more chronic disease course.
While the underlying causes of anhedonia in MDD are multifaceted, researchers have reached some consensus on both its biochemical (disruptions in neurotransmitters, e.g., dopamine) and neurophysiological (reward system dysfunction) mechanisms (Der-Avakian & Markou, 2012; Tye et al., 2013). In addition, affective dynamics, specifically temporal fluctuations of affect, have provided important insights into a potential psychological mechanism driving anhedonia (Trull, Lane, Koval & Ebner-Priemer, 2015). According to the Process Model of Emotion Regulation, emotion generation is conceptualised as a dynamic process unfolding over time (Gross, 1998). The general framework of a complete emotional experience typically comprises a baseline phase, an experience phase, and a maintenance phase (Admon & Pizzagalli, 2015; Trull et al., 2015). Certain features of affective dynamics, such as affective reactivity and affective maintenance, describe how a person reacts to an emotional stimulus from its onset to extinction, encompassing the scale of the entire emotional process (Booij, Snippe, Jeronimus, Wichers & Wigman, 2018). Other concepts such as instability, variability and temporal dependency (also known as inertia) refer to moment-to-moment affective changes over a restricted period of time (Schoevers et al., 2021). In particular, the feature of inertia refers to resistance to fluctuations in emotional states (Kuppens, Allen & Sheeber, 2010). Compared to healthy populations, patients with MDD have been found to exhibit stronger inertia relating to positive affect (PA), meaning that they are likely to maintain an ongoing level of PA over time, rather than shifting to a higher level (Kuppens et al., 2010). A recent study proposed a further concept called mood drift, referring to mood change as time passes (Jangraw et al., 2023). Mood drift was measured by mood slope (i.e., change in mood per unit time), and it was reported that individuals with less negative mood drift have a higher risk for depression.
Subthreshold depression (StD) refers to the presence of several clinically relevant depressive symptoms, but which together do not meet the diagnostic criteria for MDD (Rodríguez, Nuevo, Chatterji & Ayuso-Mateos, 2012). StD attracts less attention as it generally has fewer and milder symptoms compared to MDD. However, StD has a high prevalence (approximately 11 % in the general population: (Zhang et al., 2023)), with even higher rates (ranging from 20 to 40 %) among university students (Jiang et al., 2019; Mikolajczyk et al., 2008). Unsurprisingly, individuals with StD have an increased risk of developing MDD compared with healthy populations (Tuithof et al., 2018). Importantly, anhedonia is also a core feature of StD (Rodríguez et al., 2012; Zhang et al., 2022). Qiu and colleagues (2024) demonstrated lower generalization of reward-related stimuli among StD individuals relative to HCs, leading to poorer maintenance of PA. Unlike MDD patients, who exhibit not only poor PA maintenance but also low PA reactivity to emotionally valanced stimuli in laboratory settings (Bylsma, Morris & Rottenberg, 2008), anhedonia in StD seems to be associated with a distinct pattern. We previously showed that while individuals with StD were unable to sustain PA for as long as HC, they nonetheless did experience comparable PA in direct response to positive video stimuli (Song et al., 2024). Nevertheless, it is unclear whether normal PA reactivity in StD indicates similar emotion processes to HC, which could not be ascertained from this purely behavioral study examining only the experience phase.
Therefore, in the current study, we designed a multi-stage experimental fMRI procedure comprising various phases of emotional experience to identify the precise components of PA that are impaired in StD. Additionally, to better understand the neural underpinnings of impaired PA maintenance in StD, we examined both static and dynamic functional connectivity (sFC and dFC, respectively). sFC refers to correlations in activation between different brain regions, typically during an entire resting-state scanning session (Zhang et al., 2021). It allows comparisons of brain connectivity patterns between groups or sessions (Betzel et al., 2014), which can capture state-like processes underlying PA maintenance. By contrast, dFC assesses spatiotemporal variations in brain connectivity patterns over time, allowing the assessment of changes in connectivity patterns and temporal features during the experience of PA (Allen et al., 2014; Hutchison, Womelsdorf, Gati, Everling & Menon, 2013).
The primary aim of the current study was to investigate PA dynamics in StD, and to identify their neural underpinnings, across the entire PA process (primarily in the experience and maintenance phases). We hypothesized that: (1) individuals with StD would exhibit reduced capacity in sustaining PA compared to HC, but would show normal PA reactivity; (2) compared to HC, StD would have altered dFC components specifically in the maintenance phase, but without any specific pattern in behavior (e.g., affective instability) – we also predicted that alterations in dFC would be associated with impaired PA maintenance, reflecting a functional neuroimaging marker of PA maintenance decline; and (3) group differences in sFC would occur only during the stage with the fastest decline in PA.
MethodParticipantsWith the approval of Institutional Review Board of South China Normal University, participants were recruited from universities in Guangzhou, China. Following our previous work (Song et al., 2024), our screening procedure adapted a two-stage method (Horiuchi, Aoki, Takagaki & Shoji, 2017; Yang et al., 2024) that combined the Beck Depression Inventory (BDI-II), Center for Epidemiological Studies Depression Scale (CES-D) and a Mini-International Neuropsychiatric Interview (M.I.N.I.). Participants who scored 14 or above on the BDI-II and 16 or above on the CES-D were invited to undergo a M.I.N.I., allowing researchers to assess their depressive symptoms and determine if they could be classified as part of the StD group. Individuals with a history of psychotropic medication use or any prior diagnoses of depressive episodes will be further excluded. For the healthy controls, we only included those individuals with a BDI-II score of 6 and below and a CES-D score of below 16. Further details such as the scoring method and exclusion criteria are described in the supplementary material. A final sample of 50 participants, including 25 HC and 25 StD, were selected for the present study. All participants had normal or corrected hearing and vision and the groups were matched on age and gender. The appreciation of humor (AOH) subscale of Multidimensional Sense of Humor Scale (MSHS) (Thorson & Powell, 1993) was used to examine and confirm that potential group differences in PA after movie viewing were not affected by the ability to appreciate humor.
