The ability to empathize with another person's inner experience is believed to be a central element of our social interactions. Previous research has focused on cognitive (e.g., theory of mind) and emotional (e.g., emotional contagion) empathy, and less on behavioral factors (i.e., the ability to respond empathically). Recent studies suggest that the Default Mode Network (DMN) mediates individual variability in distinct empathy-related behaviors. However, little is known about DMN activity during actual empathic responses, understood in this study as the ability to communicate our understanding of the others’ experience back to them. This study used an empathy response paradigm with 28 participants (22-37 years old) to analyze the relationship between the quality of empathic responses to 14 empathy-eliciting vignettes and patterns of attenuation in the DMN. Overall, the results suggest that high levels of empathic response, are associated with sustained activation of the DMN when compared with lower levels of empathy. Our results demonstrate that the DMN becomes increasingly involved in empathy-related behavior, as our level of commitment to the other's experience increases. This study represents a first attempt to understand the relation between the capacity for responding in a supportive way to others’ needs and the intra-individual variability of the pattern of the DMN attenuation. Here we underline the critical role that the DMN plays in high-level social cognitive processes and corroborate the DMN role in different psychiatric disorders associated with a lack of empathy.
In the literature, empathy is discussed as a multi-factor construct. Individual variance in empathy stems from the ability to mirror another's feelings (i.e., affective or emotional empathy), awareness and understanding of situational dynamics inherent in another's condition (i.e., cognitive empathy), and modulatory preservation of the distinction between self and others (i.e., self-regulatory functioning) (Batson et al., 1997; Decety & Cowell, 2015; Eisenberg et al., 2000; Ickes, 1997; Shamay-Tsoory et al., 2009). However, little attention has been given to neural correlates of different types of empathic responses.
Since the definition of empathic response varies among researchers, it is essential to clarify that in the context of the present study, the empathic response is understood as the ability to communicate our understanding of others’ experiences back to them so they feel understood (Carkhuff, 1969; Ivey et al., 2013), rather than only feeling empathy. The ability to respond empathically is critical for successful interpersonal relationships and for a variety of other social and cognitive tasks (Ivey et al., 2013; Valiente et al., 2004). Carl Rogers (1957) initiated the theoretical debate on the importance of responding empathically, and his efforts led to the operationalization of the responses into categories. Following Rogers, Carkhuff (1969) introduced an empathy scale allowing the classification of three levels of empathic response. The lowest level of empathy (i.e., subtractive empathy) expresses little or no involvement with the other's circumstance, representing a mechanical interaction in social situations that diverges from the other's perspective. When responding in a subtractive way, the other's feelings are not taken into consideration and may frequently be distorted. Carkhuff also identified a basic level of empathy (i.e., interchangeable empathy) that involves accurately comprehending of the other's experiences and feelings. At this level, there is some access to the other's inner world. However, the interchangeable response mirrors what is expressed without providing an in-depth understanding of the other's experience. The highest level of empathy (i.e., additive empathy) involves a deep understanding of the other's experience and further clarification of that inner experience (Carkhuff, 1969; Ivey et al., 2013; Truax & Carkhuff, 2008). It is of interest to note that what is meant in this study as high, moderate, and low levels of empathy were formulated on the basis of a clinical perspective (i.e., an in-depth interpretative response of the other's experience). By ‘clinical or therapeutic empathy,’ we mean the presence of both domains of empathy, namely the cognitive and affective domains underlying the experience of putting oneself closer to the other's experience when interacting in a clinical setting (Howick et al., 2018; Tan et al., 2021). In other words, when healthcare professionals and patients fell empathy for each other. Ultimately, the purpose of engaging empathically with clients in a clinical setting is to generate better health outcomes for them. Although empirical evidence to support the comparison between a clinical and a more typical definition of high-level empathy (i.e., mirroring the other's perspective to show understanding and showing concern) is sparse, these approaches do not seem to be totally dissimilar.
At the methodological level, neuroimaging techniques have been used to explore aspects of empathic processing, including positron emission tomography (PET) (Ruby & Decety, 2004; Shamay-Tsoory et al., 2005) and blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI) (Mendez & Perryman, 2003; Shamay-Tsoory et al., 2009; Tholen et al., 2020). Resting-state functional connectivity MRI (RS-fcMRI), a non-invasive method to assess brain activity in the absence of external stimuli, has also been introduced as a valuable tool for investigating brain networks underlying empathy (Bilevicius et al., 2018; Cox et al., 2012; Yue et al., 2021).
