Environmental factors such as psychosocial stress have demonstrated to have an impact on the breast cancer (BC) course. This study aims to explore the impact of psychotherapy and stressful life events (SLE) on BC survivors’ illness trajectories.
Method68 women with BC underwent Positive Psychotherapy or Cognitive-Behavioral Stress Management and 37 patients were included as a control group. The effects of distress reduction and SLE on their 5-year recurrence were investigated. Additional analyses examined the effect of receiving vs. not receiving psychotherapy and of the type of therapy on survival and disease-free interval, DFI.
ResultsA one-point decrease of the Hospital Anxiety and Depression Scale (HADS) after psychotherapy predicted a lower risk of 5-year recurrence, OR = 0.84, p = .037, 95% CI = 0.71-0.99). Also, a one point-increase in the number threatening SLE (OR = 1.92; p = .028, 95% CI = 1.07-3.43) was related to higher 5-year recurrence.
ConclusionsThe findings highlight the necessity of studying not only a given situation (i.e., psychotherapy, SLE) but its specific impact on individuals.
El estrés psicosocial ha demostrado tener un impacto en la evolución del cáncer de mama (CM). Este estudio tiene como objetivo explorar el impacto de la psicoterapia y de los acontecimientos vitales estresantes (AVE) en la supervivencia de pacientes con CM.
Método113 mujeres con CM recibieron psicoterapia positiva o terapia cognitivo-conductual para manejar el estrés y 37 se incluyeron como grupo control. Se analizaron los efectos de la reducción de la Escala de Ansiedad y Depresión Hospitalaria (HADS) y de los AVE sobre la recurrencia a los cinco años, así como el efecto de recibir psicoterapia y del tipo de enfoque d esta sobre la supervivencia.
ResultadosLa reducción de un punto en la HADS después de recibir psicoterapia predijo un menor riesgo de recurrencia, OR = 0,84, p = 0,037, IC 95% = 0,71-0,99. Además, cada aumento en el número de AVE vividos como amenazantes (OR = 1,92; p = 0,028, 95% CI = 1,07-3,43) se relacionó con una mayor recurrencia.
ConclusionesLos resultados indican la necesidad de estudiar no solo la presencia de un evento potencialmente impactante en la conducta (psicoterapia o AVE) sino el efecto especifico que ha tenido en cada individuo.
Breast cancer (BC) is the most common oncological diagnosis among women worldwide, affecting 2 million annually and presenting the highest death rate (Bray et al., 2018). Such mortality, which has increased over the past 25 years (Azamjah et al., 2019), is largely accounted for by the impact of distant metastases appearing as a recurrence of a prior episode (Liang et al., 2020).
Survival analyses are widely used in oncology to study cancer recurrence, metastases, or death. Hence, prognosis can be assessed in terms of overall survival, or survival after a given period (usually 5 years). The disease-free interval (DFI) is defined as the time between primary treatment completion and the detection of a cancer reappearance. The most powerful survival prognostic factors are those intrinsically related to the disease (grade, the tumor size, axillary nodal involvement, and negative estrogen and progesterone receptors, Lafourcade et al., 2018), followed by social factors (Dean et al., 2018) and environmental events. In this line, recent studies have investigated the negative impact of the exposure to stressful life events (SLE) on patients’ quality of life and even on cancer incidence and progression (Fagundes et al., 2017). In fact, a connection has been established between adverse childhood experiences (ACE) and BC (Holman et al., 2016), with evidence suggesting that these associations may be mediated by alterations in the immune system, such as lower interleukin-2 (IL-2) levels. Also, Crosswell et al. (2014) assessed a possible relationship between ACE and elevated inflammation (a predictor of mortality) in BC survivors, and found a positive association with different types of adversity, such as abuse, neglect and unstructured home environments.
Given the association between the aforementioned stressors and cancer, the potential effect of different psychotherapies in the cancer experience, evolution and recovery have been made (Clark et al., 2021; de la Torre-Luque et al., 2016; Ichikura et al., 2020; James et al., 2018; Lengacher et al., 2019). It has been reported that offering a psychosocial intervention during primary oncological treatment may improve the general health status of cancer patients (Chen & Ahmad, 2018) and influence long-term illness outcomes of BC patients by increasing their DFI (Andersen et al., 2008). The results from a recent meta-analysis showed that psychosocial interventions delivered early in the disease course (non-metastatic) were associated with a reduction of mortality in 41% of cases (Oh et al., 2016).
Among the evidence-based psychological treatments that help to assimilate the experience of cancer, a prominent example is cognitive behavioral stress management (CBSM; Antoni, 2004). This approach has shown to increase relaxation and positive affect and to decrease serum cortisol, anxiety, depression, thought avoidance and negative mood (Tang et al., 2020). Stress reduction through cognitive behavioral techniques is especially important during peri-traumatic periods, when the cancer threat is still present. In fact, a recent study (Wang et al., 2018) has shown that CBSM achieved greater reduction of posttraumatic stress symptoms in BC patients with higher initial cancer-specific distress.
