The eight-item Morisky Medication Adherence Scale (MMAS-8) is a structured self-report measure of medication-taking behavior that has been widely used in various cultures. In Spain, no studies to date have analyzed the psychometric properties of the scale in psychiatric care. The purpose of the present instrumental study was to determine the psychometric properties of the Spanish version of the MMAS-8 in a sample of 967 consecutive psychiatric outpatients. The scale showed adequate construct validity and results pointed to a one-factor solution in which all the items contributed to the final index of adherence. The MMAS-8 exhibited significant correlation coefficients with the 10-item Drug Attitude Inventory, Form C of the Multidimensional Health Locus of Control scale, and the Hong Psychological Reactance Scale. Moreover, the MMAS-8 was able to differentiate between various mental disorder diagnosis groups. The findings of this study suggest that the Spanish version of the MMAS-8 is a reliable and valid measure of medication adherence that can be used in a psychiatric outpatient setting.
La Escala de Adherencia a la Medicación de Morisky-8 ítems (MMAS-8) es una medida auto-informada estructurada de la conducta de toma de la medicación ampliamente utilizada en diferentes culturas. No existen estudios en España que analicen sus propiedades psicométricas en población psiquiátrica. El objetivo de este estudio es determinar las propiedades psicométricas de la versión española de la MMAS-8 en una muestra de 967 pacientes psiquiátricos en régimen ambulatorio. Los resultados mostraron una adecuada validez de constructo, con una clara tendencia a una solución monofactorial, donde todos los ítems colaboraron en el índice final de adherencia. MMAS-8 alcanzó correlaciones significativas con el Inventario de Actitudes hacia la Medicación-10 ítems, con la forma C de la Escala Multidimensional de Locus de Control sobre la Salud y la Escala de Reactancia Psicológica. También la MMAS-8 permitió diferenciar el nivel de adherencia entre diferentes trastornos psicopatológicos. Los hallazgos de este estudio indican que la MMAS-8 es una medida fiable y válida para evaluar la adherencia a la medicación y que puede ser utilizada con muestras de pacientes psiquiátricos.
Non-adherence to well-prescribed psychiatric medications compromises the effectiveness of available treatments and has been associated with poor treatment outcomes such as increased risk of relapse and recurrence as well as higher health-care costs (Geddes, Carney, & Davies, 2003; Velligan et al., 2009, 2010). At present, the extent to which patients follow psychiatric advice is a major concern and an important challenge to the practice of psychiatry. In fact, rates of non-adherence to medication in psychiatric patients range between 28 and 52% in patients with major depressive disorder, 20 and 50% in patients with bipolar disorder, and 20 and 72% in patients with schizophrenia (Julius, Novitsky, & Dubin, 2009).
Currently, there is no ‘gold standard’ measure of medication adherence, given that all the measures available have their limitations (Osterberg & Blaschke, 2005). Non-adherence can be measured directly or indirectly. Direct methods of assessing medication non-adherence detect the presence of the drug in a patient's body using assays for the drug, drug metabolites, or other markers in urine, blood, or other bodily fluids. However, such methods are rarely used because of their high cost and inability to provide feedback at the point of care (Voils, Hoyle, Thorpe, Maciejewski, & Yancy, 2011). Moreover, their results can be influenced by factors other than adherence such as drug or food interactions, physiological variability, dosing schedules, and the half-life of drugs (Roberts & Turner, 1988; Smith, Psaty, Heckbert, Tracy, & Cornell, 1999). Indirect methods measure medication non-adherence by analyzing behavior. They include electronic drug monitoring, pill counts, pharmacy refills, medical record review, directly observed therapy, clinician assessment, and self-reports. The poor availability and high cost of electronic monitoring of dosing schedules limit the feasibility of this method (Choo et al., 1999). As regards pill counts, prescriptions may be filled some time before needed and patients may not accurately recall the date medications were started; drugs may not be stored in their original containers and/or tablets from other bottles may be added to the new container (Shelly, Vik, & Maxwell, 2005). Although self-reports carry a potential risk of misstatements or response biases, they provide a reasonably accurate estimate of adherence (Osterberg & Blaschke, 2005). Self-reports have the following advantages: they are brief, inexpensive, and applicable in various settings. In addition, they can provide immediate feedback at the point of care and reveal underlying issues that contribute to non-adherence (Voils et al., 2011).
The eight-item Morisky Medication Adherence Scale (MMAS-8) (Morisky, Ang, Krousel-Wood, & Ward, 2008) is a structured self-report measure of medication-taking behavior. It was developed from a previously validated four-item scale (Morisky, Green, & Levine, 1986) and supplemented with additional items addressing the circumstances surrounding adherence behavior. This measure was designed to facilitate the recognition of barriers to and behaviors associated with adherence to chronic medications such as psychiatric drugs. The scale provides information on behaviors related to medication use that may be unintentional (e.g., forgetfulness) or intentional (e.g., not taking medications because of side effects). Besides its authors, other researchers (e.g., Gupta & Goren, 2013) have provided evidence of good psychometric properties of the scale. The MMAS-8 is currently available in 33 languages and is widely used in various types of studies (i.e., Al-Qazaz et al., 2010; Kim et al., 2014; Yan et al., 2014).
