Source monitoring refers to the ability to determine the source of memories and encompasses three subprocesses: internal source monitoring, reality monitoring, and external source monitoring. Neuroimaging studies provide valuable insights about neural correlates of source monitoring, but the causal relationship between brain and behavior is lacking. This study aimed to identify brain circuits involved in source monitoring by synthesizing the effects of brain stimulation on source monitoring as a function of the targeted brain regions or circuits.
MethodWe conducted a systematic review of interventional studies that have examined the effects of brain stimulation on source monitoring across six databases. The principal outcome was the difference of source monitoring performance between active and control stimulation conditions.
Results23 studies (920 healthy participants and 54 patients with schizophrenia) were included. Our findings revealed the involvement of i) the lateral prefrontal and temporoparietal cortices in internal source monitoring, ii) the medial prefrontal and temporoparietal cortices in reality monitoring, and iii) the precuneus and the left angular gyrus in external source monitoring.
ConclusionsThese findings deepen our understanding of the brain mechanisms of source monitoring and highlight specific stimulation targets to alleviate source monitoring deficits.
Source monitoring refers to the cognitive function that allows one to determine the source/the origin of information stored in memory, either for events or thoughts (Johnson et al., 1993). It can be distinguished from item memory, which refers to the ability to recognize or recall the previously presented information per se (i.e., its content). In other words, whereas item memory focuses on the "what" that is being remembered (i.e., the central information or item), source monitoring involves recalling the conditions under which memory for an item was acquired. It includes the external context, such as the spatial and temporal context (where and when the item was learned), the agent (from whom the item was learned), the input modality (whether the item was perceived through one or more sensory modalities or imagined), and the internal context, such as the cognitive operations performed during encoding (e.g., cognitive operations performed on the items, efforts made to imagine the items). According to the source monitoring framework developed by Johnson et al. (1993), memories do not come with labels indicating their source, but the source is rather determined based on remembering the different internal/external features associated with the memory. When the memory for the internal or external context (also called source memory) is insufficient, source monitoring relies on reconstructing/guessing the source (Kuhlmann et al., 2021).
Being able to remember and reconstruct the source of a memory is crucial in everyday life, as it allows us to distinguish fantasy from reality. However, source monitoring errors can occur in both healthy and pathological populations. An example of source misattribution is the phenomenon of unconscious plagiarism, also known as cryptomnesia, which is observed when someone unintentionally attributes something they have heard/seen somewhere as coming from their imagination. The reverse is also observed when internally generated experiences are erroneously evaluated as being real, a type of error that has been proposed as underlying hallucinations of schizophrenia (Bentall, 1990).
Source monitoring processes have been classified into three main processes based on the origin of the differentiated information (Johnson et al., 1993): 1) internal source monitoring, which refers to the ability to discriminate between different internal sources. For example, this process is involved in distinguishing between something we have said aloud and something we have imagined saying; 2) reality monitoring, which refers to the ability to discriminate between internal and external sources. This process allows us to distinguish our thoughts and imaginations from perceived real events. An example of this would be distinguishing between something we have imagined and something we have heard; and 3) external source monitoring, which refers to the ability to discriminate between different external sources. For example, this process is involved in identifying which of our colleagues has given us certain information.