Considering cancer as a long-lasting threat, post-traumatic stress and suffering may develop into a more global, sustained and existential distress, which usually appears (or remains) after primary cancer treatment termination (Martínez Arroyo et al., 2019). Hence, stress and distress in these future stages have been associated less with coping with the threat of cancer and more with accommodating cancer experience in one's psychosocial identity and the return to a new life (Ochoa-Arnedo et al., 2021). Positive vital changes, so called post-traumatic growth, appear with the positive accommodation and enhancement of these basic beliefs and worldview, with a resulting improved level of adaptation compared with pre-cancer life. Positive psychotherapy for cancer (PPC; Meibodi et al., 2021; Ochoa-Arnedo & Casellas-Grau, 2015) is one of the few psychological therapies that facilitate post-traumatic growth during this accommodation stage after primary oncological treatments (Ochoa et al., 2017). Interestingly, a recent review and meta-analysis of positive psychological interventions (Bolier et al., 2013; Chakhssi et al., 2018) has reported significant effects in promoting adaptive functioning and reducing distress in psychiatric and general populations.
Notwithstanding the extent research summarized herein, the effects of sustained distress/stress reduction in recurrence and DFI during midterm survivorship –after the end of oncological treatments–, have not been tested yet. Recently, in a randomized controlled trial (RCT) (Ochoa-Arnedo et al., 2021), we reported that PPC was more effective in reducing post-traumatic stress symptoms and distress in cancer survivors than CBSM implemented as the Breast Cancer Stress Management and Relaxation Training (B-SMART) program (Antoni, 2004). The present study is a 5-year follow up of those patients that aim to analyze the effect of the two aforementioned evidence-based psychological therapies and of SLE on cancer survival. Considering all the above, we hypothesize that for BC patients who completed their primary cancer treatment: (1) distress reduction after psychotherapy will lead to a lower risk of recurrence at 5 years, (2) the presence of SLE will be related with a higher probability of experiencing a recurrence at 5 years, and (3) receiving psychological therapy, independently of the type, will decrease the cancer recurrence and increase the DFI.
MethodParticipantsSpecific information regarding participants recruitment and inclusion/exclusion criteria can be found elsewhere (Ochoa-Arnedo et al., 2021). Still, we provide the main inclusion criteria for its relevance to the present study: (a) age >18; (b) diagnosis of a single primary cancer; (c) having completed their primary oncological treatment (i.e., surgery, chemotherapy, or radiotherapy); (d) score ≥10 on the Hospital Anxiety and Depression Scale, HADS; Zigmond & Snaith, 1983) Spanish version (Ouintana et al., 2003); and (e) being capable to understand and read Spanish. For the present study, only those participants who had available and updated follow-up information of cancer recurrence, DFI and date of the last follow-up at 5 years were retained. Patients who reported any prior cancer occurrences, any prior or current severe mental disorders (hospitalization or a formal diagnosis of psychosis, suicidal behavior, or substance dependency), or any major concurrent illnesses seriously affecting their cognitive performance (e.g., neurological disorders) were excluded.
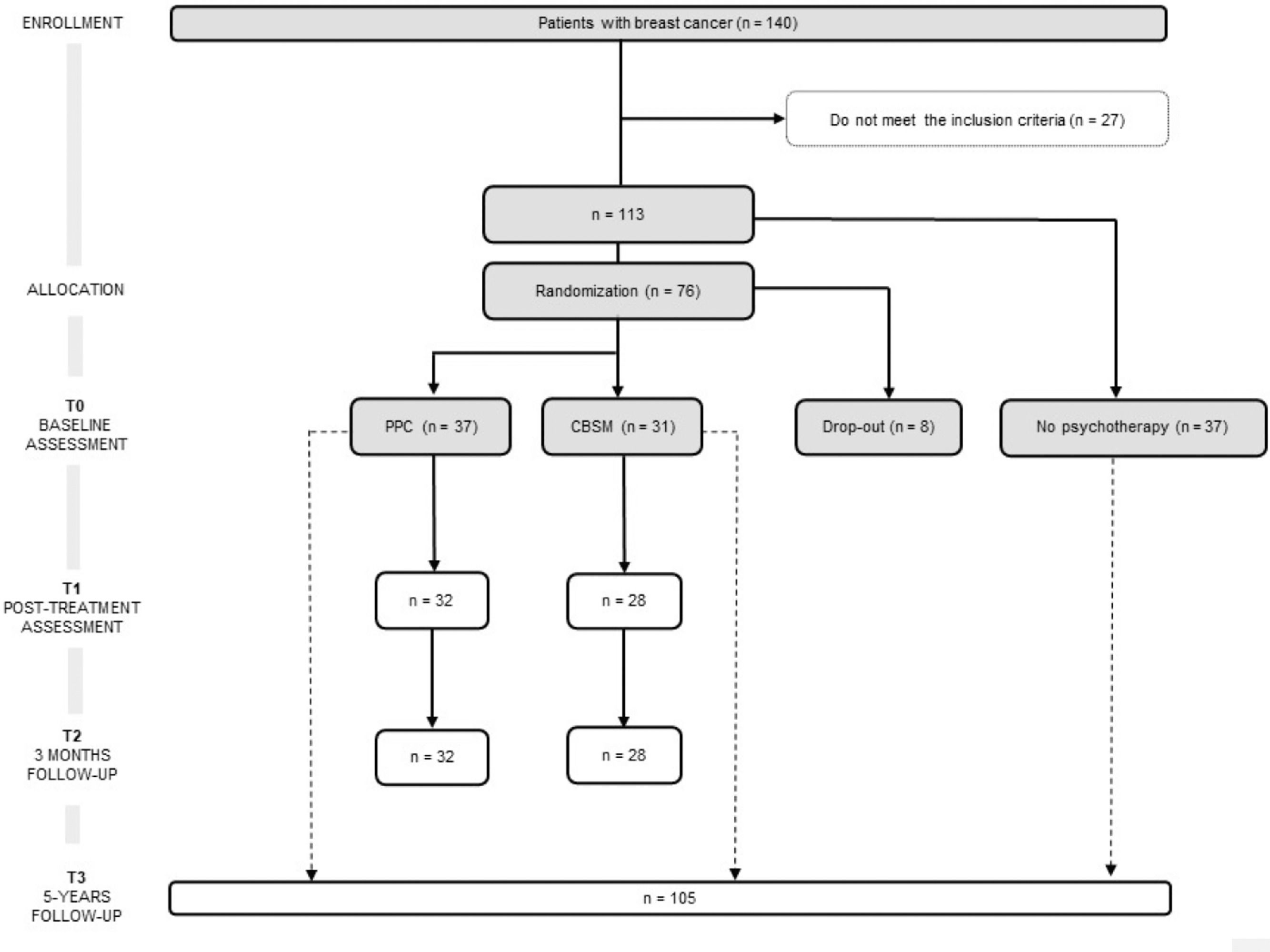
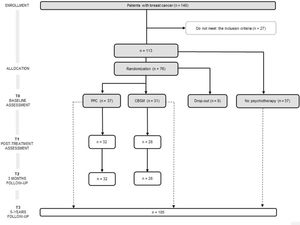
From the 196 patients recruited in the previous RCT (Ochoa-Arnedo et al., 2021), 140 agreed to participate. Of such, 113 (mean age = 50.44, SD = 9.88) complied the inclusion criteria. Among these, 37 participants were not available to undergo psychotherapy and were followed-up as Non-Psychotherapy Group (NP), registering their recurrence and DFI. In turn, 8 participants opted out from the study. The rest of participants (N = 68) were randomly assigned to one of the two psychotherapies: PPC (n = 37) or CBSM (n = 31). Participants’ CONSORT diagram is shown in Figure 1. Some patients (n = 8) did not fullfeed T1 and T2 follow-up assessments. Information regarding recurrences and DFI at 5 years were collected for 105 participants.
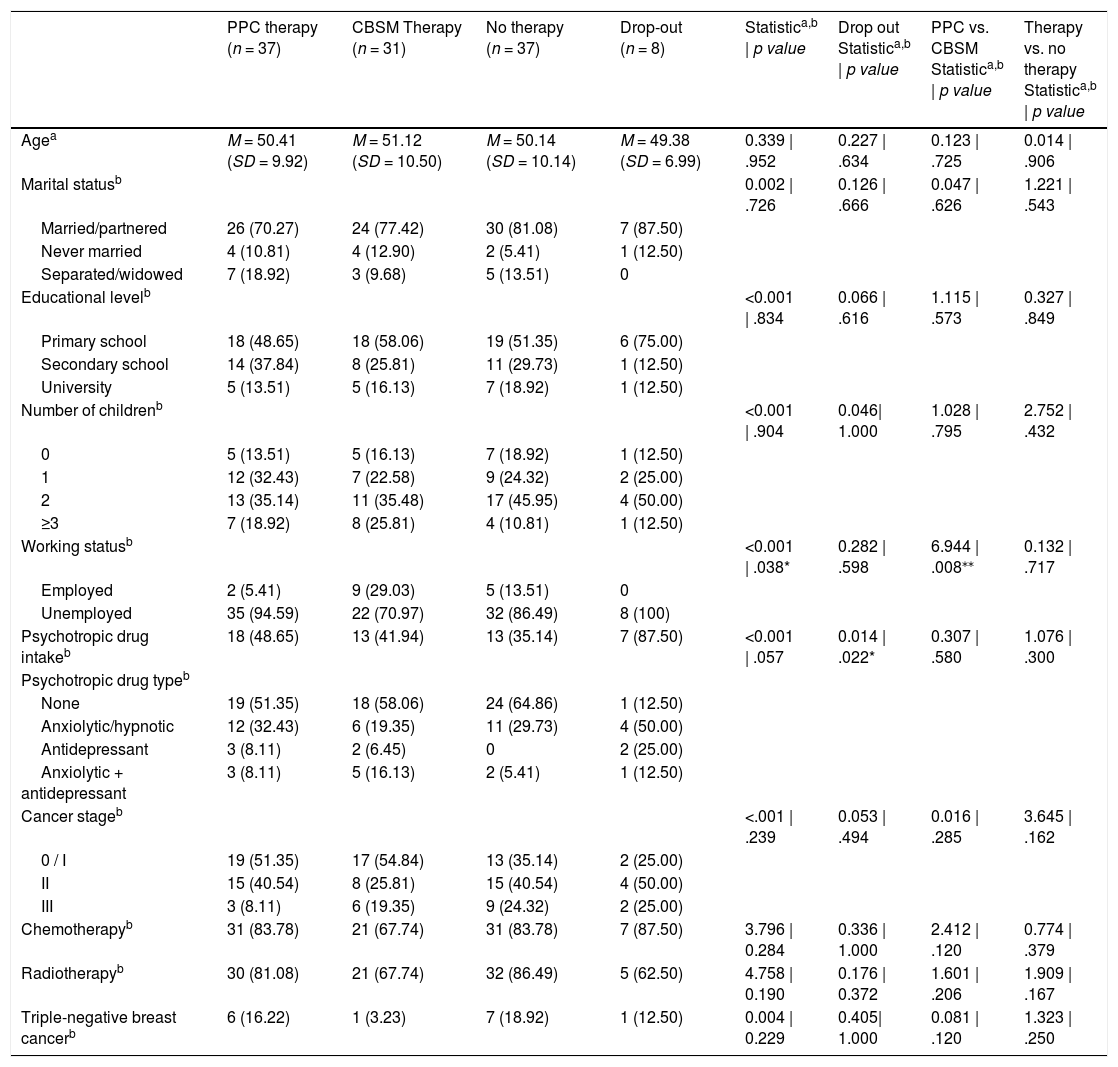
Sociodemographic and medical characteristics of the sample are summarized in Table 1 for each study group: PPC, CBSM and NP group. Drop-out participants (started psychotherapy but did not reach sufficient sessions to be included) are also described in Table 1. All participants were Hispanic or Latino Americans women with residence in Spain.
Participants characteristics.
| PPC therapy (n = 37) | CBSM Therapy (n = 31) | No therapy (n = 37) | Drop-out (n = 8) | Statistica,b | p value | Drop out Statistica,b | p value | PPC vs. CBSM Statistica,b | p value | Therapy vs. no therapy Statistica,b | p value | |
|---|---|---|---|---|---|---|---|---|
| Agea | M = 50.41 (SD = 9.92) | M = 51.12 (SD = 10.50) | M = 50.14 (SD = 10.14) | M = 49.38 (SD = 6.99) | 0.339 | .952 | 0.227 | .634 | 0.123 | .725 | 0.014 | .906 |
| Marital statusb | 0.002 | .726 | 0.126 | .666 | 0.047 | .626 | 1.221 | .543 | ||||
| Married/partnered | 26 (70.27) | 24 (77.42) | 30 (81.08) | 7 (87.50) | ||||
| Never married | 4 (10.81) | 4 (12.90) | 2 (5.41) | 1 (12.50) | ||||
| Separated/widowed | 7 (18.92) | 3 (9.68) | 5 (13.51) | 0 | ||||
| Educational levelb | <0.001 | .834 | 0.066 | .616 | 1.115 | .573 | 0.327 | .849 | ||||
| Primary school | 18 (48.65) | 18 (58.06) | 19 (51.35) | 6 (75.00) | ||||
| Secondary school | 14 (37.84) | 8 (25.81) | 11 (29.73) | 1 (12.50) | ||||
| University | 5 (13.51) | 5 (16.13) | 7 (18.92) | 1 (12.50) | ||||
| Number of childrenb | <0.001 | .904 | 0.046| 1.000 | 1.028 | .795 | 2.752 | .432 | ||||