The purpose of this study was to explore the psychometric properties of the Spanish version of the eight-item Morisky Medication Adherence Scale (MMAS-8) in a psychiatric outpatient setting. We are aware of the debate about the appropriateness of certain diagnostic labels (Pemberton & Wainwright, 2014; Robles et al., 2014), including proposals for eliminating such labels (Timimi, 2014). In this study, however, we used the major psychiatric diagnosis labels mainly for communication purposes. Specifically, we will examine the internal structure of MMAS-8 (with both exploratory and confirmatory factor analyses). For external evidences, MMAS-8 will be related or contrasted with (i) socio-demographic and contextual variables, usually associated with adherence to treatment (gender, age, educational level, treatment duration, treatment complexity, and psychiatric diagnosis); and (ii) psychological processes (self-efficacy, health locus of control, and psychological reactance). Attitude toward drugs was used a criterion for adherence.
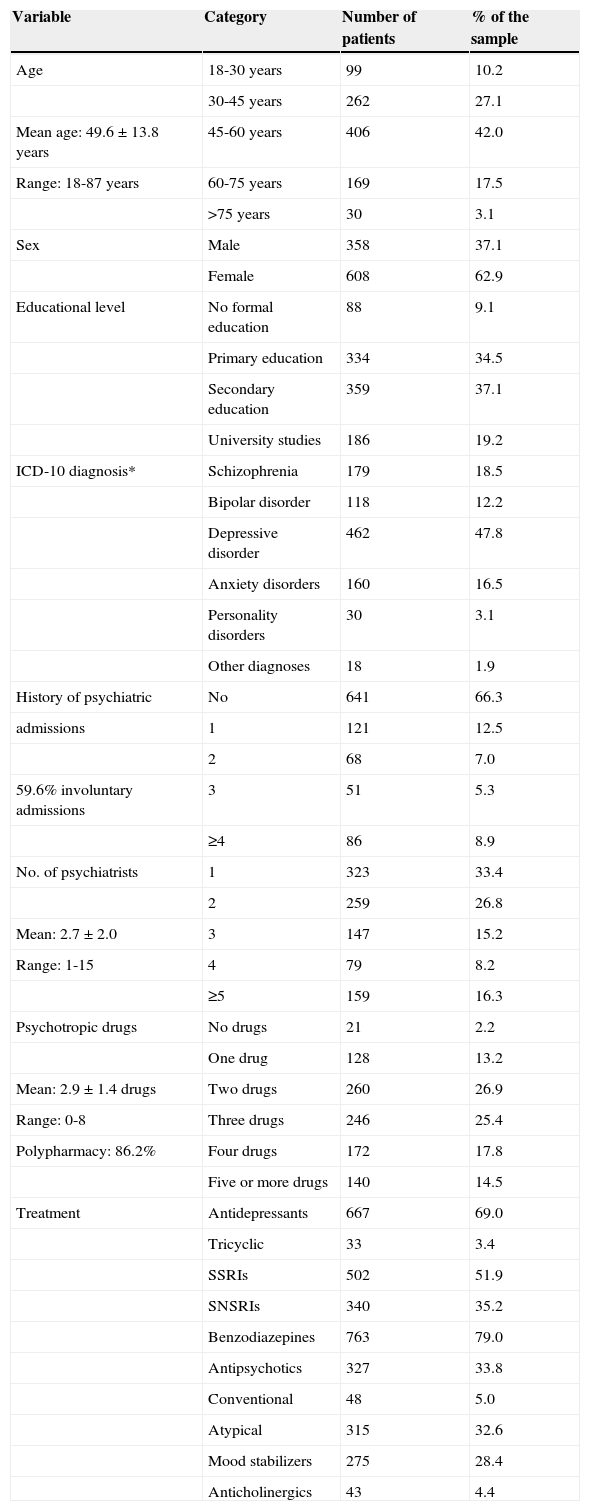
MethodParticipantsA final sample of 967 psychiatric patients accepted to participate in this study. Table 1 shows the sample distribution according to the socio-demographic and clinical variables included in the research.
Socio-demographic and clinical characteristics of the sample (N=967).