Numerous studies have attempted to identify the brain underpinnings of source monitoring, mostly using neuroimaging techniques (for a review see Mitchell & Johnson, 2009). These neuroimaging studies have unveiled a large brain network involved in source monitoring, including prefrontal, medial temporal, temporoparietal, and parietal cortices. Recent developments in data processing methods for fMRI studies allow overcoming the correlative nature of these neuroimaging techniques and inferring causal links between component populations of neural networks, cognitive processing, and behavior (Bielczyk et al., 2019). However, non-invasive brain stimulation (NIBS) techniques remain the gold standard technique to investigate the causal relationship between brain and behavior, here for source monitoring processes. NIBS allows for selectively manipulating the activity, connectivity or excitability of a target brain region and studying its consequences for cognition and behavior, thereby inferring the causal contribution of the target brain region or circuit to the studied cognitive function/behavior (Bergmann & Hartwigsen, 2021; Neige et al., 2021). Various NIBS techniques are currently available, including transcranial magnetic stimulation delivered as single pulses or repetitive stimulation trains (rTMS), theta burst stimulation (TBS), which is a form of rTMS, transcranial direct current stimulation (tDCS), and transcranial random noise stimulation (tRNS). The choice of using one technique over another depends on their properties, mechanisms of action, and presumed effects on brain functioning. For example, cathodal tDCS, continuous TBS (cTBS), low frequency rTMS (LF-rTMS) are often used for their presumed inhibitory effects, while anodal tDCS and high frequency rTMS (HF-rTMS) have been used for their presumed excitatory effects on the stimulated brain region. These putative effects are mainly derived from previous animal and human studies that investigating the aftereffects of these techniques on motor corticospinal excitability. Furthermore, NIBS can have both immediate effects during stimulation and subsequent effects that last beyond the stimulation period, depending on the stimulation parameters. NIBS can be applied either during a cognitive task (online) to modulate brain circuits in real time and assess its immediate impact on cognitive function or outside the task period (offline), allowing researchers to study how sustained changes in brain excitability influence subsequent cognitive performance.
Here, we conducted a systematic review of studies investigating the effect of NIBS on source monitoring. To identify brain circuits causally involved in source monitoring processes and to gain a more comprehensive understanding supporting these cognitive processes, we aimed to synthesize the effect of NIBS on source monitoring performance as a function of the targeted brain regions or circuits.
MethodsThis review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). The protocol was pre-registered in the PROSPERO database (CRD42022363565).