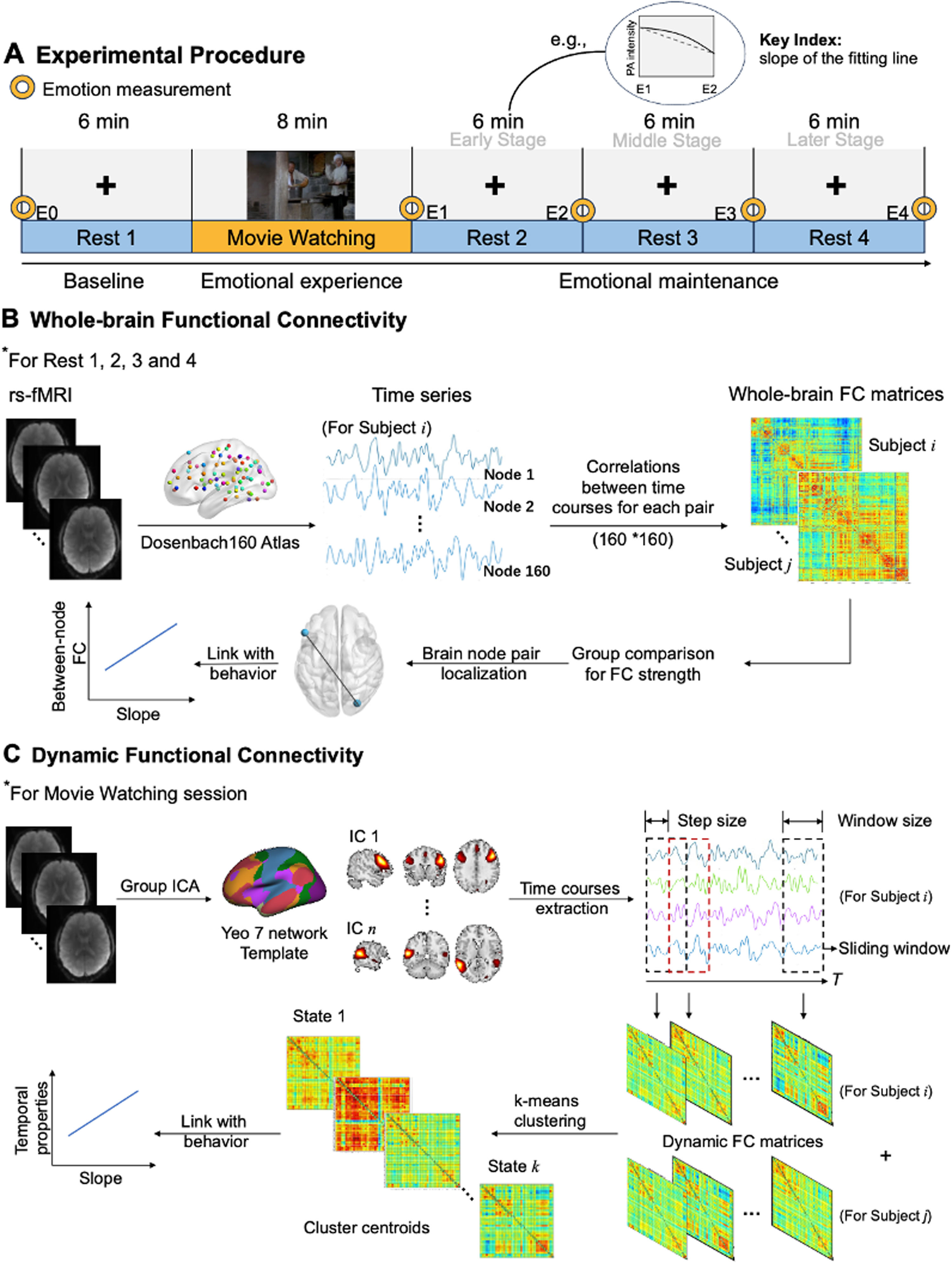
Procedures and measuresThe fMRI experimental design consisted of three phases: baseline, emotional experience and emotional maintenance (Fig. 1A). Throughout the four resting-state scanning sessions (Rest 1–4), participants viewed a fixation point on the screen and were instructed to concentrate solely on it without engaging in any other thoughts. After Rest 1, participants were scanned while watching an 8-min positive video clip, selected according to the procedure applied in our previous work (Song et al., 2024). During movie viewing, participants were instructed to rate their real-time affect continuously by pressing two buttons in the fMRI machine. The second-by-second affect ratings ranged from −100 (extremely sad) to 100 (extremely happy) were collected using the software CARMA v14.07 (Girard, 2014). Rest 2, 3 and 4, representing the early, middle and later stages of emotional maintenance, respectively, were conducted following the movie viewing period.
Schematic of experimental procedure and flowchart of functional connectivity. (A) The fMRI scanning included four resting scans and one task scan. Each resting scan lasted 6 min, with Rest 1 being conducted before the Movie Watching session, followed by Rest 2, 3 and 4. Throughout the experiment, five measurements of PA were taken, labeled as E0 to E4. (B) Whole-brain functional connectivity analyses were conducted on the data from all four resting sessions. Time series were extracted based on Dosenbach160 atlas and therefore the FC matrices for each subject were all 160×160 matrices. (C) Dynamic functional connectivity analysis was conducted on data collected during movie watching session. IC obtained by Group ICA were further selected based on Yeo 7 network template. A sliding window approach was used to estimate dynamic functional connectivity and K-means clustering was then performed on the dynamic functional connectivity estimates. State occurrences and transitions were calculated, and graphic measure was calculated for each state. FC, functional connectivity; IC, independent component.
Participants provided several ratings at the beginning of the fMRI experiment and between each of the scanning sessions (marked as E0 to E4). Specifically, they rated how happy, excited, relaxed they felt at that moment ranging from 1 (not at all) to 9 (very much). Preliminary analyses revealed consistent between-group differences and temporal trends across the scores of all the three items (Table S1). Therefore, the average rating of these three items at each time point was defined as the subjective PA intensity measurement for subsequent analysis.
fMRI data acquisitionMagnetic resonance images were acquired on a 3 Tesla Siemens MAGNETOM Prisma-fit scanner equipped with a 64-channel head coil at the Institute for brain research and rehabilitation, South China Normal University. High-resolution 3D T1- weighted anatomical images were acquired with following parameters: T1-weighted inversion recovery fast gradient echo, 208 sagittal slices, repetition time (TR) = 1800 ms, echo time (TE) = 2.07 ms, slice thickness = 0.80 mm, inversion time (TI)=900 ms, field of view (FOV)= 256×256 mm, voxel size=0.8 × 0.8 × 0.8 mm, flip angle (FA)=9°, average=1. Resting-state and movie-viewing functional images were collected using the same gradient-recalled echo-planar imaging pulse sequence (60 axial slices, TR= 1500 ms, TE=31 ms, slice thickness = 2.40 mm, FOV= 211×211 mm, voxel size=2.4 × 2.4 × 2.4 mm, FA= 70°).
Preprocessingrs-fMRI data (Rest 1, 2, 3 and 4)Data preprocessing was carried out using the toolbox for Data Processing & Analysis of Brain Imaging (DPABI, http://rfmri.org/DPABI) (Yan, Wang, Zuo & Zang, 2016), based on SPM12 (http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (version R2022b, MathWorks, Inc., Natick, MA, USA). The first 10 vol were removed to reach signal equilibrium and ensure participants had adapted to the scanning environment, resulting in a total of 310 vol. Preprocessing steps including slice timing correction, realignment, segmentation, normalization, smoothing were then performed. Details of preprocessing steps and parameters, as well as head motion, are shown in supplementary material.
Movie-viewing fMRI dataThe data preprocessing steps and parameters were similar to those for rs-fMRI. No participant exceeded an average framewise displacement (FD) > 0.5 mm or maximum translation of 3 mm or rotation of 3° (Xu et al., 2021), and therefore no participants were excluded on the basis of head motion.