| Variable | Category | Number of patients | % of the sample |
|---|---|---|---|
| Age | 18-30 years | 99 | 10.2 |
| 30-45 years | 262 | 27.1 | |
| Mean age: 49.6±13.8 years | 45-60 years | 406 | 42.0 |
| Range: 18-87 years | 60-75 years | 169 | 17.5 |
| >75 years | 30 | 3.1 | |
| Sex | Male | 358 | 37.1 |
| Female | 608 | 62.9 | |
| Educational level | No formal education | 88 | 9.1 |
| Primary education | 334 | 34.5 | |
| Secondary education | 359 | 37.1 | |
| University studies | 186 | 19.2 | |
| ICD-10 diagnosis* | Schizophrenia | 179 | 18.5 |
| Bipolar disorder | 118 | 12.2 | |
| Depressive disorder | 462 | 47.8 | |
| Anxiety disorders | 160 | 16.5 | |
| Personality disorders | 30 | 3.1 | |
| Other diagnoses | 18 | 1.9 | |
| History of psychiatric | No | 641 | 66.3 |
| admissions | 1 | 121 | 12.5 |
| 2 | 68 | 7.0 | |
| 59.6% involuntary admissions | 3 | 51 | 5.3 |
| ≥4 | 86 | 8.9 | |
| No. of psychiatrists | 1 | 323 | 33.4 |
| 2 | 259 | 26.8 | |
| Mean: 2.7±2.0 | 3 | 147 | 15.2 |
| Range: 1-15 | 4 | 79 | 8.2 |
| ≥5 | 159 | 16.3 | |
| Psychotropic drugs | No drugs | 21 | 2.2 |
| One drug | 128 | 13.2 | |
| Mean: 2.9±1.4 drugs | Two drugs | 260 | 26.9 |
| Range: 0-8 | Three drugs | 246 | 25.4 |
| Polypharmacy: 86.2% | Four drugs | 172 | 17.8 |
| Five or more drugs | 140 | 14.5 | |
| Treatment | Antidepressants | 667 | 69.0 |
| Tricyclic | 33 | 3.4 | |
| SSRIs | 502 | 51.9 | |
| SNSRIs | 340 | 35.2 | |
| Benzodiazepines | 763 | 79.0 | |
| Antipsychotics | 327 | 33.8 | |
| Conventional | 48 | 5.0 | |
| Atypical | 315 | 32.6 | |
| Mood stabilizers | 275 | 28.4 | |
| Anticholinergics | 43 | 4.4 |
Note. ICD=International Classification of Diseases; SNSRIs=Selective Noradrenaline and Serotonin Reuptake Inhibitors; SSRIs=Selective Serotonin Reuptake Inhibitors.
We assessed age, sex, educational level (no formal education, primary studies, secondary studies, university studies), history as a psychiatric patient (in years), and type of psychoactive drugs currently taken. For evaluation purposes, medications were divided into the common groups of psychotropic drugs: antidepressants (tricyclic antidepressants, selective serotonin reuptake inhibitors–SSRIs–and serotonin and norepinephrine selective reuptake inhibitors–SNSRIs–), benzodiazepines, antipsychotic drugs (conventional and atypical), mood stabilizers, and anticholinergics. We also recorded how long patients had received psychiatric treatment (in months), the number of psychiatrists who had treated them during that time, and the number of psychiatric admissions, specifying whether they were voluntary or involuntary. Diagnoses and treatment information were obtained through the prescription sheet provided to all patients after their psychiatric consultation, which included the diagnosis and each prescription given.
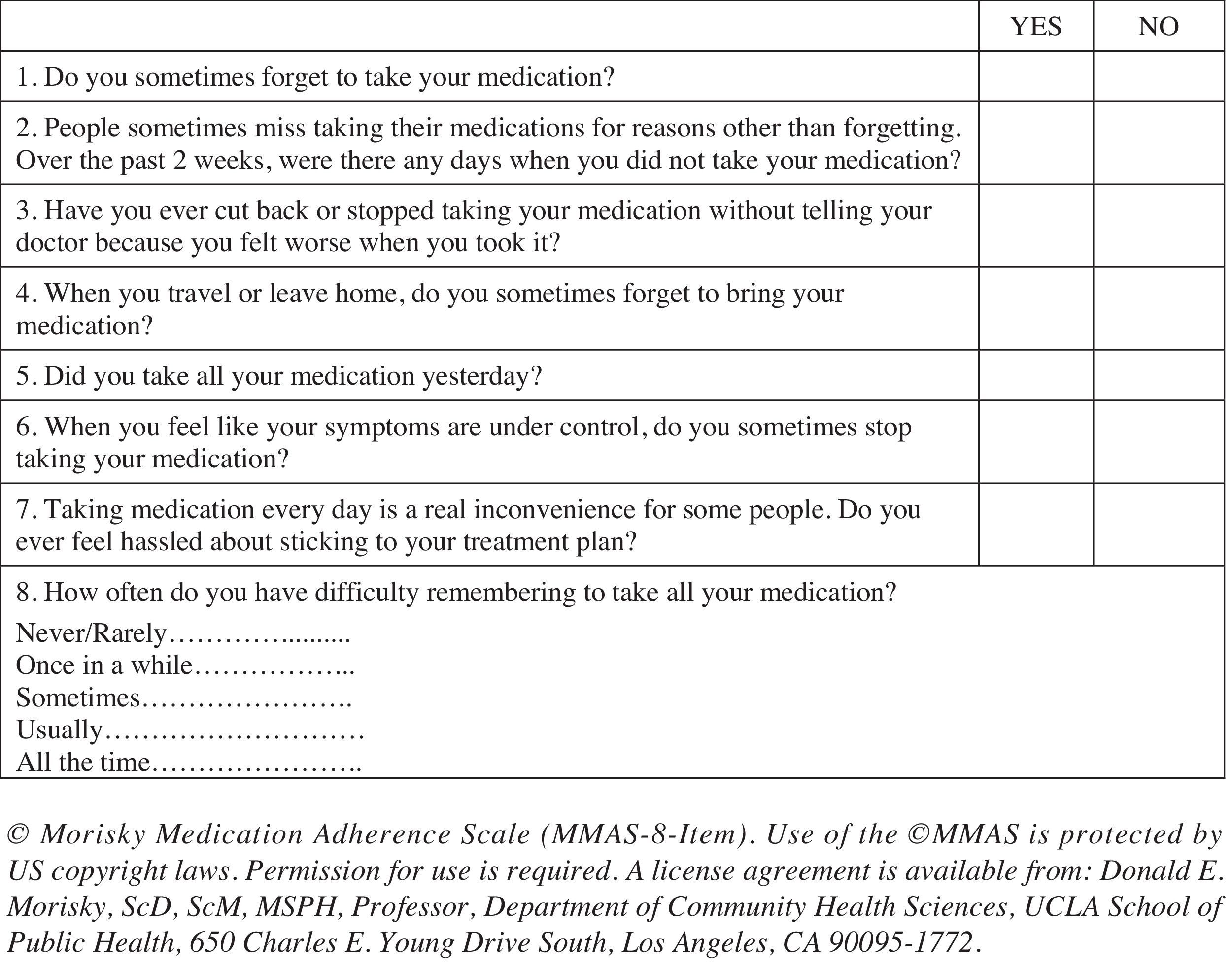
Medication adherence was tested using the Spanish version of the validated eight-item self-report Morisky Medication Adherence Scale (MMAS-8) (Morisky et al., 2008; Appendix 1). Questions are formulated to avoid a “yes-saying” bias (i.e., the wording of Item 5 is reversed to prevent the tendency to respond the same way to a series of questions regardless of their content). Response choices are “yes” or “no” for items 1 through 7 and Item 8 has a five-point Likert response scale. Each “no” response is rated as 1 and each “yes” response is rated as 0 except for item 5, in which each “yes” response is rated as 1 and each “no” response is rated as 0. For Item 8, the code (0-4) has to be standardized by dividing the result by 4 to calculate a summated score. Total scores on the MMAS-8 range from 0 to 8, with scores of 8 reflecting high adherence, 7 or 6 reflecting medium adherence, and <6 reflecting low adherence. Permission to use the scale was granted by Donald Morisky, the copyright holder of the instrument.