Literature search and selection criteriaA systematic search for articles was conducted in the PubMed, PsycINFO, Embase, Web of Science, and Science Direct databases (up to May 15, 2023) using the following keywords: ("source monitoring" OR "source memory" OR "source discrimination" OR "source attribution" OR "source recognition" OR "reality monitoring" OR "reality memory" OR "reality discrimination" OR "self-monitoring" OR "self-memory") AND ("brain stimulation" OR "transcranial electrical stimulation" OR "transcranial direct current stimulation" OR "transcranial alternating current stimulation" OR "transcranial random noise stimulation" OR "transcranial magnetic stimulation" OR "theta burst stimulation" OR "slow oscillatory stimulation" OR "transcranial ultrasound stimulation" OR NIBS OR tES OR tDCS OR tACS OR tRNS OR TMS OR rTMS OR TBS). The first 200 results in Google Scholar were also screened.
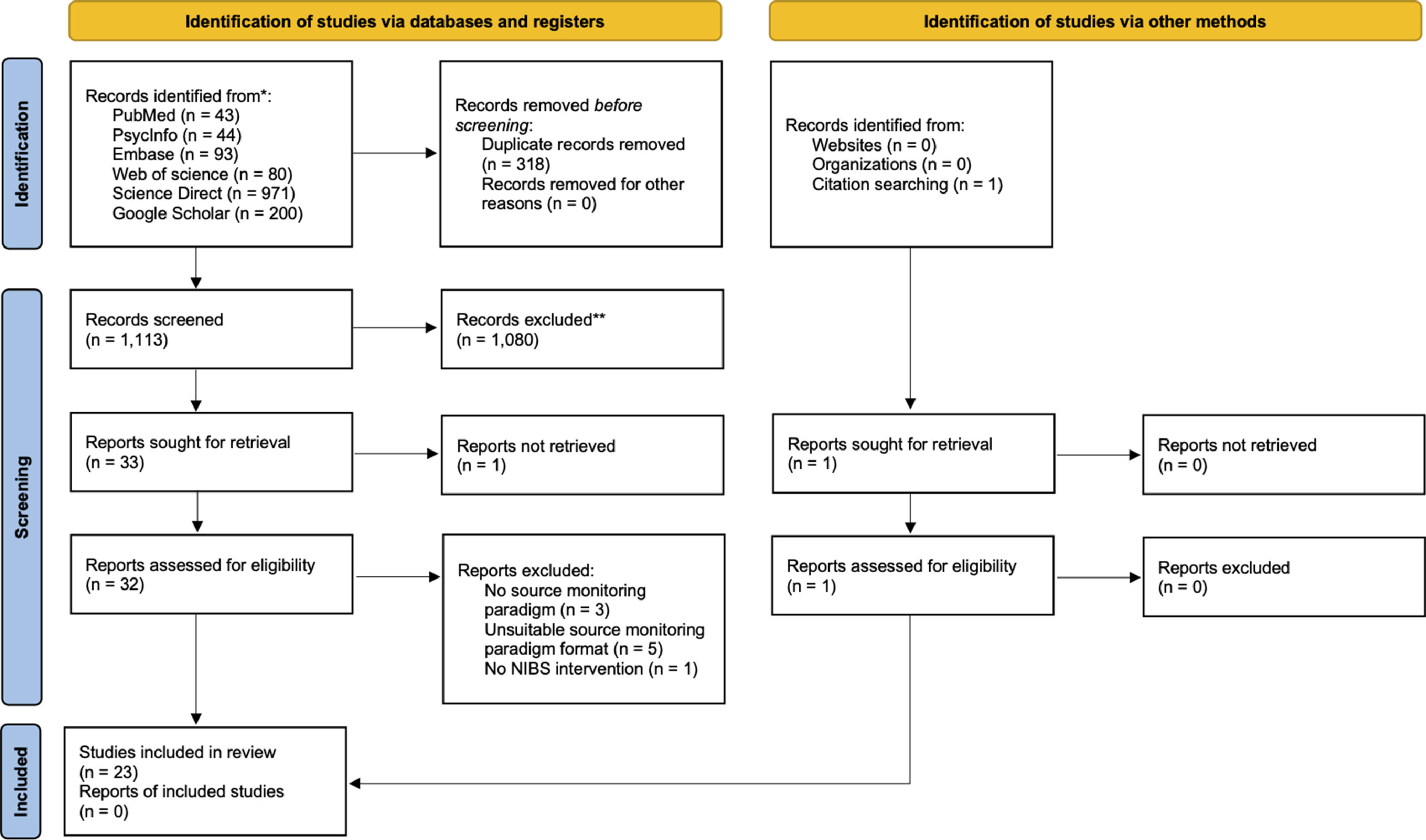
The inclusion criteria were established with the PICOS guidelines (Supplementary material, Table S1). All study designs were covered, including randomized controlled trials (RCT), case studies, open-label uncontrolled studies and prospective studies. After the removal of duplicates (M.P.), two authors (M.P. and M.M.) independently screened the titles, abstracts, and full texts of extracted articles and selected studies applying eligibility criteria, with disagreements being resolved by consulting a third author (J.B.). Inclusion criteria were: (1) investigation of at least one source monitoring subprocess (i.e., external source monitoring, internal source monitoring, reality monitoring, and source memory); (2) the use of at least one source monitoring experimental paradigm including an encoding phase where each item is presented in a single source, and a subsequent retrieval (test) phase where participants have to determine the source of items with limited multiple-choice questions for different explicitly defined sources (see Fig. 2A for an example of a typical source monitoring paradigm); (3) a detailed description of the NIBS technique used; (4) comparison of source monitoring performances of the intervention group (i.e., active stimulation) with one or more control groups, including sham stimulation, active stimulation over a control site, and baseline condition. We excluded studies not published in English and preprints.
Data extraction and main outcomeStudy design, sample size, age, sex, source monitoring paradigm, source monitoring outcomes (including measures of accuracy and error (Bröder & Meiser, 2007)), stimulation procedure, stimulation parameters and timing of stimulation with respect to the source monitoring task were extracted from the included studies. Where applicable, clinical data were also extracted. Our primary outcome was defined as the differences in source monitoring accuracy and/or error scores between the active stimulation and control conditions. Additionally, old/new recognition performance was extracted, when available, as the ability to recognize previously presented stimuli (old items) from items not previously shown (new items). These scores provide a measure of item memory and allow the dissociation between the effects on source monitoring and item memory to be explored.