Whole-brain resting-state functional connectivityWhole-brain resting-state functional connectivity (FC) analysis was carried out using GRETNA v2.0.0 (Wang et al., 2015). For each of the four rs-fMRI sessions, we divided the brain into 160 regions of interest (ROIs) according to the Dosenbach Atlas (Dosenbach et al., 2010), with each ROI representing a node. This functional template for defining ROIs covers most of the cerebral cortex and cerebellum and has been widely applied to examine whole-brain functional connectivity during rs-fMRI (Shao, Tan, Zhan & He, 2024). Using the residual images after data preprocessing, average time courses from each ROI were extracted. Pair-wise Pearson correlation coefficients were computed between all pairs of nodes, resulting a 160 × 160 matrix of FC for each subject (Fig. 1B), which was then Fisher transformed to Z-values. To investigate the group difference in FC strength over the four resting sessions, we calculated mean FC strength at the whole-brain level for each subject in each scan, i.e., the average functional connectivity across all ROIs in the brain. Independent samples t-tests were then employed to assess the presence of group differences in each scan. Correction for multiple comparisons was applied using the false discovery rate (FDR) at p < 0.05.
Dynamic functional connectivityThe preprocessed movie-viewing fMRI data were decomposed into functional networks by applying spatial group independent component analysis (ICA) using the GIFT toolbox (v 4.0.4.11) (Calhoun, Adali, Pearlson & Pekar, 2001) (Fig. 1C). Details relating to the ICA and choice of components are shown in supplementary material. Thirty-eight meaningful independent components (ICs) were identified and were then sorted into seven functional networks based on the Yeo et al., 2011 template as previously described (Li, Ran & Chen, 2023): the default mode network (DMN), frontoparietal network (FPN), visual network, sensorimotor network (SMN), ventral attention network (VAN), dorsal attention network (DAN), and limbic network.
Postprocessing steps were applied to time courses of the selected 38 ICs to remove remaining noise sources, including detrending, despiking, and low‐pass filtering with a high-cutoff frequency of 0.15 Hz (Allen et al., 2014).
Based on the results of the ICA, dynamic functional connectivity (dFC) was conducted using sliding window approach in the GIFT toolbox. The data were divided into sliding windows of 28 TRs (42 s), in steps of 1 TR, convolved with a Gaussian distribution of sigma 3 TR. According to previous studies, window sizes between 30 and 60 s are appropriate because they can identify cognitive states and capture additional variations in functional connectivity not found with larger window sizes (Hutchison et al., 2013; Wilson et al., 2015). Then, the graphical LASSO method was used to apply a L1 regularization with 100 repetitions to the inverse covariance matrix to minimize within‐window noise (Friedman, Hastie & Tibshirani, 2008). Prior to further analyses, the functional connectivity matrices underwent a Fisher's Z-transformation.
As dFC connectivity patterns (states) recur across time and subjects, we employed a k-means clustering algorithm (Fig. 1C) to cluster the windowed functional connectivity (FC) matrices across the subjects, with the algorithm being repeated 100 times (Allen et al., 2014). The L1 distance (Manhattan distance or City distance) function was applied to estimate the similarity between windowed FC matrices, as it has been verified to be suitable for high-dimensional data (Aggarwal, Hinneburg & Keim, 2002). The optimal number of cluster centroids was determined to be four (Fig. S2 in supplementary material) based on the elbow criterion of cluster validity index (Allen et al., 2014).
Functional connectivity of emotion regulation-related ROIsTo further explore the neural correlates of emotion regulation and positive emotional experience and maintenance, a ROI based correlation approach was applied to evaluate temporally correlated BOLD signal between 14 predetermined ROIs using DPABI. The analyses were performed on functional MRI data collected during two sessions: a movie-watching session and a resting-state session (Rest 2). The Rest 2 session was selected based on hypothesis in the current study and prior findings indicating its significance in the time course of positive emotion in StD (Song et al., 2024). The selection of these ROIs was based on previous research demonstrating their involvement in emotion regulation, particularly in populations exhibiting depressive symptoms (Park et al., 2019; Wu, Li & Wang, 2024). These 14 ROIs encompass 10 cortical regions and 4 subcortical regions as defined by the Harvard-Oxford Atlas, including the bilateral – insula, dorsolateral prefrontal cortex (dlPFC), medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), ventromedial prefrontal cortex (vmPFC), amygdala, accumbens (Table S2). Compared with other brain atlases, the Harvard-Oxford Atlas provides more refined parcellation of the subcortical regions, such as amygdala and accumbens, which are considered key regions related to emotion regulation.
For each participant, the mean time series was extracted from each ROI by averaging the time series of all voxels within the ROI. Functional connectivity between each pair of ROIs was computed as the Pearson correlation coefficient between their time series, resulting in a 14 × 14 FC matrix for each participant. After applying Fisher's Z transformation, group differences in FC values between the HC and StD groups were assessed using independent samples t-tests. To investigate the relationship between functional connectivity and emotional maintenance, Pearson correlation analysis was performed to assess the association between the FC values of ROI pairs exhibiting significant group differences and K1 (a metric of early-stage emotional maintenance, as defined in later section).
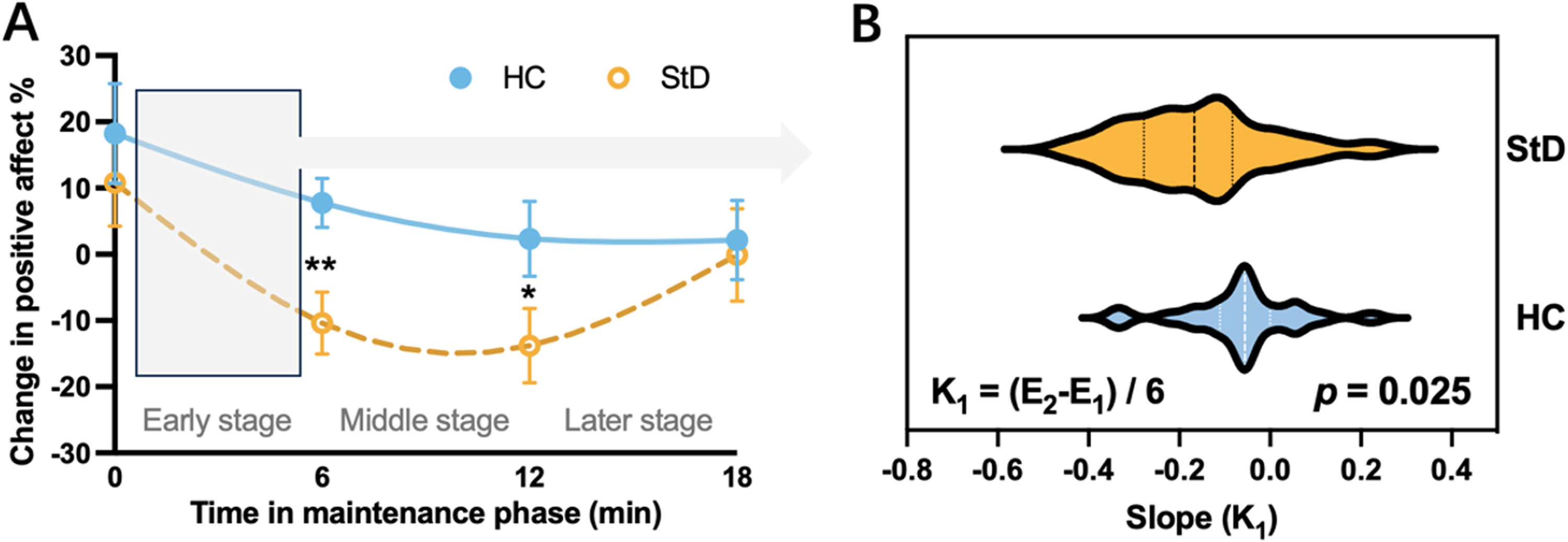
Statistical analysisBehavioral analysisDemographic and clinical variables, including age, gender and depression severity (BDI-II and CES-D), were examined using SPSS 25.0 (Chicago, IL, United States). PA differences between the groups were examined in three steps. First, a repeated-measures analysis of variance (ANOVA) including group and time was employed to determine whether there was a difference in subjective PA intensity between the two groups across the five timepoints (E0∼E4). Then, the effectiveness of the mood induction (i.e., movie watching) and group differences in affective reactivity were tested using a group (HC, StD) by time (E0, E1) repeated-measures ANOVA. Third, to test whether individuals with StD showed aberrant PA maintenance, we calculated the slope of the simulated line of emotional response of each subject in each stage (i.e., early, middle, later stage, see Fig. 1A), specifically the slope of affect change. For example, the slope K1 of the participants in the early stage was obtained by dividing the difference between their value at E2 and their value at E1 by the time span (i.e., 6 min). In addition to slope K2 of the middle stage and slope K3 of the later stage, we also calculated slope K4 as an overall index of PA maintenance. The equations are shown below. Independent samples t-tests were then used to examine group differences.