Four self-report questionnaires were used as validity criteria in the study: the Hong Psychological Reactance Scale (HPRS), Form C of the Multidimensional Health Locus of Control scale (MHLC-C), the General Self-Efficacy Scale (GSE), and the 10-item Drug Attitude Inventory (DAI-10).
The Hong Psychological Reactance Scale (Hong & Faedda, 1996) is a 14-item self-report questionnaire that was developed to measure individual differences in reactance proneness, that is, individuals’ trait propensity to experience psychological reactance. According to the concept of psychological reactance (Hong & Faedda, 1996), when an individual's freedom is threatened, the individual will be motivated to restore his or her perceived loss of freedom. Participants indicated the extent to which they endorsed each cognitive or affective statement on a five-point Likert scale (from 1=strongly disagree to 5=strongly agree). In our study we used the validated Spanish version of the scale (Cronbach's alpha: Affective Psychological Reactance= .76; Cognitive Psychological Reactance= .62) (De las Cuevas, Peñate, Betancort, & De Rivera, 2014).
Form C of the Multidimensional Health Locus of Control scale (MHLC-C; Wallston, Stein, & Smith, 1994) was used to assess patients’ perception about who or what controls their depression outcomes. The MHLC-C is an 18-item general purpose, condition-specific locus of control self-report scale that can easily be adapted for use with any medical or health-related condition to assess individuals’ beliefs on what influences their health. It is composed of four subscales: an internal locus of control subscale–Internality–and three external locus of control scales–Chance, Doctors, and Other (powerful) People–that measure control variables with regard to participants’ health. Each item includes a belief statement about the patient's medical condition with which she/he may agree or disagree through a six-point Likert scale ranging from strongly disagree (1) to strongly agree (6). We used the validated Spanish version of the scale (Cronbach's alpha: Internal=.67; Chance=.62; Doctors=.58; Other People=.41) (Doku-Ramírez, Fonseca-Parra, González-Gil, Gualdrón-Alba, & Cifuentes-Villalobos, 2012).
The General Perceived Self-Efficacy Scale (GPSE; Schwarzer & Jerusalem, 1995) is a ten-item self-report scale that measures general self-efficacy as a prospective and operative construct. In contrast with other scales that were designed to assess optimism, this scale explicitly refers to personal agency, that is, the belief that our own actions are responsible for successful outcomes. Each item is scored from 1 (not at all true) to 4 (completely true). The summary score ranges from 10 to 40, with highest scores indicating high self-efficacy. We used the validated Spanish version of the scale (Cronbach's alpha= .90) (Bäßler & Schwarzer, 1996; Sanjuán, Pérez, & Bermúdez, 2000).