Among Resting-State Networks (RSNs) indexed by RS-fcMRI, the default mode network (DMN) is of particular interest because it has been associated with a diversity of social cognition tasks (Buckner & DiNicola, 2019; Mars et al., 2012; Schilbach et al., 2008; Yeshurun, Nguyen, & Hasson, 2021). Researchers describe the DMN as an intrinsic functional connectivity circuit that triggers internally oriented cognitive processes and involves widely distributed brain regions such as the dorsal and ventral medial prefrontal cortex (dmPFC/vmPFC), the posterior cingulate cortex extending to the precuneus (PCC/pC), the lateral, inferior, and medial parietal cortex, the bilateral angular gyri (Greicius et al., 2003), and portions of the medial and lateral temporal cortices (Buckner et al., 2008; Gusnard & Raichle, 2001; Sheline et al., 2009). The DMN was first explored in the context of a task-induced deactivation (TID) paradigm (Raichle et al., 2001; Shulman et al., 1997). These studies reported a reduction of blood flow into the DMN when individuals engaged in externally-directed attention tasks in comparison to the rest condition. These results suggested the DMN deactivation during active cognitive tasks (Shulman et al., 1997). However, in contrast with the deactivation paradigm, other studies revealed that some psychological tasks yield little or no deactivation of the DMN when compared to resting periods (Buckner, 2012; Chiou et al., 2020) and that the DMN may play an essential role in high level social cognitive processes such as self-referential thought, the judgment of others’ beliefs, focus on the internal representation of affective states, and social processing (D'Argembeau et al., 2007; Gusnard et al., 2001; Mars et al., 2012; Schilbach et al., 2008; Sheline et al., 2009). In sum, the DMN has been considered necessary in the social understanding of others (Li et al., 2014). It has been suggested that the DMN manifests a stable feature of the individual (Almgren et al., 2018), reflecting thoughts and feelings, and could help predict behavior within specific contexts (Bar, 2009; Gusnard & Raichle, 2001; Nierhaus et al., 2009; Raichle & Gusnard, 2005). The DMN has also been associated with prosocial personality traits. For example, extraversion and agreeableness, typically codified as prosocial orientations, were found to positively relate with the DMN both at the functional (Sampaio et al., 2014) and structural level (Coutinho et al., 2013). In the literature, functional connectivity of the DMN is relatively stable across subjects and time (Fox & Raichle, 2007; Greicius et al., 2003). However, there is evidence for inter and intra-individual variability during goal-directed behaviors (Wicker et al., 2003).
One might reasonably expect that the empathic response ability is influenced by the individual pattern of functional connectivity of the DMN since this resting network is involved in the same social cognitive processes implied in empathy (Mars et al., 2012; Schilbach et al., 2008). Buckner and Carroll (2007) found that tasks related to social or self domains were associated with less attenuation of the DMN. Another study explored brain networks underlying working memory and social content and reported that high social load was associated with sustained activation (i.e., less attenuation) of the DMN when compared to resting-state patterns (Meyer et al., 2012).
In sum, activation patterns in the DMN may be associated with emotional and cognitive domains of empathy. However, little is known about DMN activity during actual empathic responses, specifically about the role of each of the DMN nodes in different levels of empathic response, in a clinical perspective (i.e., according with the empathic counseling style). Therefore, the central objective of this study is to analyze the relationship between the participant's functional attenuation profile in different nodes of the DMN and corollary levels of empathic response, according to Carkhuff's clinical perspective of the empathic response. Specifically, an empathy response paradigm was used to analyze the relationship between the quality of the empathic response (i.e., additive empathy; interchangeable empathy; subtractive empathy) and patterns of attenuation in the DMN. This study aims to demonstrate that a high level of empathic response correlates with less attenuation of DMN activity than moderate and low empathic response. Our first hypothesis is that high levels of empathic response are associated with almost the same activation of the DMN as in a resting state. As our second hypothesis, we thus expect that moderate levels of empathic response are associated with more attenuation of the DMN than in a resting state. The third hypothesis is that low levels of empathic response are related to high attenuation of the DMN when comparing it to rest. And finally, our fourth hypothesis is that when comparing conditions, high levels of empathic response are associated with a higher activation (or less attenuation) of the DMN in comparison to moderate and low levels.
Materials and methodsParticipantsTwenty-eight right-handed healthy participants were recruited from an online university (n = 14; enrolled in a post-graduate course in Psychology) and community (n = 14; namely secondary teachers, nurses, and managers)) pool. Three participants were excluded due to scanning errors (image acquisition and/or imaging artifacts). In the resulting sample, 52% were female (women: mean age = 26.46 years, ranging from 22 to 37 years; men: mean age= 29.42 years, ranging from 25 to 39 years). Laterality was assessed with the Edinburgh Handedness Inventory (Oldfield, 1971). None of the subjects reported a history of psychiatric or substance use disorder. The study was approved by the internal ethical committee.