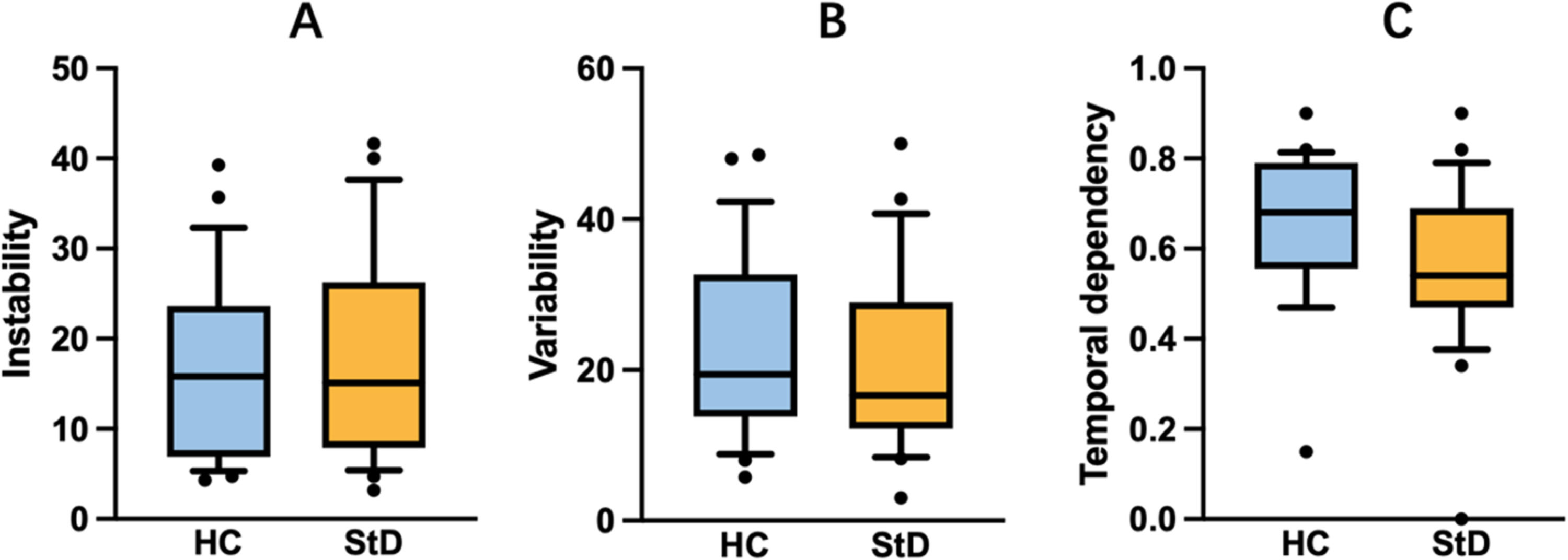
Affective fluctuations (i.e., dynamics) during the period of movie viewing were examined not only in the brain (i.e., via dFC) perspective but also behaviorally. We computed instability, variability and temporal dependency for each participant using data collected by CARMA v14.07. Instability, qualified by the root mean square of successive differences (RMSSD) of affect time-series data, refers to the changes from one moment to the next. As suggested by Schoevers and his colleagues (2021), variability and temporal dependency, qualified by variance and autocorrelation respectively, are regarded as two subcomponents of instability. Independent-samples t-tests were then used to examine group differences.
Temporal propertiesTo analyze the temporal properties of dFC states, we computed three indices for each subject: mean dwell time, fraction time and number of transitions. Mean dwell time is defined as the average time a subject remained in a certain state without switching to another state; fraction time is defined as the percentage of total time a subject spent in a certain state; and number of transitions is defined as the number of times the subject changed between states. Independent samples t-tests were conducted to check whether there were any differences between HC and StD on these temporal properties. False discovery rate (FDR) was used to corrected p-values and the threshold for statistical significance was set at p < 0.05.
In addition, subject-specific medians corresponding to each group-level state were estimated and independent-samples t-tests were used to compare the connectivity strength of each state at each pairing (703 pairings; p < 0.05, FDR correction) between HC and StD.
Link between mood drift and temporal propertiesJangraw and colleagues (2023) proposed a concept called “mood drift” by computing change in mood per minute (slope), reflecting mood drift over time. Based on prior results (Song et al., 2024), we speculated that mood drift in individuals with StD might be different, and that any difference might be related to brain dynamics during movie viewing. Therefore, we examined the relationship between temporal affective properties and mood drift. We defined mood drift (change of slope) as the change from K1 to K2 and then to K3, which were calculated in units of six minutes (the duration of one resting scan). To test this, we conducted a linear mixed model (LMM) analysis using R software (version 4.1.2, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/)). The formula used in the model is shown below (variable “tp” is further replaced based on the actual results):
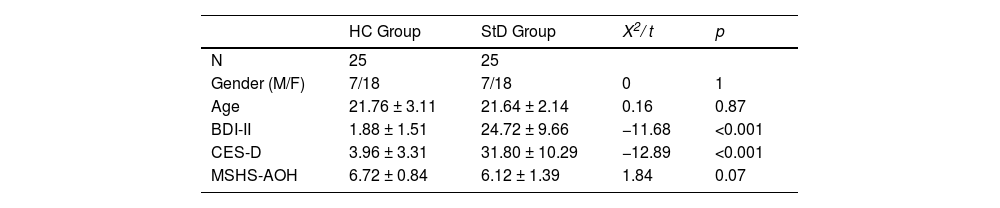
where Y ij represents the slope (estimated change in mood per resting session) in person i at time j, and β0 is the intercept. Time is the time in units of six minutes from the start of the first resting session after movie viewing. b0i and b1i are the random intercept and random slope of i, respectively. ϵij represents the random error term of person i at time j, and tp the temporal properties.ResultsDemographic and clinical characteristicsThe demographics and clinical characteristics of the participants in the current study are shown in Table 1. There were no significant group differences in age (t(48)= 0.16, p = 0.87) and gender (X12 = 0, p = 1). In the AOH scale of MSHS, the HC group exhibited a numerically higher mean score compared to StD group, although the difference only narrowly missed significance (t(48) = 1.84, p = 0.07); consequently, AOH level was included as a covariate in the analysis.