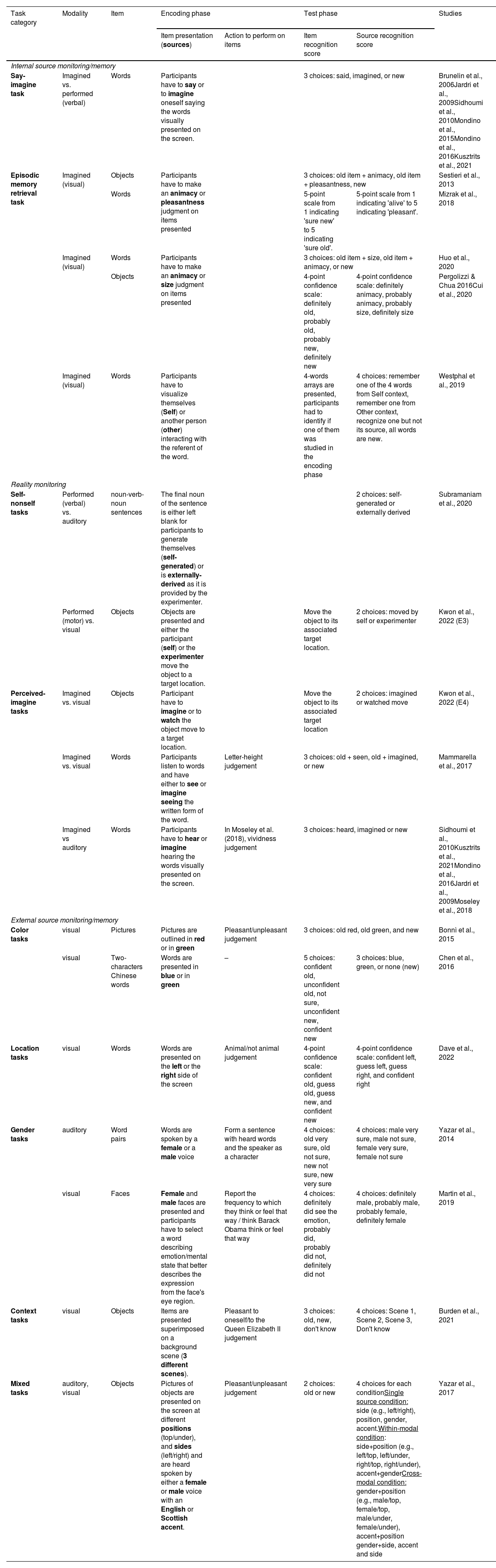
The studies were then classified and summarized descriptively according to the subtype of source monitoring studied, categorized into the 3 subtypes defined by Johnson et al. (1993): internal source monitoring, when discriminating between different self-generated information, such as imagined or performed actions; reality monitoring, when discriminating between a self-generated information and an externally-generated information; and external source monitoring when discriminating between different externally-generated information. An alternative classification of source monitoring has been proposed based on stimulus modality, i.e., how stimuli are presented to or encoded by the participant (Damiani et al., 2022). For example, stimuli from external sources may be presented as either auditory or visual, and stimuli from internal sources may be categorized as imagined or performed. This categorization is provided as a reference for each of the paradigms outlined in Table 1.