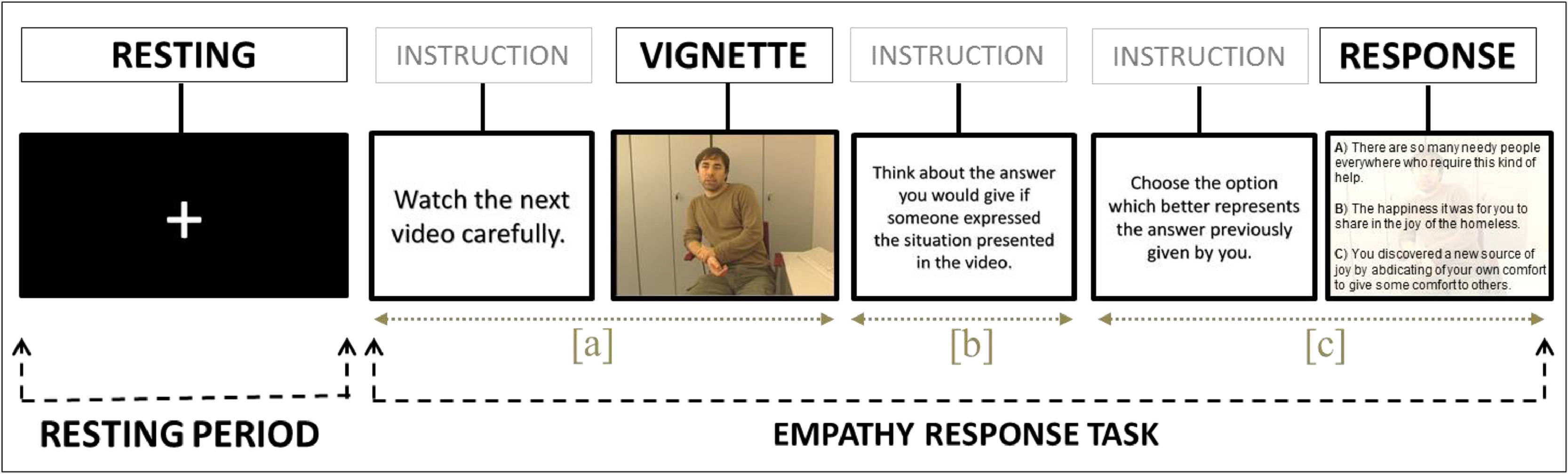
Functional MRI empathy response paradigmOliveira-Silva and Gonçalves (2011) described the paradigm used in this study, which includes 14 trials. During each trial, there was a first segment to trigger the empathy networks when participants were asked to watch a vignette for 27 s, which portrayed actors narrating an emotional life event. Then, in a second and most important segment, participants are expected to conceive an appropriate response mentally, during 10 s. And in the final segment, they choose one from among three empathic response options (additive; interchangeable; subtractive) according to the similarity between the option and the response previously conceived (see Fig. 1). This final segment allows the classification of the triggered empathy level in that experience. The response options remained on the screen until the participant made a response. The 14 experimental trials were followed by interleaved resting periods (e.g., visual fixation) lasting 10 s. Fixation on a crosshair is considered a reliable method to activate the DMN (i.e., Spreng, 2012).
Schematic demonstration of the task performed inside the scan and the different segments. Each one of the 14 blocks comprises one ‘resting period’ (i.e., a 10-second period when subjects were asked to passively fixate on a cross on the center of the screen) and one trial of the ‘empathy response task’ (i.e., initially participants were required to [a] attentively watch a short emotionally loaded vignette, lasting 27 s; then [b] internally conceive an appropriate response during 10 s; and finally, [c] to choose one among 3 options according to its similarity with the response previously conceived, which varied between trials and between participants (minimum of 13 and maximum of 27 s). The resting period was contrasted only with segment 'b' since we considered that segment 'a' works as a trigger to empathize with someone else's condition. Then this information is processed during the 10 s when participants are asked to conceive a response. Finally, they had to choose one of the options to allow the classification of the empathy level. On average, the total procedure lasted 16,3 min. The vignettes and response choices were presented in a randomized, counterbalanced order to control for order effects. Here we exemplify three levels of empathy (i.e., subtractive, interchangeable, and additive; from the less empathic to the more empathic, respectively). After providing a response, participants started a new trial with an unseen vignette. For further details, see Oliveira-Silva and Gonçalves (2011). The actor who played the role of the simulated patient, and appears in this figure, provided written informed consent, transferring the copyright of the produced material to the research group.