| 0 | 5 (13.51) | 5 (16.13) | 7 (18.92) | 1 (12.50) | ||||
| 1 | 12 (32.43) | 7 (22.58) | 9 (24.32) | 2 (25.00) | ||||
| 2 | 13 (35.14) | 11 (35.48) | 17 (45.95) | 4 (50.00) | ||||
| ≥3 | 7 (18.92) | 8 (25.81) | 4 (10.81) | 1 (12.50) | ||||
| Working statusb | <0.001 | .038* | 0.282 | .598 | 6.944 | .008⁎⁎ | 0.132 | .717 | ||||
| Employed | 2 (5.41) | 9 (29.03) | 5 (13.51) | 0 | ||||
| Unemployed | 35 (94.59) | 22 (70.97) | 32 (86.49) | 8 (100) | ||||
| Psychotropic drug intakeb | 18 (48.65) | 13 (41.94) | 13 (35.14) | 7 (87.50) | <0.001 | .057 | 0.014 | .022* | 0.307 | .580 | 1.076 | .300 |
| Psychotropic drug typeb | ||||||||
| None | 19 (51.35) | 18 (58.06) | 24 (64.86) | 1 (12.50) | ||||
| Anxiolytic/hypnotic | 12 (32.43) | 6 (19.35) | 11 (29.73) | 4 (50.00) | ||||
| Antidepressant | 3 (8.11) | 2 (6.45) | 0 | 2 (25.00) | ||||
| Anxiolytic + antidepressant | 3 (8.11) | 5 (16.13) | 2 (5.41) | 1 (12.50) | ||||
| Cancer stageb | <.001 | .239 | 0.053 | .494 | 0.016 | .285 | 3.645 | .162 | ||||
| 0 / I | 19 (51.35) | 17 (54.84) | 13 (35.14) | 2 (25.00) | ||||
| II | 15 (40.54) | 8 (25.81) | 15 (40.54) | 4 (50.00) | ||||
| III | 3 (8.11) | 6 (19.35) | 9 (24.32) | 2 (25.00) | ||||
| Chemotherapyb | 31 (83.78) | 21 (67.74) | 31 (83.78) | 7 (87.50) | 3.796 | 0.284 | 0.336 | 1.000 | 2.412 | .120 | 0.774 | .379 |
| Radiotherapyb | 30 (81.08) | 21 (67.74) | 32 (86.49) | 5 (62.50) | 4.758 | 0.190 | 0.176 | 0.372 | 1.601 | .206 | 1.909 | .167 |
| Triple-negative breast cancerb | 6 (16.22) | 1 (3.23) | 7 (18.92) | 1 (12.50) | 0.004 | 0.229 | 0.405| 1.000 | 0.081 | .120 | 1.323 | .250 |
Note: Values are percentages with ns in parentheses unless otherwise indicated. PPC = positive psychotherapy for cancer; CBSM = Cognitive Behavioral Stress Management.
The study was approved by the Ethics Committee of the leading institution and recruiting center (Institut Català d'Oncologia). All participants gave their written consent after being informed about the study aims and procedures in accordance with the 2008 version of the Declaration of Helsinki.
MeasuresHospital Anxiety and Depression Scale (HADS). Measures anxiety and depression in people with physical illnesses (Zigmond & Snaith, 1983), and it has been used to assess mood in cancer patients with its overall score interpreted as psychological distress. There are seven items for both anxiety and depression, with total scores ranging from 0 to 21. Items are rated on a 4-point severity scale, and each question is scored between 0 (no impairment) and 3 (severe impairment). The tool was validated in a Spanish oncological sample (Requena et al., 2009), finding good internal reliability for both subscales (Cronbach's α of .82 and .84 for anxiety and depression respectively).