Summary of experimental paradigms used in the included studies.
| Task category | Modality | Item | Encoding phase | Test phase | Studies | ||
|---|---|---|---|---|---|---|---|
| Item presentation (sources) | Action to perform on items | Item recognition score | Source recognition score | ||||
| Internal source monitoring/memory | |||||||
| Say-imagine task | Imagined vs. performed (verbal) | Words | Participants have to say or to imagine oneself saying the words visually presented on the screen. | 3 choices: said, imagined, or new | Brunelin et al., 2006Jardri et al., 2009Sidhoumi et al., 2010Mondino et al., 2015Mondino et al., 2016Kusztrits et al., 2021 | ||
| Episodic memory retrieval task | Imagined (visual) | Objects | Participants have to make an animacy or pleasantness judgment on items presented | 3 choices: old item + animacy, old item + pleasantness, new | Sestieri et al., 2013 | ||
| Words | 5-point scale from 1 indicating 'sure new' to 5 indicating 'sure old'. | 5-point scale from 1 indicating 'alive' to 5 indicating 'pleasant'. | Mizrak et al., 2018 | ||||
| Imagined (visual) | Words | Participants have to make an animacy or size judgment on items presented | 3 choices: old item + size, old item + animacy, or new | Huo et al., 2020 | |||
| Objects | 4-point confidence scale: definitely old, probably old, probably new, definitely new | 4-point confidence scale: definitely animacy, probably animacy, probably size, definitely size | Pergolizzi & Chua 2016Cui et al., 2020 | ||||
| Imagined (visual) | Words | Participants have to visualize themselves (Self) or another person (other) interacting with the referent of the word. | 4-words arrays are presented, participants had to identify if one of them was studied in the encoding phase | 4 choices: remember one of the 4 words from Self context, remember one from Other context, recognize one but not its source, all words are new. | Westphal et al., 2019 | ||
| Reality monitoring | |||||||
| Self-nonself tasks | Performed (verbal) vs. auditory | noun-verb-noun sentences | The final noun of the sentence is either left blank for participants to generate themselves (self-generated) or is externally-derived as it is provided by the experimenter. | 2 choices: self-generated or externally derived | Subramaniam et al., 2020 | ||
| Performed (motor) vs. visual | Objects | Objects are presented and either the participant (self) or the experimenter move the object to a target location. | Move the object to its associated target location. | 2 choices: moved by self or experimenter | Kwon et al., 2022 (E3) | ||
| Perceived-imagine tasks | Imagined vs. visual | Objects | Participant have to imagine or to watch the object move to a target location. | Move the object to its associated target location | 2 choices: imagined or watched move | Kwon et al., 2022 (E4) | |
| Imagined vs. visual | Words | Participants listen to words and have either to see or imagine seeing the written form of the word. | Letter-height judgement | 3 choices: old + seen, old + imagined, or new | Mammarella et al., 2017 | ||
| Imagined vs auditory | Words | Participants have to hear or imagine hearing the words visually presented on the screen. | In Moseley et al. (2018), vividness judgement | 3 choices: heard, imagined or new | Sidhoumi et al., 2010Kusztrits et al., 2021Mondino et al., 2016Jardri et al., 2009Moseley et al., 2018 | ||
| External source monitoring/memory | |||||||