Demographics and clinical characteristics of the sample (mean ± standard deviation).
| HC Group | StD Group | X2/ t | p | |
|---|---|---|---|---|
| N | 25 | 25 | ||
| Gender (M/F) | 7/18 | 7/18 | 0 | 1 |
| Age | 21.76 ± 3.11 | 21.64 ± 2.14 | 0.16 | 0.87 |
| BDI-II | 1.88 ± 1.51 | 24.72 ± 9.66 | −11.68 | <0.001 |
| CES-D | 3.96 ± 3.31 | 31.80 ± 10.29 | −12.89 | <0.001 |
| MSHS-AOH | 6.72 ± 0.84 | 6.12 ± 1.39 | 1.84 | 0.07 |
Note: A Pearson's chi-square test (X2) was employed for gender comparison. Independent sample t tests were used for age, BDI-II, CES-D and MSHS-AOH comparisons. N, number of subjects; M, male; F, female; BDI-II, Beck Depression Inventory-II; CES-D, Center for Epidemiological Studies Depression Scale; MSHS-AOH, appreciation of humor subscale of Multidimensional Sense of Humor Scale.
We calculated the average rating across three items (excited, happy, relaxed) at each time point (E0 to E4). First, we tested the effectiveness of mood induction and differences in affective reactivity using a group-by-time (E0, E1) repeated-measures ANOVA. PA intensity was significantly increased from baseline after watching the videos (F(1,48) = 16.77, p < 0.001), indicating that PA was effectively induced by the video material. There was no significant group-by-time interaction (F(1,48) = 0.25, p = 0.62), reflecting a similar magnitude of PA change from E0 to E1 between the two groups. This pattern of results suggests that while baseline PA was lower in the StD group, these participants were able to experience an increase in PA induced by the movie.
To examine overall potential group differences in PA intensity across the entire time course, a group-by-time (E0 to E4) repeated-measures ANOVA was conducted. The StD group consistently provided lower PA intensity ratings than the HC group (F(1,48) = 21.53, p < 0.001), as well as a main effect of time (F(4192) = 9.30, p < 0.001), manifested as a significant increase in PA following the mood induction and a continuous decrease in the subsequent courses. Critically, a significant group by time interaction also emerged (F(4192) = 3.73, p = 0.010), indicating the two groups showed different patterns of change, reflecting more PA in the HC group compared with StD following the offset of the mood induction manipulation (E1 to E4).
During the eight-minute (480 s) movie viewing period, real-time affect of the participants was collected. Participants adjusted their rating by pressing the up and down keys to make the current rating reflect their current emotion. Participants were instructed to press the keys only when they perceived a change in their emotions and felt the need to adjust the rating. In fact, they often maintained the same score for a short period of time through withholding a button press. If a participant did not react during a particular second, the CARMA v14.07 which is responsible for recording second-by-second affect would record the participant's emotion for that second at the same level as the previous second. Ultimately, each participant had 480 ratings. After examining the frequency of affective changes, and also the timescale of plot changes during the movie, we down-sampled the measurement into 20-second blocks, computing the average score over each block, resulting in 24 ratings for each subject. We then calculated instability, variability and temporal dependency. However, there were no significant group differences (instability: t(48) = −0.25, p = 0.81; variability: t(48) = 0.25, p = 0.81; temporal dependency: t(48) = 1.88, p = 0.07) (Fig. 2). Performing this analysis without down-sampling (i.e., with 480 ratings per participant) did not change the results (all ps > 0.05).
Impairment in sustaining positive affect in StDChanges in mood throughout the maintenance phase were calculated separately for each participant as the percentage of change from E0 for each of the four subsequent ratings, thus controlling for baseline PA. A mixed ANOVA was conducted with group as a between-subjects factor, and the four post-baseline sampling time points as repeated measures. There was a significant main effect of time (F(3144) = 4.29, p = 0.006), and the StD group percentage change in PA was lower overall than in the HC group (significant main effect of group: F(1,48) = 6.80, p = 0.01). No significant group-by-time interaction was found (F(3144) = 0.78, p = 0.505).
Importantly, as shown in Fig. 3A, in the HC group positive mood was not noticeably attenuated for at least 18 min after the movie, reflecting a sustained PA response. We compared the difference between the two groups on the slope in each of stage (early, middle and later stage). Independent samples t-tests showed that during the first six minutes after watching the positive video clip, the StD group experienced the largest decline in PA, which was significantly steeper than in the HC group (t(48) = 2.32, p = 0.025, Fig. 3B). However, there was no significant difference in the subsequent rate of decline in PA between the two groups. This indicates that despite an immediate increase in PA following exposure to a positive stimulus, StD individuals were more likely to regress quickly to their baseline PA state.
Group differences in PA reactivity and maintenance. (A) Mixed ANOVA revealed that both groups exhibited a significant increase in positive mood immediately following the mood induction manipulation (that is, movie viewing), yet positive mood was sustained over time in HC but not StD individuals (change in positive affect % > 0). (B) The slope of early stage in StD group was significantly different from that in HC group. **p < 0.01, *p < 0.05.