The Stressful Life Events Inventory (SLEI; Pérez-Sales et al., 2012). Collects information about the number and impact (threat and influence on one's lifetime trajectory) of 34 extreme life experiences, mostly related to trauma, loss, and crisis that participants may have experienced through their life. Although all patients had in common the cancer experience, the subjective experience of threat and the influence on one's life experience may vary significantly. Also, other extreme vital experiences were also collected. SLEI indexes were total number of extreme experiences, number of threat experiences (extreme or severe), number of extreme experiences with high influence in their life, number of past positive experiences and total number of positive experiences lived with an extreme influence on life.
InterventionsCognitive-Behavioural Stress Management (CBSM) is based on the Breast B-SMART program (Antoni et al., 2001), which focuses on facilitating the control and assimilation of stress. Although the original design consisted of 10 sessions, during its adaptation to Spanish population its implementation was more accepted by patients and therapists when performed over 12 sessions (Ochoa‐Arnedo et al., 2006). Therefore, in the present study the program consisted of 12 weekly, 90-minute sessions. Each group comprised 8–12 disease-free patients after completing their primary cancer treatments.
Positive Psychotherapy for Cancer (PPC) aims to facilitate post-stress growth through psychotherapeutic methods associated with the development of positive life changes after cancer. Sessions are conducted in group and spread across four modules. This therapy is also delivered weekly, over 12 90-minute sessions, and with groups of the same size. The PPC program is manualized, and the guide is available in Spanish (Ochoa et al., 2010) and English (Ochoa-Arnedo & Casellas-Grau, 2015).
ProcedureParticipants, recruited from Institut Català d'Oncologia (ICO), were allocated to either PPC or CBSM in a two-step block randomization procedure (see Figure 1). Both, participants and psychotherapists, were aware of the allocated arm, while data managers and assessors were blind, as it is recommended for trials of psychological interventions (Guidi et al., 2018). The interventions were led by clinical psychologists who had been trained in the administration of CBSM and PPC, while their performance was supervised by two experts in the application of the techniques (Antoni, 2004; Ochoa et al., 2010). Treatment integrity was assessed by the two supervisors, either via closed-circuit television cameras or by videotaping a random selection of 25% of the sessions in each group. Therapy adherence was registered in an ad-hoc questionnaire adapted from the revised Cognitive Therapy Scale (Blackburn et al., 2001).
Participants from the therapy groups were assessed at baseline (T0), immediately after psychological treatment (T1) and at 3-month (T2). After 5 years (T3), their recurrence and DFI were registered. Participants from the Non-Psychotherapy group were assessed at T0 and at T3.