| Color tasks | visual | Pictures | Pictures are outlined in red or in green | Pleasant/unpleasant judgement | 3 choices: old red, old green, and new | Bonnì et al., 2015 | |
| visual | Two-characters Chinese words | Words are presented in blue or in green | – | 5 choices: confident old, unconfident old, not sure, unconfident new, confident new | 3 choices: blue, green, or none (new) | Chen et al., 2016 | |
| Location tasks | visual | Words | Words are presented on the left or the right side of the screen | Animal/not animal judgement | 4-point confidence scale: confident old, guess old, guess new, and confident new | 4-point confidence scale: confident left, guess left, guess right, and confident right | Dave et al., 2022 |
| Gender tasks | auditory | Word pairs | Words are spoken by a female or a male voice | Form a sentence with heard words and the speaker as a character | 4 choices: old very sure, old not sure, new not sure, new very sure | 4 choices: male very sure, male not sure, female very sure, female not sure | Yazar et al., 2014 |
| visual | Faces | Female and male faces are presented and participants have to select a word describing emotion/mental state that better describes the expression from the face's eye region. | Report the frequency to which they think or feel that way / think Barack Obama think or feel that way | 4 choices: definitely did see the emotion, probably did, probably did not, definitely did not | 4 choices: definitely male, probably male, probably female, definitely female | Martin et al., 2019 | |
| Context tasks | visual | Objects | Items are presented superimposed on a background scene (3 different scenes). | Pleasant to oneself/to the Queen Elizabeth II judgement | 3 choices: old, new, don't know | 4 choices: Scene 1, Scene 2, Scene 3, Don't know | Burden et al., 2021 |
| Mixed tasks | auditory, visual | Objects | Pictures of objects are presented on the screen at different positions (top/under), and sides (left/right) and are heard spoken by either a female or male voice with an English or Scottish accent. | Pleasant/unpleasant judgement | 2 choices: old or new | 4 choices for each conditionSingle source condition: side (e.g., left/right), position, gender, accent.Within-modal condition: side+position (e.g., left/top, left/under, right/top, right/under), accent+genderCross-modal condition: gender+position (e.g., male/top, female/top, male/under, female/under), accent+position gender+side, accent and side | Yazar et al., 2017 |
The quality of the included studies was assessed independently by two authors (M.P. and M.M.) using the standard quality assessment criteria tool with the 14-item checklist for quantitative studies (Kmet et al., 2004). Disagreements on quality assessment were resolved by discussion until a consensus was reached. Pre-consensus interrater agreement was calculated for the total score of each study using an intraclass correlation coefficient (ICC).
ResultsSearch resultsThe initial search identified 1431 records through databases and 1 through citation searching (Fig. 1). After removing duplicates and excluding ineligible studies, 33 full texts were assessed for inclusion based on eligibility criteria. A total of 23 studies (21 RCTs and 2 case reports) met our inclusion criteria and were included in the review. The studies included a total number of 920 healthy participants and 54 patients with schizophrenia. Detailed characteristics of the included studies are provided in Table 2.
Characteristics and individual results of the included studies (n = 23).
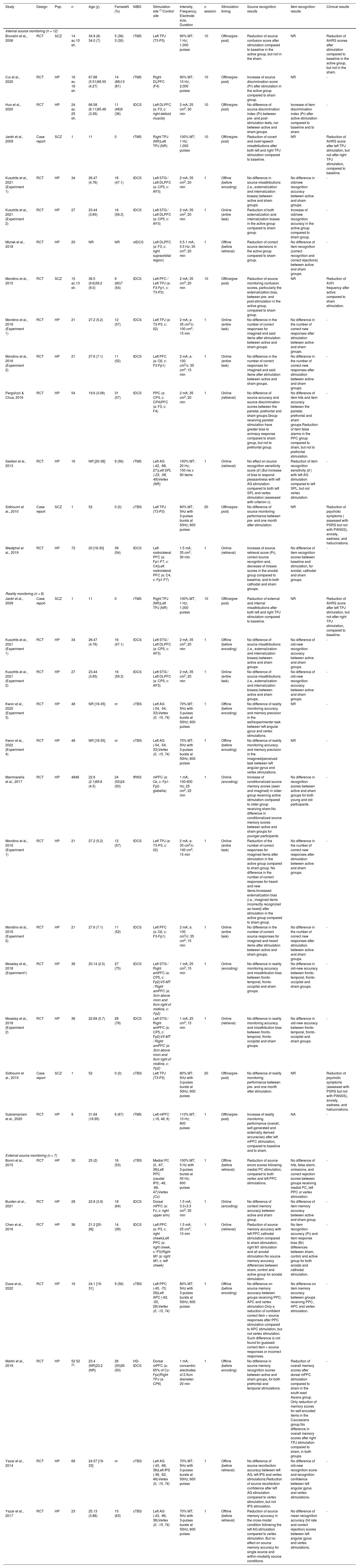