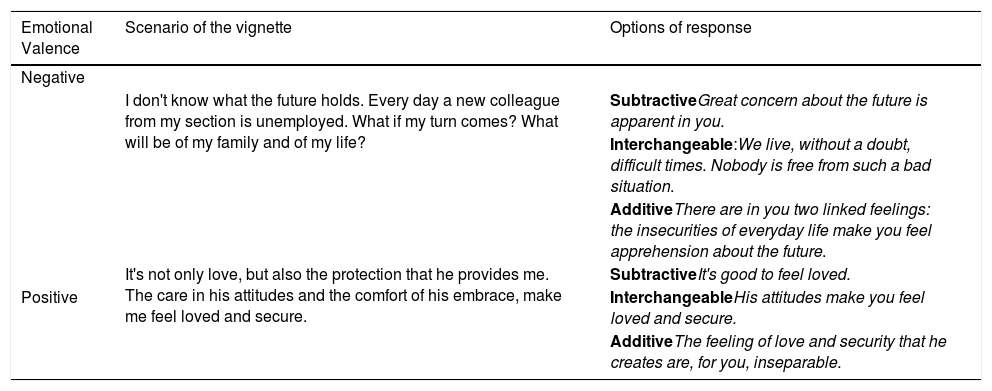
The 14 vignettes, used as stimuli for the empathic response, were selected from a larger dataset of empathy-eliciting vignettes (Oliveira-Silva & Gonçalves, 2011) based on self-reporting ratings and psychophysiological measures collected from a different sample of 97 participants. Regarding the self-report ratings, only the vignettes rated as highly positive, highly negative, and high attention-demanding were selected for this study. The psychophysiological measure used to determine the vignettes, according to the level of arousal elicited during viewing, was the skin conductance level. Only vignettes which elicited an increase in the SCL of at least 50% from the resting baseline were selected for this study. Each vignette lasted 27 s and portrayed a hypothetical situation (i.e., the actors play a character based on a scenario given to them). All actors provided written informed consent, transferring the copyright of the produced material to the research group. Their participation was compensated with monetary benefits. The scenarios were developed based on the clinical experience of the last author of this study, who has many years of experience as a clinical psychologist. Vignettes differed in content and emotional valence (i.e., six presented negative/adverse circumstances and eight presented positive/favorable ones). The vignettes were pseudo-randomized, counterbalancing the sequence as well as the actors and emotional valence. Each vignette conveys sufficient information to elicit a choice from among three levels of empathic response. As explained previously, these responses were developed based on Carkhuff's operational definition of the levels of empathy during a social interaction (Carkhuff, 1969). See below Table 1 for examples of the scenarios used in the paradigm.
Example of the scenarios used for the empathy response task.
Note: The entire instrument is available on request to the first author.
Participants were provided an explanation of the procedure and signed a written informed consent following the Declaration of Helsinki before practicing the empathy response task on a laptop outside the magnet room. Four supplementary vignettes presented in randomized order were used for practice. For each vignette, participants were asked to put themselves in the main character's position and imagine what they would do in each situation.
The software package “Presentation” (Neurobehavioral Systems, Inc., Albany, CA) was used to administer the empathy response task, to register the participant's responses, and to synchronize the paradigm with the scanner sequence (one trigger per new task event). Vignettes and response choices were presented in a counterbalanced order. Participants’ responses were recorded through a digital Lumina response device (model: LU-400 PAIR; Cedrus Corporation, San Pedro, CA), designed specifically for use in fMRI. Participants were instructed to indicate their answer choice (i.e., A, B or C) by pressing the appropriate key of the response pad. After the scanning sessions, participants were debriefed on how they felt during the experiment and on the objectives of the study.
fMRI acquisition, pre-processing, and analysesAcquisitionThe fMRI session was carried out on a 3T MRI system (Achieva 3.0T Philips Medical Systems, Netherland), with a gradient booster, a standard eight-channel head coil, and a custom head cushion to achieve head stabilization. A 2D echo-planar pulse sequence based on BOLD effect (Repetition Time = 3000 ms; Echo Time = 35 ms; Flip Angle = 90º; Field of View = 235 mm; Bandwidth = 3926 Hz/Px; Matrix Acquisition = 72×74; Pixel Size = 3.2; Slice Thickness = 3.2 mm; no gap between slices) was collected for each run while patients performed the empathy response task. The task stimuli were projected onto a screen located at the head of the MRI device using an LCD digital projector. The head coil was fitted with a mirror system, which allowed participants to view the screen. The fMRI acquisition was electronically synchronized with the task onset.
PreprocessingPrior to any analysis, all the acquisitions were visually inspected in order to exclude any critical head motion or brain lesions. The fMRI preprocessing was conducted using SPM8 (Statistical Parametrical Mapping, version 8, http://www.fil.ion. ucl.ac.uk) analysis software. The functional images were realigned to the mean image with a six-parameter rigid-body spatial transformation, estimated at 0.9 quality and 4 mm separation. The motion threshold for individual slices of all data was 1.5 mm of translation and 1.5 degrees of rotation in any direction. Using the Echo Planar Imaging (EPI) template provided by SPM, data were spatially normalized to the MNI (Montreal Neurological Institute) standard coordinate system. Several authors have shown that EPI-based normalization can provide similar or better results than normalization using T1-weighted images (Calhoun et al., 2017; Grabner et al., 2014; Huang et al., 2010). Images were then re-sampled to 2*2*2 mm3 and smoothed to decrease spatial noise with an 8 mm full-width half-maximum Gaussian kernel.