Statistical analysesAll statistical analyses were conducted using Stata 14.0 (StataCorp, 2009). Sociodemographic and medical differences between groups (i.e., receiving therapy versus not receiving therapy, and specific type of therapy) were compared using Kruskal Wallis (given the non-normality observed in data distribution) and chi-squared tests, as appropriate. Categorical variables were compared using Fisher exact test when sample sizes were small. DFI was calculated from the day of primary oncological treatment completion to the day when the recurrence was confirmed, or alternatively, to the last day of the 5-year survival period studied when the person stayed healthy, and no recurrence was informed.
Logistic regression models were used to: (1) evaluate the effect of changes in HADS score before and after psychotherapy on the risk of 5-year recurrence and (2) explore the effect of different SLE on the risk of 5-year recurrence,
All variables included in Table 1 were examined as potential confounding variables and were introduced in the main analysis if a) the specific group comparison was statistically significant or b) if the variable was demonstrated to have an impact on patient survival in previous literature (i.e., age, cancer stage and oncological treatment type). Logistic regression models were used to (3) measure the magnitude of the association between receiving therapy (therapy vs. no therapy) and the risk of 5-year recurrence, and (4) explore the effects of the type of therapy on the risk of 5-year recurrences.
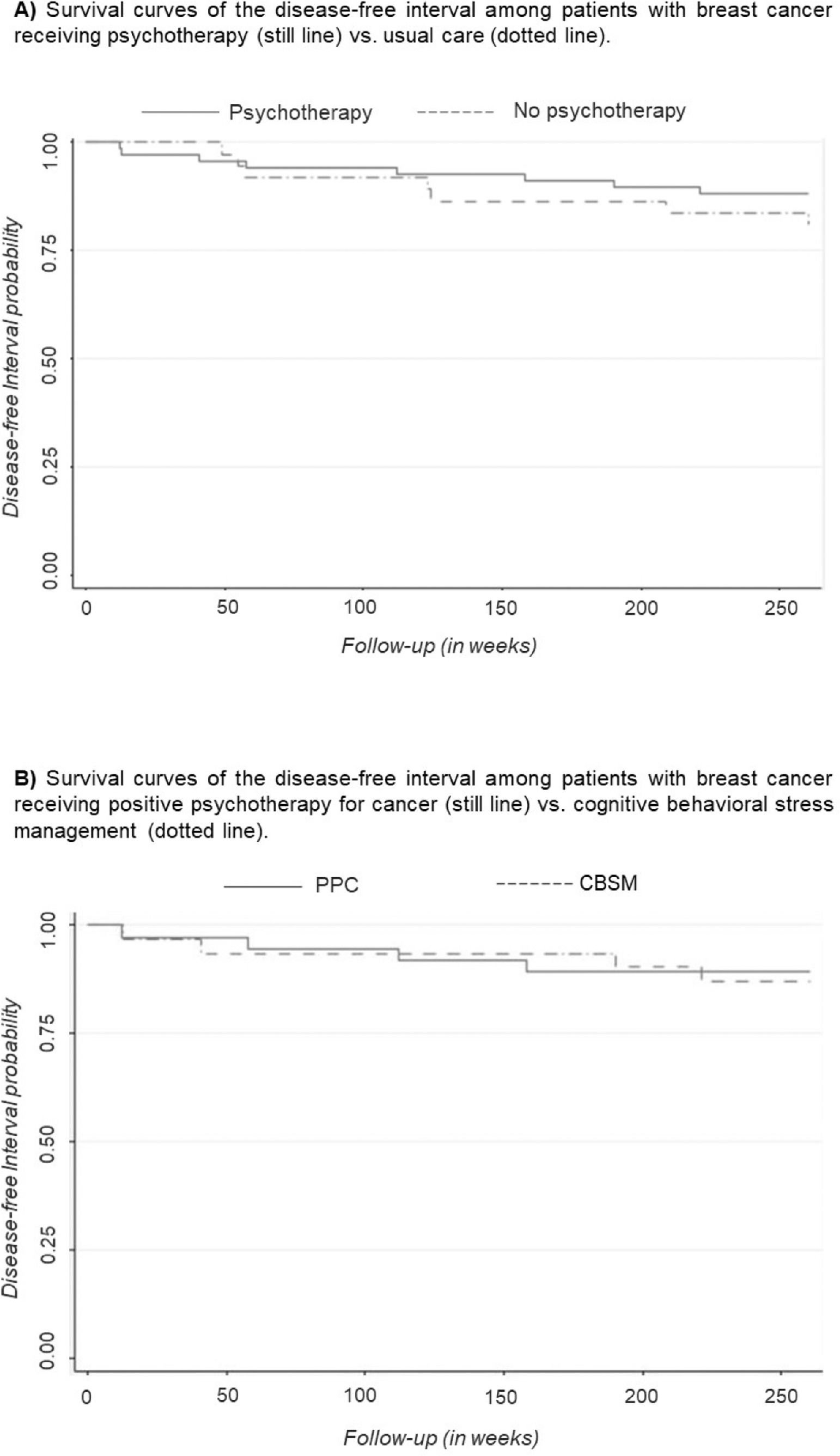
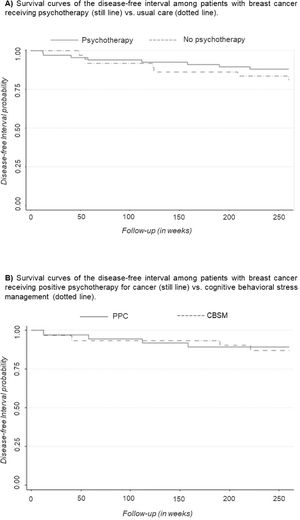
Kaplan Meier plots were used to visualize 5-year DFI curves and log-rank tests were performed to compare these curves between therapy and non-therapy groups and the type of therapy received.