| Study | Design | Pop. | n | Age (y) | FemaleN (%) | NIBS | Stimulation site (1)Control site | Intensity, Frequency, Electrode size, Duration | n session | Stimulation timing | Source recognition results | Item recognition results | Clinical results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Internal source monitoring (n = 12) | |||||||||||||
| Brunelin et al., 2006 | RCT | SCZ | 14 ac.10 sh. | 34.9 (8) 34.0 (7) | 5 (36) 3 (30) | rTMS | Left TPJ (T3-P3) | 90% MT; 1 Hz; 1,000 pulses | 10 | Offline(pre-post) | Reduction of source confusion score after stimulation compared to baseline in the active group, but not in the sham. | NR | Reduction of AHRS scores after stimulation compared to baseline in the active group, but not in the sham. |
| Cui et al., 2020 | RCT | HP | 16 ac. 16 sh. | 67.88 (5.51)66.50 (4.27) | 14 (88)13 (81) | rTMS | Right DLPFC (F4) | 90% MT; 10 Hz; 2,000 pulses | 10 | Offline(pre-post) | Increase of source discrimination score (Pr) after stimulation in the active group compared to sham group. | NR | - |
| Huo et al., 2020 | RCT | HP | 24 ac. 25 sh. | 66.58 (6.11)65.48 (3.39) | 11 (46)9 (36) | tDCS | Left DLPFC (a: F3, c: right deltoid muscle) | 2 mA; 25 cm2; 30 min | 10 | Offline(pre-post) | No difference of source discrimination index (Pr) between pre- and post-stimulation tests, nor between active and sham groups. | Increase of item discrimination index (Pr) after active stimulation compared to baseline and to sham. | - |
| Jardri et al., 2009 | Case report | SCZ | 1 | 11 | 0 | rTMS | Right TPJ (NR)Left TPJ (NR) | 100% MT; 1 Hz; 1,000 pulses | 10 | Offline(pre-post) | Reduction of covert and overt speech misattributions after both left and right TPJ stimulation compared to baseline. | NR | Reduction of AHRS score after left TPJ stimulation, but not after right TPJ stimulation, compared to baseline. |
| Kusztrits et al., 2021 (Experiment 1) | RCT | HP | 34 | 26.47 (4.76) | 16 (47.1) | tDCS | Left STG / Left DLPFC (a: CP5, c: AF3) | 2 mA; 35 cm2; 20 min | 1 | Offline (before encoding) | No difference in source misattributions (i.e., externalization and internalization biases) between active and sham groups. | No difference in old/new recognition accuracy between active and sham groups. | - |
| Kusztrits et al., 2021 (Experiment 2) | RCT | HP | 27 | 23.44 (3.65) | 16 (59.3) | tDCS | Left STG / Left DLPFC (a: CP5, c: AF3) | 2 mA; 35 cm2; 20 min | 1 | Online (entire task) | Reduction of both externalization and internalization biases in the active group compared to sham group. | Increase of old/new recognition accuracy in the active group compared to sham group. | - |
| Mizrak et al., 2018 | RCT | HP | 20 | NR | NR | otDCS | Left DLPFC (a: F3, c: right supraorbital region) | 0.5-1 mA; 5.5 Hz; 35 cm2; 20 min | 1 | Offline (before retrieval) | Reduction of correct source decisions in the active group compared to sham group. | No difference of item recognition (correct recognition and correct rejections) between active and sham groups. | - |
| Mondino et al., 2015 | RCT | SCZ | 15 ac.13 sh. | 36.5 (9.6)39.2 (9.0) | 9 (60)7 (54) | tDCS | Left PFC / Left TPJ (a: F3-Fp1, c: T3-P3) | 2 mA; 35 cm2; 20 min | 10 | Offline(pre-post) | Reduction of source monitoring confusion scores, particularly the externalization bias, between pre- and post-stimulation in the active group, compared to sham group. | NR | Reduction of AVH frequency after active compared to sham stimulation. |