Statistical analysesBefore starting the statistical analysis, trials were categorized according to the empathic category of the response option (i.e., subtractive, interchangeable, and additive). Three different contrasts were created for the deactivation analysis, consisting of a reverse subtraction, resting periods (10 s each) minus each of the empathy task periods. In order to maximize the detection power and ensure that the comparison periods have the same length, only the segments requiring the conceive of an internal response after watching the vignettes with a length of 10 s were considered. The motion correction parameters were included as covariates of non-interest in order to increase sensitivity and reduce the effects of motion artifacts. The significant functional maps, as well as age and gender (as nuisances’ covariates), were entered into a second-level random-effects group analysis using an analysis of variance (ANOVA) with the empathy type as a factor, conducted using SPM8 (Statistical Parametrical Mapping, version 8, http://www.fil.ion. ucl.ac.uk) running under Matlab® R2014b. This second-level analysis generated a statistical map of the magnitude of attenuation (i.e., resting period vs. task periods) with a within-subject factor (empathic category of the response with three levels: subtractive, interchangeable, and additive). Then, in order to examine the hypothesis that the DMN areas would deactivate more during trials in which the participants provided a low level of empathic responding compared with trials when they provided higher levels of empathic responding, post hoc t-contrasts were performed to compare the functional response of each empathy category with the other two empathy response categories (controlling for age and gender). In order to select only the brain areas within the DMN, results were masked with the DMN template provided by the Group Independent Component Analysis (ICA) 2.0 of fMRI Toolbox - GIFT (Buckner et al., 2008; Raichle et al., 2001). In other words, the DMN was defined as comprising four key anatomical regions: the mPFC, the PCC, and the left and right inferior parietal lobule. The final components identified by ICA, for resting state analysis, were sorted and spatially correlated with the Default Mode template from GIFT for DMN identification and visually inspected. We thus identified for each individual the best-fit component that corresponded to the DMN. The results were corrected for multiple comparisons using the Monte Carlo simulation run via AlphaSim in the AFNI software, resulting from 5,000 iteractions, which generated a probability of alpha = .05, a voxel-wise probability threshold of p < .001, with a minimum cluster size of 62 voxels.
ResultsEmpathy response taskRegarding the descriptive statistics of the participants’ performance in the empathy task, the mean of the subtractive type responses was 3.9 (range: 2.7-5.0 out of 14); the mean of interchangeable responses was 3.9 (range: 2.7-5.0 out of 14); the mean of additive responses was 2.0 (range: 6.1-8.3 out of 14). The trials duration differed among conditions because of the variability in the response period (minimum of 13 and maximum of 27 s. Regarding the duration of the entire task, we had a minimum of 11. 6 min and a maximum of 14.93 min. After the scanning session, participants were thoroughly and carefully debriefed to ensure that they have understood the task. For instance, they were asked to describe their thoughts for some of the scenarios. The debriefing indicates that all participants feel they could identify themselves with at least one of the answer options.
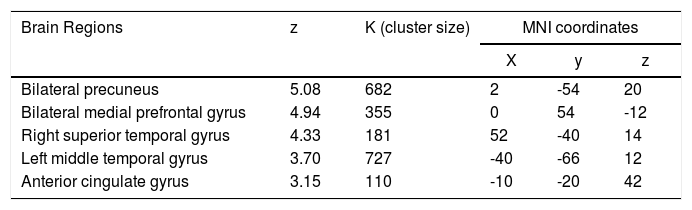
Group-level analysisAnalysis of the group-level changes map of the DMN, comparing the resting periods and the task periods from the twenty-five subjects regardless of the task-induced effects, revealed significant regions of attenuated activity in classic DMN components, namely the bilateral precuneus, bilateral medial prefrontal cortex, the anterior cingulate gyrus, the right superior temporal gyrus, and the left middle temporal gyrus (p < 0.05 corrected for multiple comparisons, with a Monte Carlo-based method) (see Table 2).
Group statistics of DMN deactivation.
Note: Each cluster´s z value, size and coordinates in Montreal Neurological Institute (MNI) space (x, y, z), are given. All correlations were significant at a p<0.05 threshold corrected for multiple comparisons using Monte Carlo method.
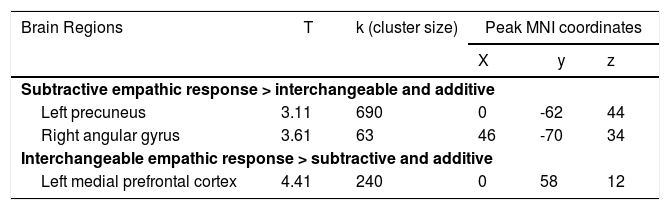
After the within-subjects ANOVA analysis (including the empathic response categories, (i.e., subtractive, interchangeable, and additive), the task-induced changes within the DMN were assessed by comparing each empathic category with the two other (i.e., additive > interchangeable and subtractive; interchangeable > subtractive and additive; subtractive > interchangeable and additive). This analysis allowed us to determine which specific DMN regions deactivated more in each specific empathic category compared to the other two distinct categories. Results showed that for the additive empathy response conditions, no significant attenuation of DMN areas was manifested. In other words, there was no statistically significant suspension of the DMN during trials in which subjects displayed the highest level of empathy compared with the interchangeable and subtractive types of empathy response.