Statistical significance was assumed at a p-value <.05 and Odds Ratio (OR) and 95% Confidence Interval (CI) were presented in both main and additional analyses.
ResultsComparisons revealed no significant baseline (T0) differences between groups except for working status, where the percentage of unemployed patients receiving PPC were higher than those receiving CBSM (p = .008). Also, psychotropic drug intake was higher in those patients dropping out of the study (p = .022). Since age and stage at diagnosis are known to be highly associated with recurrence and survival, these two variables were later included in multivariate regression models.
Effects of HADS reduction after psychotherapy in the risk of 5-year recurrenceLogistic regression models adjusted for HADS at baseline revealed that a reduction in HADS score between T0, (M = 22.38, SD = 6.26) and T2, (M = 17.77, SD = 7.25), predicted a significant lower risk of 5-year recurrence (OR = 0.84; p = .037, 95% CI = [0.71-0.99]). When the model was also adjusted for age, stage at diagnosis and the number of extreme and threatening SLE the effect remained significant (OR = 0.83; p = .045, 95% CI = [0.69, 1.00]). When this analysis was stratified by the type of therapy (PPC vs CBSM), the magnitude of the effect was not significantly different (p = .550) between groups: CBSM, OR = 0.90 vs. PPC, OR = 0.70.
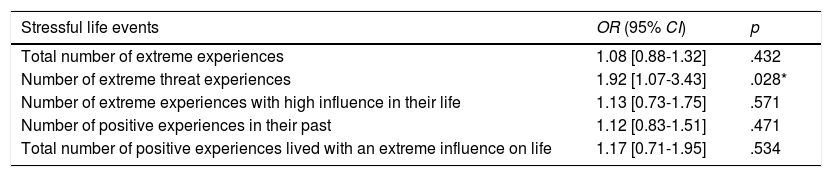
Effect of SLE on 5-year recurrenceWhen analyzing the relationship between different type/number of extreme life experiences and 5-year recurrence, it was found that a one-point increase in the number of threatening SLE increased by 92% the risk of recurrence (1.92, p = .027, 95% CI = [1.07-3.43]). The effect remained significant after repeating the analysis adjusting for age and stage at diagnosis (OR = 2.00, p = .023, 95% CI = [1.10-3.64]). For more details regarding the SLE subtypes and their associated statistics, see Table 2.
Univariate logistic models and cancer recurrence at 5 years (N = 113).
| Stressful life events | OR (95% CI) | p |
|---|---|---|
| Total number of extreme experiences | 1.08 [0.88-1.32] | .432 |
| Number of extreme threat experiences | 1.92 [1.07-3.43] | .028* |
| Number of extreme experiences with high influence in their life | 1.13 [0.73-1.75] | .571 |
| Number of positive experiences in their past | 1.12 [0.83-1.51] | .471 |
| Total number of positive experiences lived with an extreme influence on life | 1.17 [0.71-1.95] | .534 |
Note: OR = odds ratio; CI = confidence interval.
The analysis of the odds ratio showed that the psychological interventions reduced the risk of 5-year recurrence around 43%, although this result did not reach statistical significance (OR = 0.57, p = .321, 95 %CI = [0.19-1.73]).
Effects of receiving versus not receiving psychotherapy on DFIKaplan-Meier survival curves were used to compare DFI according to the presence of therapy (shown in Figure 2A). The survival curve for participants receiving psychotherapy was slightly higher than the curve for participant who did not receive psychotherapy, suggesting that the DFI experience is slightly superior for participants receiving psychotherapy. However, the log-rank test performed to compare the curves indicated no significant differences between groups (p = .335).
Effects of psychotherapy type on 5-year recurrenceThe specific type of psychotherapy did not influence 5-year risk of recurrence, OR = 0.818, p = .790, 95% CI = [0.187-3.581].
Effects of psychotherapy type on DFIKaplan-Meier survival curves were used to compare DFI according to the type of therapy received (shown in Figure 2B). The survival curves for participants were very similar in both psychotherapies, indicating no association between the type of psychotherapy and DFI. This result was confirmed by the lack of statistical significance in the log-rank test (p = .809).