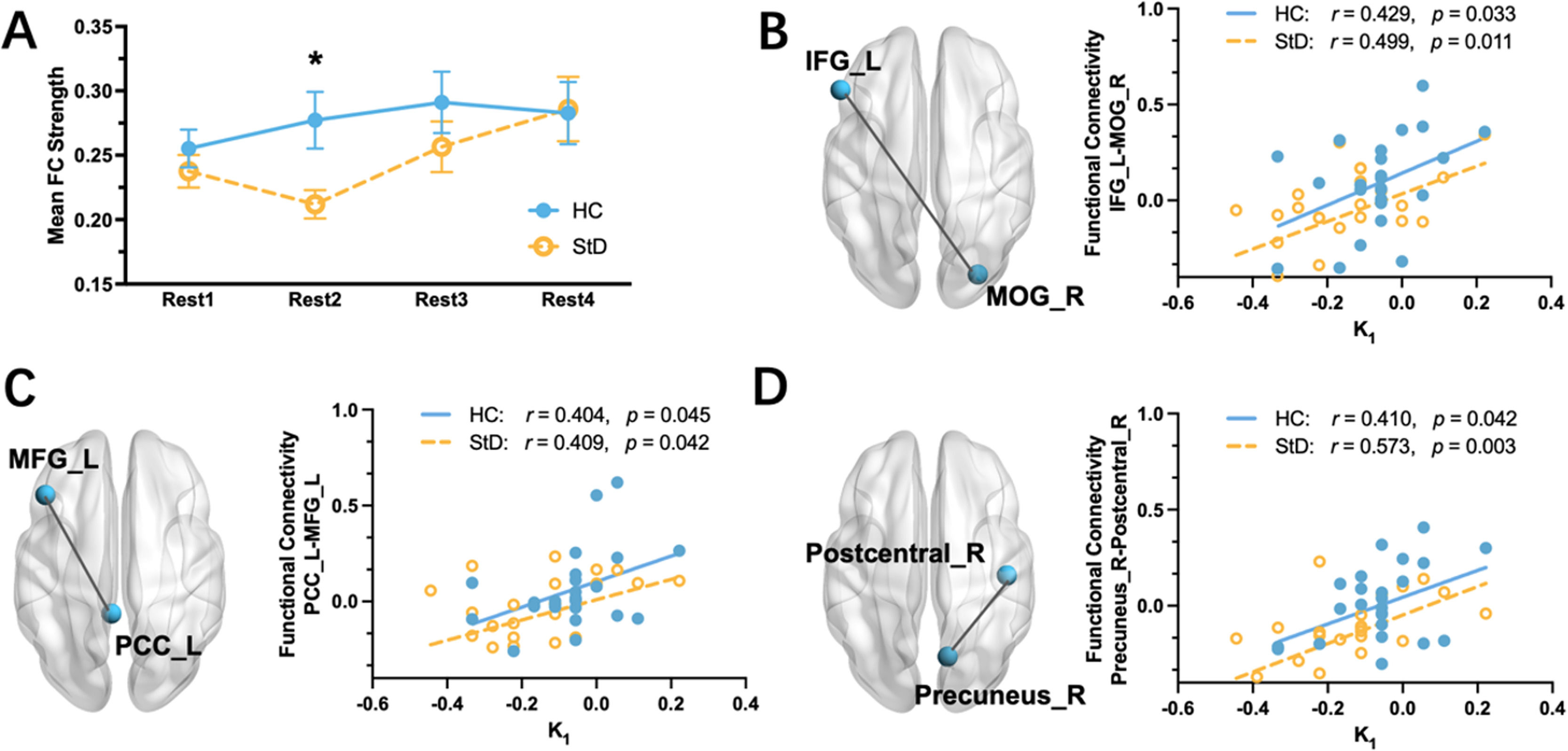
All results of functional connectivity analyses are reported at p < 0.05 (FDR corrected). During the four resting sessions, only Rest 2 (early stage, Fig. 1A) showed a significant group difference in mean FC strength (MHC = 0.28, MStD = 0.21, t(48) = 2.59, p = 0.01, Fig. 4A), coinciding with the significant decline in PA (described above) during the initial six minutes after movie viewing in the StD group. Therefore, we focused on changes occurring within Rest 2, aiming to determine the brain networks associated with the sharp decline in PA. To minimize the effect of baseline difference, we calculated differences in correlation coefficients between the groups for all node pairs based on functional connectivity data of Rest 2 minus Rest 1. For 360 node pairs showing significant group differences (p < 0.05, FDR correction), we extracted the corresponding z-values for each participant to conduct correlational analyses with the behavioral results of K1 (slope in the early stage). Ultimately, we identified a connection between the left inferior frontal gyrus (IFG, ROI 43 in Dosenbach Atlas, MNI = −52, 28, 17) and the right middle occipital gyrus (MOG, ROI 136 in Dosenbach Atlas, MNI = 29, −81, 14), a connection between the left posterior cingulate gyrus (PCC, ROI 20, MNI = −5, −43, 25) and the left middle frontal gyrus (MFG, ROI 44, MNI = −44, 27, 33), as well as a connection between the right precuneus (ROI 29, MNI = 11, −68, 42) and the right postcentral gyrus (ROI 108, MNI = 46, −20, 45) (Fig. 4B–D). Functional connectivity between these regions showed significant positive relationships with K1 in both the HC group (ps < 0.05) and StD (ps < 0.05) groups (Fig. 4B–D).
Whole-brain functional connectivity results. (A) Group comparisons in mean FC strength across four resting sessions. HC and StD showed a significant difference in Rest 2. (B) Scatter plot depicting the relationship between the left IFG – right MOG functional connectivity and K1 in the HC and StD groups. (C) Scatter plot depicting the relationship between the left MFG - left PCC functional connectivity and K1 in the HC and StD groups. (D) Scatter plot depicting the relationship between right precuneus - right postcentral gyrus functional connectivity and K1 in the HC and StD groups IFG, inferior frontal gyrus; MOG, middle occipital gyrus; MFG, middle frontal gyrus; PCC, posterior cingulate gyrus; L, left hemisphere; R, right hemisphere; *p < 0.05.
Furthermore, we conducted a series of meta-analytic connectivity modeling using the BrainMap Sleuth 3.0.4 software, with a 6-mm radius centered around these ROIs for data retrieval. Our analysis revealed that the right IFG was primarily connected to the left fusiform gyrus, while the right MOG exhibited strong connectivity with both the cuneus and the left fusiform gyrus. Additionally, further decoding of the coordinate-based brain function summary via Neurosynth indicated that the functional roles of these regions are primarily related to memory, retrieval, and episodic memory. For detailed results, please refer to Table S3 and Fig. S1 in the Supplementary materials.
Lower number of state transitions in STD during movie viewingThirty-eight ICs were identified in ICA analysis which were then grouped into the following seven networks based on their anatomical and functional properties: DMN, FPN, SMN, Limbic, Visual, DAN, VAN. Spatial maps and the specific distributions of the 38 ICs are shown in Fig. S3 and Table S4.