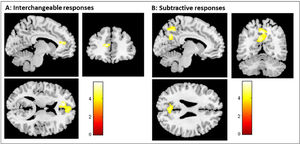
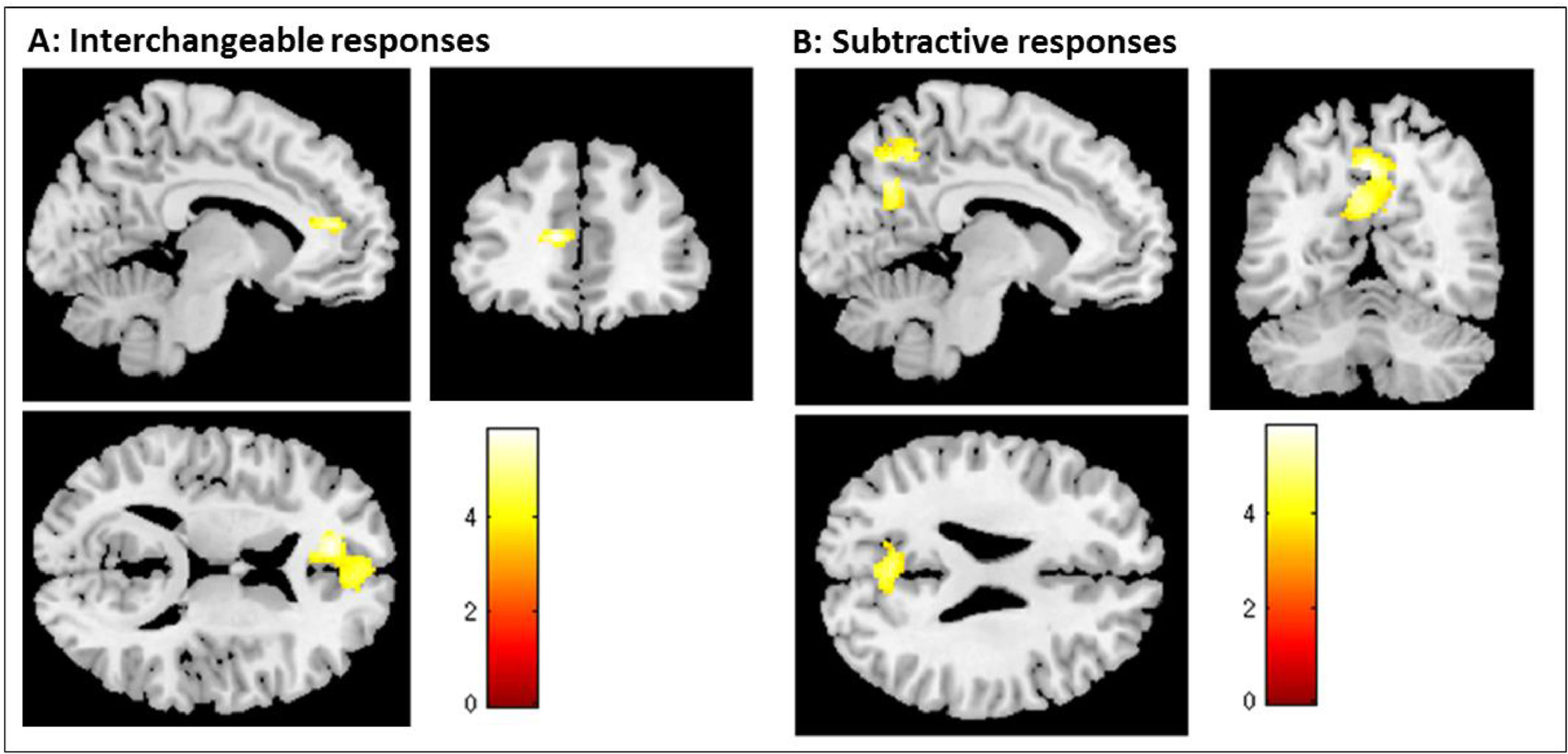
Additionally, the interchangeable level of empathy led to a significant attenuation of the left mPFC (left: x = 0, y = 58, z = 12), and the subtractive level of empathy was associated with a significant attenuation of the left pC (x = 0, y = -62, z = 44); and the angular gyrus (x = 46, y = -70, z = 34) (see Table 3). To illustrate the association between each empathy response category and DMN regions, the task-induced changes by empathic category are shown in Fig. 2, where one can see the contrast of empathy conditions (i.e., the comparison of each empathic category with the two other).