The ten-item Drug Attitude Inventory (DAI-10; Hogan, Awad, & Eastwood, 1983) was developed to measure subjective responses and attitudes of psychiatric patients toward their treatment. It indicates whether patients are satisfied with their medication and evaluates their understanding of how the treatment is affecting them. The inventory has ten highly specific items of subjective experience presented as self-report statements with which the patient agrees or disagrees. Response options are true/false, and each response is scored as +1 if correct or -1 if incorrect. The final score is the grand total of positive and negative points. Total scores comprise values from -10 to 10, with higher scores indicating more positive attitudes toward medication. A positive total score means a positive subjective response while a negative total score means a negative subjective response. The DAI-10 has been found to be correlated with both clinician-rated adherence and biochemical measures of adherence (Nielsen, Lindström, Nielsen, & Levander, 2012). In this study, the DAI-10 scale was used as a validity criterion of adherence. We used the validated Spanish version of the scale (Cronbach's alpha= .67; Robles García, Salazar Alvarado, Páez Agraz, & Ramírez Barreto, 2004).
ProcedureFrom October 2013 to May 2014, 1,220 consecutive psychiatric outpatients who attended two Community Mental Health Centers on Tenerife Island (Canary Islands, Spain) were invited to participate in the study; of these, 967 accepted. 70% of patients who refuse to participate reported that they have no time to participate while 30% give no explanation about. Interviews were held in the waiting room previously to patients’ psychiatric consultation during a period of about 25minutes. Participants received a full explanation of the study, after which they signed an informed consent document that had been approved by the local ethics committee. Next, participants filled out a brief socio-demographic and clinical survey and completed the 8-item Morisky Medication Adherence Scale.
Data analysisVarious statistical analyses were conducted. Frequency analyses were used to describe the sample. To analyze the internal structure of MMAS-8, both exploratory and confirmatory factor analyses were performed. For external evidences, depending the nature of variables, different strategies were used: for continuous variables, Pearson correlation analyses were executed; and for dichotomous variables, ANOVAs and chi-square tests were used to conduct intergroup comparisons when needed.
ResultsA two-step analysis strategy was carried out to determine the factorial structure of MMAS-8. First, an exploratory factor analysis (EFA) was carried out with a random sample (50%). Second, a confirmatory factor analysis (CFA) was performed with the rest of the sample to test the structure found with EFA.
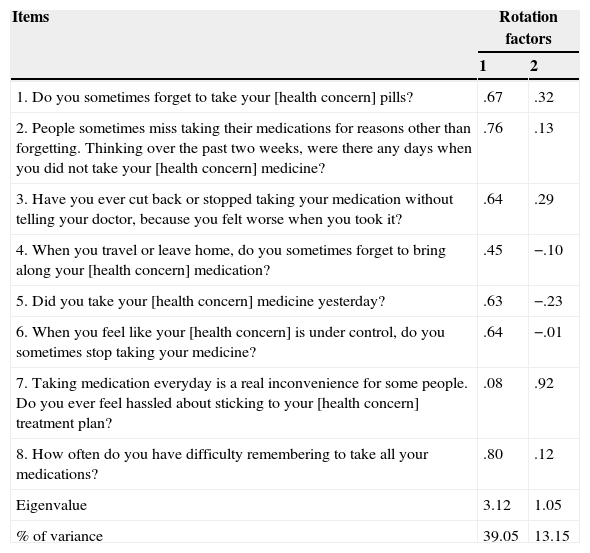
The items of the MMAS-8 were subjected to exploratory factor analysis. A principal component analysis was used to extract the factors of the measure. An oblique rotation method was selected because there were potential correlations between items measuring the same construct. The structure obtained a Kaiser-Meyer-Olkin coefficient of .83, with a χ2(28)=875.68, p=.000. These data indicated that it was possible to conduct the factor analysis. Table 2 summarizes the factor structure obtained.
Factor structure of the MMAS-8. Oblique (oblimin) rotation of the initial factor extraction using the principal component method. 50% random sample (n=480).
| Items | Rotation factors | |
|---|---|---|
| 1 | 2 | |
| 1. Do you sometimes forget to take your [health concern] pills? | .67 | .32 |
| 2. People sometimes miss taking their medications for reasons other than forgetting. Thinking over the past two weeks, were there any days when you did not take your [health concern] medicine? | .76 | .13 |
| 3. Have you ever cut back or stopped taking your medication without telling your doctor, because you felt worse when you took it? | .64 | .29 |
| 4. When you travel or leave home, do you sometimes forget to bring along your [health concern] medication? | .45 | −.10 |
| 5. Did you take your [health concern] medicine yesterday? | .63 | −.23 |
| 6. When you feel like your [health concern] is under control, do you sometimes stop taking your medicine? | .64 | −.01 |
| 7. Taking medication everyday is a real inconvenience for some people. Do you ever feel hassled about sticking to your [health concern] treatment plan? | .08 | .92 |
| 8. How often do you have difficulty remembering to take all your medications? | .80 | .12 |
| Eigenvalue | 3.12 | 1.05 |
| % of variance | 39.05 | 13.15 |
Results showed a two factor solution, but, as can be observed, results tend to a one-factor solution: using an item selection criterion of ≥.30 loading coefficients, only one item did not fall within the one-factor solution. It was Item 7, which dealt with an emotional aspect of adherence (i.e., feeling hassled about treatment schedule). Internal consistency (Cronbach's alpha) reached a level of .75. This coefficient increased slightly if Item 7 was deleted (α=.77), and the item-total correlation for this item was .16, which was highly significant (p=.000). The contribution of the various items to the total score on the MMAS-8 was always significant.