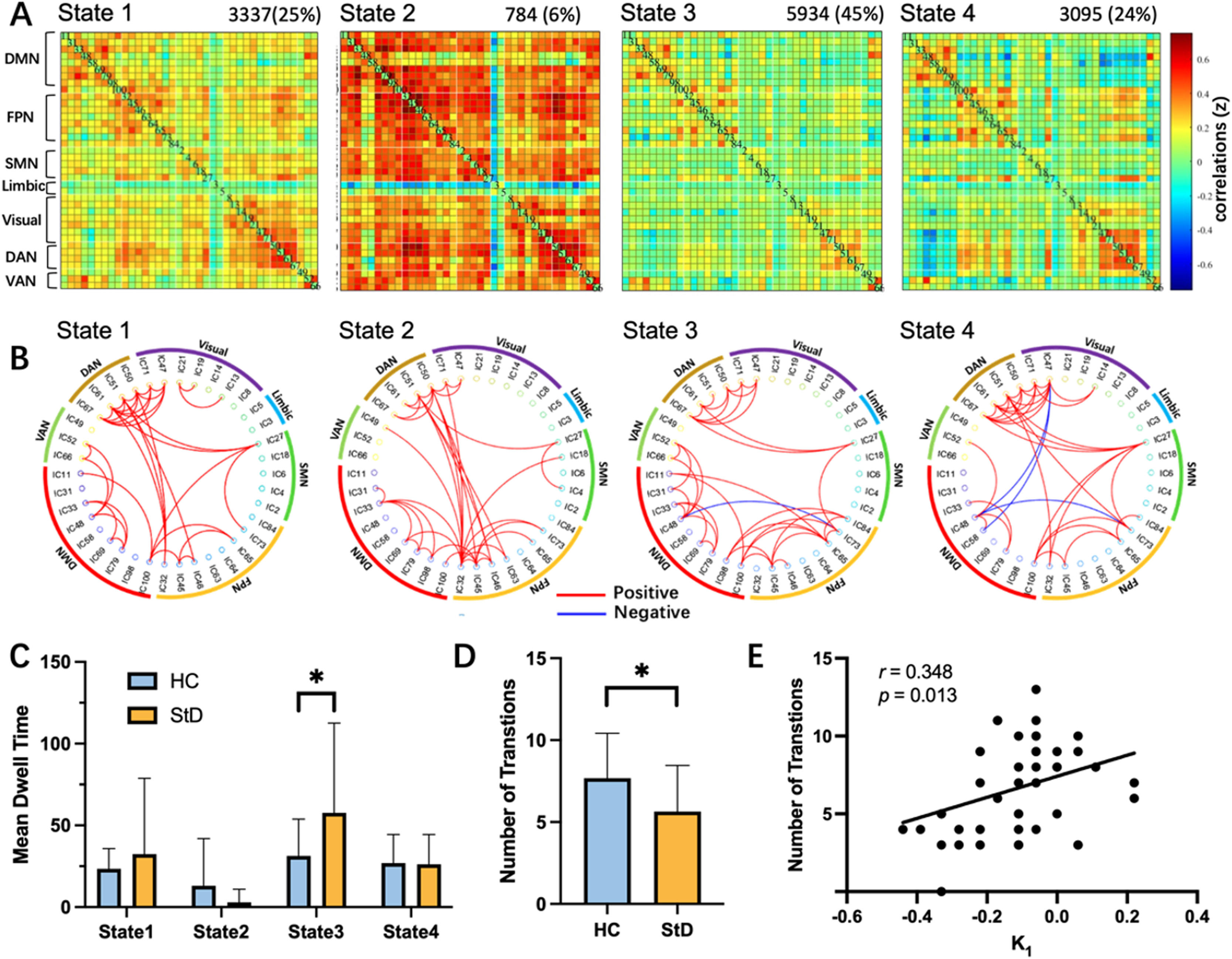
Temporal propertiesWe identified four different structured functional connectivity states with distinct features through a k-means clustering analysis (Fig. 5A). For example, state 3 was most frequent accounting for 45 % of all states, characterized by sparse connections between the networks – within-network were mainly located in DMN, FPN, and DAN, but were relatively weak. Fig 5B depicts the 5 % strongest positive or negative functional network connectivity values to characterize the different patterns of each state. Group-specific cluster centroids for each state are shown in Fig. S4.
Dynamic functional connectivity results. (A) Visualized cluster centroids of each state obtained from clustering analysis. The total number of occurrences and percentage of occurrences are listed above. (B) Graphical representation of the strongest 5 % functional network connectivity in each state. (C) Group comparison of dFC states in terms of mean dwell time. (D) Group comparison of dFC states in terms of number of transitions. (E) Pearson correlation between number of transitions and K1. DMN, default mode network; FPN, frontoparietal network; SMN, sensorimotor network; DAN, dorsal attention network; VAN, ventral attention network. *p < 0.05, FDR correction.
Independent samples t-tests were used to compare dynamic FC features between the groups. For state 3, the StD group had a significantly longer mean dwell time than the HC group (t(48)= −2.22, p = 0.031, FDR correction, Fig. 5C). The number of transitions was significantly higher in the HC group than in the StD group (t(48) = 2.60, p = 0.012, FDR correction, Fig. 5D). No significant group differences were observed in fraction time. Detailed results comparing temporal properties between the groups are shown in Table S5.
We further explored whether the group difference in brain temporal dynamics during movie viewing was related to subsequent positive emotional maintenance. In the behavioral analysis described above, the early stage was identified as the critical period of disrupted positive emotional maintenance, as the PA in StD group showed a marked decline over this stage. Therefore, we focused on the link between PA decline and dFC indices. As shown in Fig. 5E, there was a significant positive correlation between the number of transitions and K1 across all participants (r = 0.348, p = 0.013, FDR correction). This connection trended towards statistical significance even after accounting for group differences.
As a supplementary analysis, we compared connection strengths within and between networks between the groups. As reported above, state 3 showed a significant group difference in dwell time: in this state there were only four between-network connections that showed a significant difference in the StD > HC contrast; by contrast, in the HC > StD contrast for state 3 there were more group differences for both within- and between-network connections (Fig. S5). Further details are shown in the supplementary materials.
Interaction between early PA decline and number of dFC transitionsAs described above, we observed fewer transitions in the StD group, which was related to lower PA maintenance in the early stage. Therefore, number of transitions may be a core feature in experiencing and sustaining PA. We also aimed to assess the relationship between the number of transitions and mood drift in later stages (change from K1 to K2 and to K3). Therefore, we created a linear mixed model, replacing the variable “tp” in Eq. (3) with “number of transitions”.
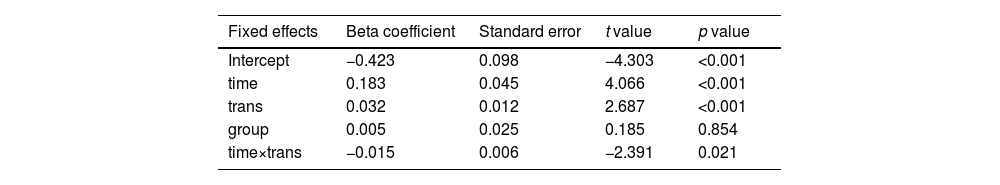
During the emotional maintenance phase, decline in PA (slope) participants slowed down over time across all participants (Estimate = 0.183, SE = 0.045, t(48) = 4.066, p < 0.001). In other words, as is evident in Fig 3A, PA decline was fastest in the early stages, after which the rate of mood decline slows. Importantly, there was a significant interaction between time and the number of transitions on PA slope even after controlling for the group factor (Estimate = −0.015, SE = 0.006, t(48) = −2.391, p = 0.021, Table 2). This indicates that the downward drift in slope over time is significantly moderated by the number of transitions, with greater transition numbers associated with a weaker decline in PA slope.
Group differences in emotion regulation-related regionsThe mean FC matrices for HC group and StD group during the movie-watching session and Rest 2 session are illustrated in Figure S6. Significant group differences in FC were identified for specific ROI pairs, and the corresponding t-values and p-values are reported in Table S6. The FC values of ROI pairs with significant group differences were further examined for their relationship with emotional maintenance (K1). A significant correlation was found during the Rest 2 session, where the FC between the right insula and left mPFC was significantly associated with K1 (r = 0.413, p = 0.04, FDR correction).
DiscussionThe current study focused on PA dynamics on the time course of positive emotion in individuals with StD. At the brain level, we considered distinct features of static and dynamic functional connectivity, applying them to various stages of positive emotion, to investigate the different brain patterns between individuals with StD and HC throughout the process of positive emotion. Furthermore, we connected these brain patterns to significant behavioral outcomes, leading to some intriguing discoveries.
Anhedonia in StD: normal PA reactivity but deficits in sustaining PAOur results in behavioral analyses replicated previous findings, indicating that individuals with StD exhibit a diminished ability to sustain PA compared to HC, while maintaining normal reactivity to positive stimuli (Song et al., 2024). These parallels were also observed in a group that has recovered from MDD but still experiences mild depressive symptoms (Admon & Pizzagalli, 2015). Notably, our study revealed a phased pattern in the maintenance of PA in StD, with a sharp decline in PA occurring within the initial six minutes of the maintenance phase in the current study. This suggests that the key distinguishing feature of StD in terms of anhedonia may lie in the reduced capacity to sustain positive emotions rather than a diminished immediate hedonic response.