DMN areas presenting a task-induced attenuation during the performance of the empathy response task, according to the level of empathy in the response (results corrected for multiple comparisons).
Notes. Coordinates are in MNI space.
Group-level analyses of the Default Mode Network (DMN). Brain regions are showing a significant effect of the empathy response categories in the attenuation pattern of the DMN (i.e., the comparison of each empathic category with the two other). A - shows the result from a t-test comparing interchangeable with subtractive and additive responses (i.e., interchangeable < subtractive and additive). B - shows the result from a t-test comparing subtractive with interchangeable and additive responses (i.e., subtractive < interchangeable and additive). Additive empathy response conditions showed no significant attenuation of DMN areas when compared with subtractive and interchangeable responses. The color bar shows the T value thresholded at p < .05 corrected for multiple comparisons.
The present study explored the association between the patterns of attenuation of the DMN in a block-design task and the quality of participants’ empathic responses. Participants performed an empathy task alternated with resting periods. Affect-laden vignettes were presented with empathic responses options belonging to one of the three categories: subtractive, interchangeable, and additive empathy, ranging from less to more empathic. Our hypothesis was that high levels of empathic response (i.e., additive empathy) would be associated with less attenuation of DMN activity (i.e., less deactivation). Consistent with this hypothesis, when participants provided additive empathic responses (i.e., the highest level of empathy) to vignettes portraying emotional contexts, no significant attenuation, i.e., deactivation of activity in the DMN was found. These results suggest that the DMN was still engaged during the performance of additive empathy responses. In contrast, interchangeable and subtractive empathic responses were associated with a higher degree of attenuation in the DMN activity (i.e., higher deactivation).
Our results generally resonate with previous findings showing that the DMN is implicated in social-cognition tasks (Iacoboni et al., 2004; Spreng & Grady, 2010; Straus et al., 2019). The DMN has been characterized as a brain network consistently deactivated during goal-directed tasks (Greicius et al., 2003; Raichle et al., 2001). However, Andrews-Hanna (2012) argued that the attenuation patterns (i.e., the decreased activation) within the DMN during attention-demanding tasks depend on the specific nature of the task. Still before that, Spreng and Grady (2010) reported an association between empathy-related abilities (i.e., theory of mind, autobiographical memory, and prospection) and patterns of DMN activity. Collectively, these studies outline the crucial role of the DMN for social-related processes.
Different nodes of the DMN are involved in social cognitive functions. For example, the mPFC has been implicated in two central processes of the empathic response: (1) the ability to imagine a scenario or outcome to predict and generate the relevant affective response; (2) the capacity to decide about the affect of one's self or the other (Seger et al., 2004). Indeed, several authors have argued that higher levels of empathic response, represented in this study in the additive responses, may be associated with the capacity for differentiating between self and others (Decety & Svetlova, 2012; Hoffman, Kurtines, & Gewirtz, 1991; Lamm et al., 2019), and further an ability to provide personal input along with an in-depth understanding of the other's experience.
The posterior nodes of the DMN, including the PCC along with the pC, have been implicated in the integration of external information into an autobiographical emotional context (Cavanna & Trimble, 2006), aiding in the differentiation between self and others’ experiences (Jackson et al., 2006; Ruby & Decety, 2004). Likewise, the angular gyrus, considered by some authors a key node of the DMN (Greicius et al., 2003; Vincent et al., 2006), has been implicated in many mental states (e.g., believes, feelings and thoughts, regarding the self as well as the others) and processes (e.g., memory retrieval and theory-of-mind) highly associated with empathic functions (Frith & Frith, 2003). Advanced levels of additive empathy may require the ability to integrate another's experience into the personal experience to provide a clarifying frame of reference. The PCC is activated during social processing (Britton et al., 2006), subjective processing of affect in empathic responses (Prehn-Kristensen et al., 2009), and pain-related empathy (Jackson et al., 2005; Lamm et al., 2011). Additive levels of empathy may require activation of the cingulate cortex and angular gyri in order to facilitate resonance with the emotional experience of another person.
These results, therefore, need to be interpreted with caution due to the limited number of trials categorized as additive. Indeed, the number of additive responses was expected to be considerably lower than the number of interchangeable and subtractive responses due to the potential downsides and costs to oneself of high empathy (Tone & Tully, 2014). Furthermore, the low number of additive responses may be potentially related to the specific definition of empathy used in the clinical approach, based on Carkhuff's categorization of the ability to provide an empathic response (Carkhuff, 1969). Although the frequency of empathy responses is not the primary focus of this paper, further studies need to be carried out to replicate these findings and to clarify how other operationalization of this construct may yield different results. Also, although the professional actors playing the roles in the vignettes were chosen based on comparability regarding sex and age, the level of self-identification between participants and actors was not evaluated. Since self-identification processes might have affected participants’ understanding or the effort to imagine how to handle the situations, future studies should consider this assessment, besides controlling for actors’ age and gender congruence. Another suggestion is to include the evaluation of participants’ engagement level with the actors to minimize confounder variables.