DiscussionThe present study aimed to explore the influence of stress and distress in BC evolution. It was found that a reduction in psychological distress after the psychotherapy had a positive impact in cancer recurrence. Also, the findings revealed that the higher the number of threateningSLE the higher the cancer recurrence at 5-year follow-up.
The results presented herein suggest that the distress improvements after psychotherapy observed in the previous study (Ochoa-Arnedo et al., 2021) may be related with lower cancer recurrence at 5 years. This finding supports the literature exposing the relationship between psychological stress and cancer progression (Mravec et al., 2020b) and also adds evidence on the therapeutic options that reduce stress-related signaling and therefore the recurrences (Mravec et al., 2020a). Considering that chronic stress is associated with relevant behavioral and biological processes, it is plausible that the distress reduction promoted psychological adaptation favoring better outcomes of BC patients. In this sense, it has been demonstrated that psychological distress can influence tumor progression via epigenetic changes, neuroendocrine disturbances or immune surveillance (McGregor & Antoni, 2009) . In fact, a reduction in serum cortisol and increases in cellular immune functioning has been reported in BC patients undertaking CBSM (McGregor et al., 2004).
It has also been found that a one-point increase in the number of threatening SLE was related to a higher risk for cancer recurrence at 5 years. This association has been reported in several studies with robust results (Fischer et al., 2018; Kocic et al., 2015; Kruk, 2012). From biobehavioral models of cancer, these findings could be explained by the impact that threatening SLE have on the immune system and with the activation of the endogenous catecholamine pathway; processes linked with tumor growth. To perceive events as threatening is associated with an increase of signaling in the sympathetic nervous system (Cole et al., 2015) and dysregulations of the Hypothalamic-Pituitary-Adrenal (HPA) axis (Fagundes et al., 2017), some of the main biological stress regulation systems. In patients with advanced cancer, abnormalities in HPA-axis functioning has been related to hastened mortality, as well as to alterations in cortisol and catecholamines, which act as ligands for immune cells and down regulate cellular immune function (Cole et al., 2015). Our finding is also in line with a recent study that reported how ACE are associated with poorer survival in patients with cancer diagnosis (Steel et al., 2020).
When exploring the effect of receiving psychotherapy (any type) on the risk of cancer recurrence after 5 years, it was found that the risk diminished a 43%. Although the percentage is clinically relevant, this effect was not statistically significant. These findings highlight that solely attending to psychotherapy intervention would not have an impact on cancer recurrence, but the stress reduction that some patients experienced did. This important distinction might have contributed to some of the heterogeneity reported in RCT exploring the effectiveness of adjunct psychotherapy on cancer survival (Chen & Ahmad, 2018; Gudenkauf & Ehlers, 2018). In any case, the non-therapy group was conformed with patients voluntarily not receiving therapy for individual reasons so these results may be biased, and this hypothesis should be tested in a controlled trial that also randomized the control group.
This is the first limitation that must be mentioned in this study, since the inclusion of a randomized control group not receiving psychotherapy would be necessary to truly ascertain its impact on cancer recurrence and survival. However, this option involves serious ethical implications hard to be faced in healthcare settings. Also, our sample was composed of women with BC only, a circumstance that may limit the generalization of the results. More heterogeneous samples of man and woman survivors of different cancer types should be studied to explore the effects of the employed psychotherapies in other populations. Also, the present study did not collect biological data, which may help to investigate the neurobiological underpinnings of such improvement in cancer outcomes. Therefore, future studies taking a more comprehensive approach (i.e., measuring psychosocial and biological markers) might shed more light on the pathway of distress and stress impact on cancer survival. Finally, the number of participants involved in the present study (68 in the therapy group and 37 in the control group) may have limited the probability to observe significant results related to the effect of the therapy on 5-year recurrence even if the magnitude of the association seemed important.
In summary, our results provide valuable information on the influence that stress reduction and threatening stressful experiences may have on cancer recurrence. In this regard, it is important to highlight the relevance of patient experience, as only those SLE considered threatening were risk factors for recurrence. Likewise, the sole attendance to psychotherapy did not show a significant effect on cancer recurrence, but the resulting distress reduction did. Thus, psychotherapy interventions able to reduce distress such as PPC or CBSM, and with potential to reconceptualize threatening experiences in post-traumatic growth (e.g., PPC), may be protective factors against worse cancer outcomes. More research is needed to ascertain this connection, preferably through long-term follow-ups.
FundingThe study has been supported by the Carlos III Health Institute under the FIS grants PI15/01278 and PI19 / 01880, co-financed by the European Regional Development Fund (ERDF) “a way to build Europe”. This work has also been supported by the Secretaria d'Universitats i Recerca of the Generalitat de Catalunya and The European Social Fund under the FI grant 2020 FI_B 00288. Consolidated research group: Research in health services in cancer, 2017SGR00735.
We thank all the patients and their relatives for their participation in this study, and CERCA Program for institutional support. We also thank the biostatistics service of IDIBELL for their kind guidance, specially to Dr Cristian Tebé.