Aberrant PA maintenance in StD is associated with altered brain dynamics during experience phaseDespite individuals with StD experiencing a similar level of positive affect (PA) change as HC in response to the movie, distinct neural processes were observed between the two groups following PA induction. Analysis using dFC revealed that individuals with StD exhibited increased mean dwell time in state 3, suggesting a preference for remaining in a relatively disassociated connectivity state. Additionally, StD transitioned less frequently among different states than HC, which correlated with a faster decline in PA during the early maintenance phase. A recent study on healthy subjects demonstrated that movie viewing triggers rich brain states and frequent transitions between states depending on the scene description (Meer, Breakspear, Chang, Sonkusare & Cocchi, 2020). It has been suggested that reduced transition frequency in patients with affective disorders may be linked to challenges in emotion regulation (Dai et al., 2023; Li et al., 2021). Moreover, other work has associated a lower number of transitions with various cognitive domains including attention, memory and visuospatial abilities (Fiorenzato et al., 2019; Li et al., 2017). Therefore, frequent transitions among brain states could be fundamental for individuals in perceiving, processing, and regulating emotional stimuli, thereby ensuring the PA they acquire is maintained time.
Another notable finding is the significant association between increased number of transitions and weak trend in slope change (mood drift). Mood drift has been identified as significantly associated with risk of depression (Jangraw et al., 2023). The sensitivity of mood to the passage of time is a long-intuited phenomenon (Nunokawa, 1996), and faster mood drift might predict a lack of the sense of constraint during psychological tasks or rest periods. We speculate that individuals with a faster mood drift might be due to inattention to their obtained PA, leading to the frequent mind-wandering during the maintenance phase. These findings highlight the importance of investigating effective interventions or methods to adjust brain dynamics during experiencing phase, potentially improving mood drift in StD.
FC between certain regions might be the critical targets related to PA maintenanceFour rs-fMRI sessions in the current study represented baseline and different stages of maintenance. Through a sFC approach, we observed a weaker mean FC strength in Rest 2, during which PA declined the most in StD. After controlling for the baseline difference, lower FC was identified between left IFG and right MOG, left PCC and left MFG, as well as right precuneus and right postcentral gyrus in StD compared to HC during Rest 2. Further analysis revealed that stronger FC between these identified node pairs was associated with a slower decline in PA. Prior research consistently found an activation in left IFG during tasks related to emotion regulation (Guha et al., 2020; Li et al., 2021; Morawetz, Bode, Baudewig, Kirilina & Heekeren, 2016). The down regulation of emotion was associated with decreased coupling between left IFG and certain regions while up regulation of emotion was exactly the opposite (Morawetz, Bode, Baudewig & Heekeren, 2017). A study on StD subjects demonstrated reduced the left IFG activity when processing negative emotional stimuli compared to HC (Li, Wei, Sun, Zhang & Qiu, 2017), further suggesting its potential role in positive emotion and its maintenance. The right MOG, as one of components in the occipital lobe, was responsible for visual information perception and processing (Ren et al., 2019; Tanaka, Kida, Inui & Kakigi, 2009). Some studies additionally indicated the right MOG frequently involved in emotional processing, particularly in an affective disorder (Adleman et al., 2013; Barahona-Corrêa et al., 2020; Perlman et al., 2013). Therefore, FC between the left IFG and the right MOG may facilitate the transmission and integration of visual information during emotion regulation, aiding individuals in effectively utilizing the information to maintain PA. In addition, meta-analysis based on BrainMap databases revealed that the left MFG was mainly connected to the left precentral gyrus and bilateral inferior parietal lobule, which are mainly related to memory, task, item and control, while the left PCC was mainly related to retrieval. The two connected regions might contribute to the conscious and proactive memory retrieval. Data retrieval from Neurosynth database indicated that the left PCC, left MFG, and right postcentral gyrus primarily relate to memory, retrieval and episodic memory, suggesting their collaboration in aiding individuals to recall watched scenes and extract relevant memories, thereby lasting experienced PA. Although there may be potential issues with inferring from brain regions to specific cognitive functions in reverse, aggregated evidence such as combining BrainMap and Neurosynth in this study can enhance the robustness of the results. Furthermore, reverse inference can to some extent deepen our understanding of these identified brain regions and drive the generation of new hypotheses that can be tested in future experiments (Poldrack, 2006).
Limitations and future directionsThere are several limitations to the current work that merit comment. First, our study utilized a video clip to induce PA, a widely accepted method (Fernández-Aguilar, Navarro-Bravo, Ricarte, Ros & Latorre, 2019). However, it remains unknown whether differences in brain activity patterns observed in StD hold true with other types of emotion-inducing materials, such as words, sentences, pictures, or music. Future research should apply different mood induction materials for validation. Second, we note a possible difference in temporal dependency between StD and HC, although this did not achieve statistical significance but might in a larger sample. The current study relied also on a relatively small sample size, which may limit the potential to separate underlying effects and identify small-to-moderate effect sizes. A more rational set of real-time affect rating ranges might also help identify potential effect. Additionally, the substantial noise present during MRI scanning could be a potential factor influencing emotional processing (McJury, 2022). Future research with larger sample sizes and noise reduction in MRI could further investigate the affective dynamics in StD. Third, temporal properties may serve as potential neuroimaging markers for identifying StD, but further validation is needed. For instance, techniques such as machine learning could be employed to evaluate the accuracy of these indicators in predicting StD (Sato et al., 2023). Fourth, further study should pay attention to collecting and analyzing psychometric measures related to emotional regulation, as this would help better link the observed deficits in positive emotions in individuals with StD to emotional regulation. Finally, many studies suggest that the development of depression could be viewed as a continuous spectrum, with StD lying between no depressive symptoms and MDD (Baumeister, 2010). Therefore, including a diagnosed depression group, along with the application of dimensional analyses in future studies would help enhance the exploration of the depressive spectrum, clarify the differences between StD and MDD, as well as identify potential markers or thresholds that distinguish between these levels of severity.
ConclusionsThe present study investigated PA dynamics in StD from behavioral and neuroimaging perspectives, linking them together to elucidate the underpinnings of anhedonia in StD. StD subjects exhibited diminished capacity to maintain PA and displayed aberrant brain patterns during both the experience and maintenance phases. FC between the left IFG and the right MOG, the left PCC and the left MFG, as well as the right precuneus and the right postcentral gyrus indicated the brain networks involved in PA maintenance. Moreover, lower number of transitions during positive affective processing was related to subsequent faster PA decline and mood drift. This implies that difficulties in sustaining PA in StD are not solely linked to the maintenance phase but also to early emotional experiences. These results provide insight into the understanding of anhedonia in individuals with StD and highlight the potential for future research to explore interventions targeting alterations in brain states during emotional processing and mood drift.
This study was supported by National Key R & D Program of China [grant number SIT2030-Major Projects 2022ZD0214300], Nature Science Foundation of China [grant number 32271139], and Natural Science Foundation of Guangdong Province of China [grant number 2023A1515011331].