For this study, by taking the whole-time interval of the vignette, mental elaboration, and response as a unique phenomenon, the aim was to capture all the phases of the empathic response. From the authors’ perspective, and also following Carl Roger's view (Rogers, 1986), empathy is a process rather than a state. This could be precisely the major strength of this study because it provides insights into what is happening in the DMN when the participants engage with an empathic target in the context of an ecological setting, and not only when participants are processing emotional stimuli, or rating their own emotional state, for example. In other words, the main goal of this study was to capture the empathic response as it unfolds in real life. Therefore, the ‘full block’ approach allowed to include (1) the moment in which the empathizer is exposed to the target (which represents the moment when the relationship between the target and the empathizer is established and when the empathizer may adopt the other's perceptual world (for more about this see Ivey et al., 2013); (2) the moment in which the empathizer decides to respond and elaborates a possible response to the target in order to communicate his/her own sense of the target's world; and, finally, (3) the moment when an empathic response is chosen (as close as possible to the response that was elaborated earlier).
Together, those results highlight the role of the DMN in social cognitive processes, and particularly in empathy-related behaviors. High levels of empathic response were found to be associated with sustained activation in several areas of the DMN. Additive empathy requires the observer to connect with the inner experience of another, while maintaining an appropriate cognitive and emotional distance. Activation patterns in the DMN during additive empathic responses may elucidate the neurobiological correlates for various social cognitive processes involved in high level empathy. These could include an ability to resonate with both the semantic (i.e., the middle left temporal gyrus) and emotional (i.e., the ACC) aspects of another person's experience while simultaneously regulating an appropriate emotional (i.e., the mPFC) and cognitive (i.e., the PCC and pC) distance.
When considering the interchangeable empathy responses, considered a minimal level of empathy required for a dyadic (interpersonal relationship) functioning (Ivey et al., 2013), or in other words, when one is able to recognize the other's outward feelings and experiences accurately but may fail in identifying or conveying more profound affects or meanings, we found that most of the DMN areas were recruited, except the left mPFC. Finally, regarding the subtractive empathy responses, corresponding to moments when one does not tune in on the other's experience ignoring the other's reference framework, our results showed that two nodes comprising the DMN were deactivated or presented a higher attenuation (i.e., the left pC and the right angular gyrus). Whereas those two empathic categories reflect the lowest levels of empathy, our results showed that part of the DMN remained activated, or in other words, did not showed attenuation during the interchangeable and subtractive trials. Thus, one can interpret that even during low levels of empathy, part of the DMN is still recruited because of the social nature of this process (i.e., detect, attend, and respond to the other people's condition).
Considered collectively, the results of this study provide further evidence that the brain areas within the DMN are actively required and seem to have a specific role during many cognitive tasks. Furthermore, our results demonstrate that the DMN becomes increasingly involved in empathy-related behavior as our level of commitment with the other's experience increases.
This study represents a first attempt to understand the relation between the capacity for responding in a supportive way to others’ needs and the intra-individual variability of the pattern of the DMN attenuation. Our results supported our initial hypothesis that high levels of empathic responding would be associated with the recruitment of the DMN areas and reinforces that the intrasubject functional variability within the DMN may be modulating empathy-related behaviors.
Author ContributionsAll authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.





![Schematic demonstration of the task performed inside the scan and the different segments. Each one of the 14 blocks comprises one ‘resting period’ (i.e., a 10-second period when subjects were asked to passively fixate on a cross on the center of the screen) and one trial of the ‘empathy response task’ (i.e., initially participants were required to [a] attentively watch a short emotionally loaded vignette, lasting 27 s; then [b] internally conceive an appropriate response during 10 s; and finally, [c] to choose one among 3 options according to its similarity with the response previously conceived, which varied between trials and between participants (minimum of 13 and maximum of 27 s). The resting period was contrasted only with segment Schematic demonstration of the task performed inside the scan and the different segments. Each one of the 14 blocks comprises one ‘resting period’ (i.e., a 10-second period when subjects were asked to passively fixate on a cross on the center of the screen) and one trial of the ‘empathy response task’ (i.e., initially participants were required to [a] attentively watch a short emotionally loaded vignette, lasting 27 s; then [b] internally conceive an appropriate response during 10 s; and finally, [c] to choose one among 3 options according to its similarity with the response previously conceived, which varied between trials and between participants (minimum of 13 and maximum of 27 s). The resting period was contrasted only with segment](https://static.elsevier.es/multimedia/16972600/0000002300000001/v2_202305311206/S169726002200028X/v2_202305311206/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)