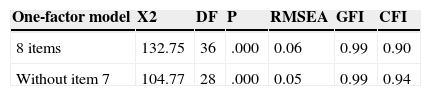
In this sense, it was tested, with a CFA strategy, two one-factor solutions: one with all the eight items, and one without item seven. For that, the rest of 50% random sample was used. The coefficients obtained are collected in Table 3. Both models achieved acceptable levels of adjust (except chi square), with better coefficients for a solution without item seven.
Confirmatory factor analyses (CFA) of MMAS-8, testing two models of one-factor solutions. 50% random sample (n=479).
| One-factor model | X2 | DF | P | RMSEA | GFI | CFI |
|---|---|---|---|---|---|---|
| 8 items | 132.75 | 36 | .000 | 0.06 | 0.99 | 0.90 |
| Without item 7 | 104.77 | 28 | .000 | 0.05 | 0.99 | 0.94 |
Note. χ2=Chi square; DF=Degree of freedom; p=probability; RMSEA=Root Mean Square Error of Approximation; GFI=Goodness of Fit Index; CFI=Comparative Fit Index.
To analyze the contribution of each item to the total score of the MMAS-8, we deleted each item one by one and compared the scores on the MMAS-8 of the three adherence groups: high adherence (HA), medium adherence (MA), and low adherence (LA). In all cases, deleting any items resulted in a significance decrease in the MMAS-8 total score. ANOVAs yielded the following coefficients when the following items were deleted: item 1, F (2, 948)=1179.65, p=.000; item 2, F (2, 948)=1743.15, p=.000; item 3, F (2, 948)=1415.92, p=.000; item 4, F (2, 948)=2050.68, p=.000; item 5, F (2, 948)=2128.71, p=.000; item 6, F (2, 948)=2046.32, p=.000; item 7, F (2, 948)=1289.34, p=.000; and item 8, F (2, 948)=1832.88, p=.000.
Considering attitudes toward medication (DAI-10 scale) as an adherence-to-treatment criterion, correlations with the items of the MMAS-8 and the total score were as follows: .12 with item 1; .17 with item 2; .22 with item 3; .09 with item 4; .16 with item 5; .18 with item 6; .25 with item 7; .25 with item 8; and .30 with the total score. All these coefficients reached statistical significance (p=.01). The ANOVA included only 949 patients since 18 patients did not complete the DAI-10 as they were not currently taking any psychiatric drug.
In addition, to test whether the three adherence groups according to the MMAS-8 scored significantly differently in attitudes toward medication, we performed an ANOVA. Results revealed that 236 patients were classified into the HA group, with a mean score (M) in the DAI-10=5.19 (standard deviation, SD=3.77); 444 were classified into the MA group, M=3.6 (SD=3.79); and 269 were classified into the LA group, M=1.96 (SD=4.38). The coefficients obtained reached statistical significance (F (2, 948)=41.7; p=.000), with a moderate effect size (η2=.081); patients with greater adherence obtained higher scores in positive attitude to drugs. Anyway, cautions must be taken because DAI total score had not a normal distribution (Kolmogorov-Smirnov's z=4.48, p=.000).
To assess external evidences of validity, the MMAS-8 was compared to various socio-demographic variables, such as gender, age, educational level, treatment duration (in months), treatment complexity (i.e., number of different drugs prescribed), and diagnosis group. No differences were obtained in the MMAS-8 total score as a function of gender [F (2, 948)=2.07) or adherence group (i.e., high, medium, low): χ2 (2)=3.99)]. However, a significant correlation was found between the scale and age (rxy=.19; p=.000): adherence increased with patient age. No significant correlations were found as a function of educational level (rxy=.04), treatment complexity (rxy=-.01), or treatment duration (rxy=.05). Yet, when the statistical analysis was conducted considering the level of adherence, we found significant differences between the three groups as a function of treatment duration (F (2, 948)=4.75, p=.003; η2=.01). Bonferroni tests revealed that patients with high adherence (M=118.65 months, SD=112.16) and patients with medium adherence (M=121.17, SD=120.72) underwent longer treatments than those with low adherence (M 95.54, SD=94.09). However, the high standard deviation indicates that these data should be taken with caution, as they show an irregular distribution of scores.
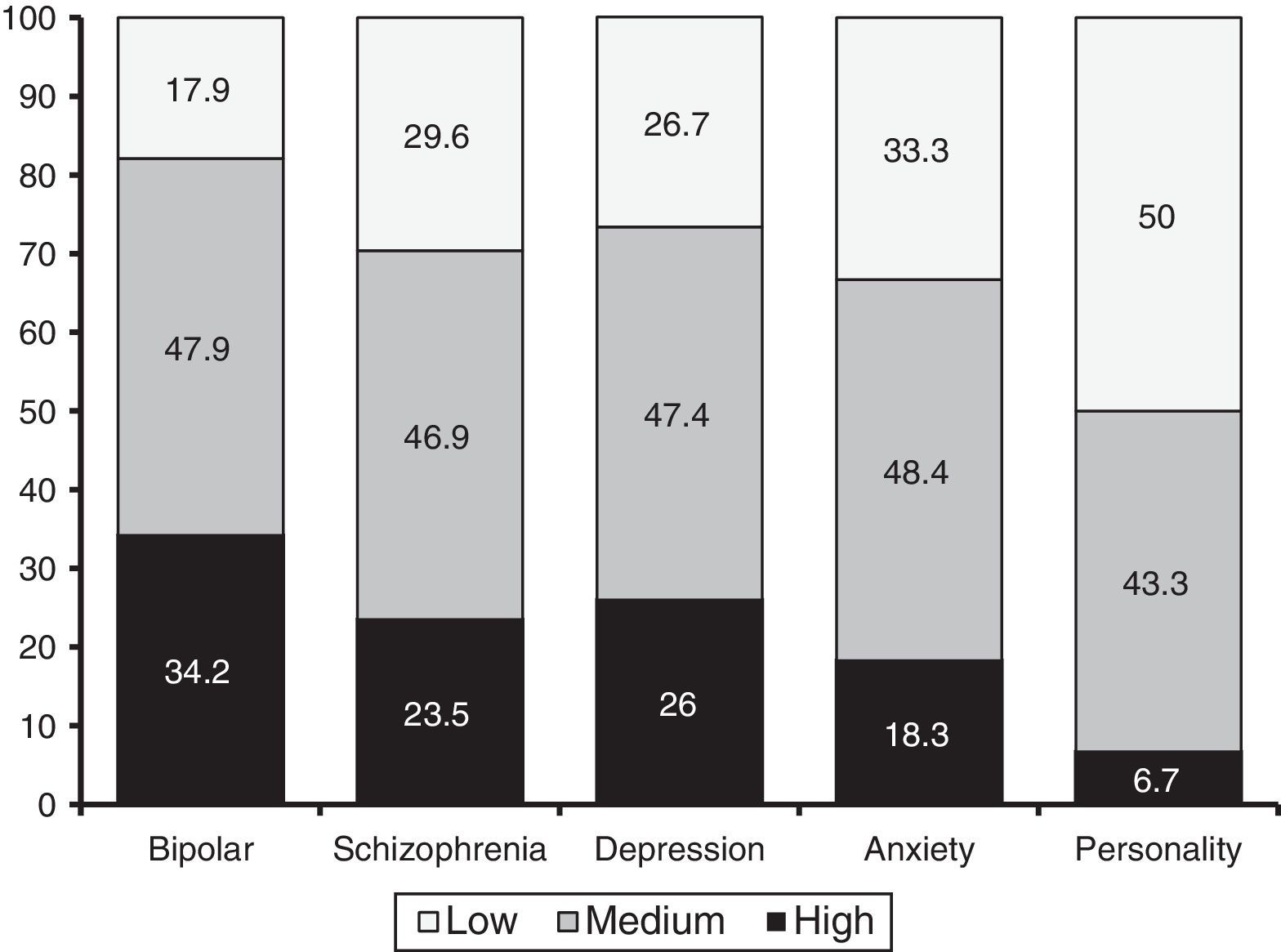
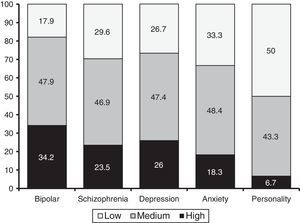
As regards diagnosis groups, an ANOVA revealed that the MMAS-8 was able to differentiate between the five major groups of disorders (i.e., schizophrenia, bipolar disorder, depression, anxiety, and personality disorders): F (4, 932)=6.35; p=.000; η2=.027). According to the Bonferroni test, patients with bipolar disorder exhibited the highest adherence (M=6.8, SD=1.42). This score was significant higher than that of patients with personality disorders (M=5.35, SD=1.58) and anxiety disorders (M=6.13, SD=1.7). In addition, patients with schizophrenia (M=6.41, SD=1.42) and patients with depressive disorders (M=6.36, SD=1.62) scored higher than patients with personality disorders. Figure 1 shows the percentages of patients (per diagnosis) with high, medium and low levels of adherence. Such percentages were statistically significant (χ2 (8)=22.77, p=.004).
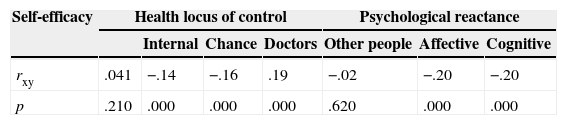
Finally, we performed correlations between the MMAS-8 and three different psychological processes that may affect adherence: level of self-efficacy, health locus of control, and psychological reactance. Table 3 summarizes the coefficients obtained.
Although the MMAS-8 seemed to be independent of self-efficacy, it exhibited correlations with health locus of control and psychological reactance. According to health locus of control, adherence was positively correlated with patients’ confidence in their doctors and negatively correlated with patients’ belief that their health depends on chance. Adherence was negatively correlated with patients’ belief that their health depends on themselves. Psychological reactance was negatively correlated with adherence, which decreased as reactance–both affective and cognitive–increased.
DiscussionTo our knowledge, this is the first study that evaluated the psychometric properties of MMAS-8 among psychiatric patients. The original MMAS-8 was tested by Morisky et al. (2008) on a sample of hypertensive patients, and it was found that the scale was reliable with good predictive validity and sensitivity. Other studies had evaluate the eight-item MMAS in hypertensive patients (De Oliveira-Filho, Moriski, Neves, Costa, & De Lyra, 2014; Hacıhasanoğlu-Aşılar, Gözüm, Capık & Morisky, 2014; Korb-Savoldelli et al., 2012), in patients taking warfarin (Wang, Kong, & Ko, 2012), in myocardial infarction patients (Yan et al., 2014), in diabetes patients (Sakthong, Chabunthom, & Charoevisuthiwongs, 2009), in HIV-positive patients (Södergârd et al., 2006), and in patients with Parkinson's disease (Fabbrini et al., 2013). Most of these studies had shown satisfactory psychometric properties, with good convergent validity with good test–retest reliability and with acceptable sensitivity and specifity (Table 4).
Correlation coefficients between total scores on the MMAS-8 and three psychological processes: self-efficacy, health locus of control, and psychological reactance.
| Self-efficacy | Health locus of control | Psychological reactance | |||||
|---|---|---|---|---|---|---|---|
| Internal | Chance | Doctors | Other people | Affective | Cognitive | ||
| rxy | .041 | −.14 | −.16 | .19 | −.02 | −.20 | −.20 |
| p | .210 | .000 | .000 | .000 | .620 | .000 | .000 |
Note. rxy=Pearson correlation coefficient; p=probability.
The main objective of this paper was to report the psychometric properties of the Spanish version of the MMAS-8 in a sample of outpatients with psychiatric disorders. The MMAS-8 scale is relatively simple and practical to use in mental health clinical settings. Leaving aside the problems inherent to Likert-type scales, which can confound some results (i.e., Hartley, 2014), the MMAS-8 scale showed adequate construct validity, with a clear trend toward a one-factor solution; all the items contributed to the final index of adherence. However, Item 7, the only item related to emotions about drug adherence (instead of behavioral adherence) did not clearly fall within the one-factor structure, perhaps because it is more related to attitudes toward medication than to behaviors directly related to adherence. More specific analyses should be conducted about this.
As regards criterion validity, the scale showed significant correlations with positive attitudes of patients toward their treatment, revealing that as patients become more satisfied with their medication they start to better understand how the treatment is helping them and increase their adherence. Differential validity showed an increase in adherence as patients aged, and patients exhibited a higher adherence to longer treatments. This latter fact should be understood as a post hoc effect when evaluating intentional groups. In other words, we did not assess a sample over time and note that treatment adherence decreased over time; instead, we used a cross-sectional study to evaluate patients receiving medical treatment who continued attending consultations to follow their treatment. This is particularly clear in patients with the most chronic disorders: bipolar disorder and schizophrenia, who realize that regular attendance to consultations is a way of better managing their disease.
We did not observe any relationships between gender, education level or complexity of treatment and non-adherence in our sample, probably as a result of the particular characteristics of the type of sample studied. Moreover, the MMAS-8 was able to differentiate between the different mental disorder diagnosis groups. As regards convergent validity, locus of control exhibited distinctive correlation patterns with adherence and psychological reactance was negatively correlated with adherence. Results confirmed that psychiatric outpatients with higher psychological reactance (both affective and cognitive) were more likely to be non-adherent than patients with lower levels of psychological reactance. Individuals with low reactance generally follow instructions and advice, while individuals with high reactance frequently confront any guidance or assistance. People with high psychological reactance typically tend to focus on their own resources, personal decisions and initiatives (i.e., internal attribution of change), while people with low psychological reactance frequently seek external help and support to achieve their goals (i.e., external attribution of change).
Our study has some limitations related to the methodology used that should be noted. First, although a high rate of participation was recorded in our study, our results may be affected by a selection bias. Specifically, there may be differences in psychological features between individuals who agreed to participate in the study and those who did not. Second, our study included a convenience sample of consecutive psychiatric outpatients attending community mental health centers and therefore may not be representative of the entire population of psychiatric patients. Third, all the questionnaires applied were self-reports, which carries a potential risk of misstatement or response biases. A further limitation is the fact that we only used one method to estimate adherence. It should be noted that many direct and indirect methods are currently available for measuring adherence. Each method has advantages and disadvantages, and no method is considered to be the gold standard.
The present study advances the evidence of the validity of the Spanish version of MMAS-8 in psychiatric outpatients and broadens previous research demonstrating the internal reliability and predictive validity of the MMAS-8 with regard to psychiatric drug treatment adherence. This eight-item self-report questionnaire is simple and can be included into routine psychiatric clinical practice. It is likely to be useful to identify patients at risk for medication adherence issues and specifically low adherence in outpatient psychiatric care. Further studies should focus on testing other psychometric properties of the instrument and warrant better assess medication-taking behaviors in different population and settings.
Available online 25 December 2014