| Mondino et al., 2016 (Experiment 1) | RCT | HP | 21 | 27.2 (5.2) | 12 (57) | tDCS | Left TPJ (a: T3-P3, c: 02) | 2 mA; a: 35 cm2/c: 100 cm2; 15 min | 1 | Online (entire task) | No difference in the number of correct responses for imagined and said items after stimulation between active and sham groups. | No difference in the number of correct new responses after stimulation between active and sham groups. | - |
| Mondino et al., 2016 (Experiment 2) | RCT | HP | 21 | 27.6 (7.1) | 11 (52) | tDCS | Left PFC (a: O2, c: F3-Fp1) | 2 mA; a: 100 cm2/c: 35 cm2; 15 min | 1 | Online (entire task) | No difference in the number of correct responses for imagined and said items after stimulation between active and sham groups. | No difference in the number of correct new responses after stimulation between active and sham groups. | - |
| Pergolizzi & Chua, 2016 | RCT | HP | 54 | 19.6 (3.06) | 31 (57) | tDCS | PPC (a: CP3, c: CP4)PFC (a: F3, c: F4) | 2 mA; 35 cm2; 20 min | 1 | Online (retrieval) | No difference of source accuracy and source discrimination scores between the parietal, prefrontal and sham groups.Group receiving parietal stimulation have greater bias to animacy response compared to sham group, but not to prefrontal group. | No difference of item hits and item accuracy between the parietal, prefrontal and sham groups.Reduction of item false alarms in the PPC group compared to sham, but not to prefrontal stimulation. | - |
| Sestieri et al., 2013 | RCT | HP | 16 | NR [20-38] | 9 (56) | rTMS | Left AG (-42, -68, 27)Left SPL (-23, -58, 49)Vertex (NR) | 100% MT; 20 Hz; 150 ms x 90 items | 1 | Online (retrieval) | No effect on source recognition sensitivity score (d’).But increase of bias to respond pleasantness with left AG stimulation compared to both left SPL and vertex stimulation (assessed with criterion c). | Reduction of item recognition sensitivity (d’) with left AG stimulation compared to left SPL, but not vertex stimulation. | - |
| Sidhoumi et al., 2010 | Case report | SCZ | 1 | 52 | 0 (0) | cTBS | Left TPJ (T3-P3) | 80% MT; 5Hz with 3-pulses bursts at 50Hz; 600 pulses | 20 | Offline(pre-post) | No difference of source monitoring performance between pre- and one month after stimulation. | NR | Reduction of psychotic symptoms ( assessed with PSRS but not with PANSS), anxiety, sadness, and hallucinations. |
| Westphal et al., 2019 | RCT | HP | 72 | 20 [18-30] | 39 (54) | tDCS | Left rostrolateral PFC (a: Fp1-F7, c: C4)Left rostrolateral PFC (a: C4, c: Fp1-F7) | 1.5 mA; 35 cm2; 30 min | 1 | Online (retrieval) | Increase of source retrieval score (Pr), correct source recogntion and, decrease of misses scores in the anodal group compared to baseline, and to both cathodal and sham groups. | No difference of item recognition scores between baseline and stimulation, for anodal, cathodal and sham groups. | - |
| Reality monitoring (n = 8) | |||||||||||||
| Jardri et al., 2009 | Case report | SCZ | 1 | 11 | 0 | rTMS | Right TPJ (NR)Left TPJ (NR) | 100% MT; 1 Hz; 1,000 pulses | 10 | Offline(pre-post) | Reduction of external and internal misattributions after both left and right TPJ stimulation compared to baseline. | NR | Reduction of AHRS score after left TPJ stimulation, but not after right TPJ stimulation, compared to baseline. |
| Kusztrits et al., 2021 (Experiment 1) | RCT | HP | 34 | 26.47 (4.76) | 16 (47.1) | tDCS | Left STG / Left DLPFC (a: CP5, c: AF3) | 2 mA; 35 cm2; 20 min | 1 | Offline (before encoding) | No difference of source misattributions (i.e., externalization and internalization biases) between active and sham groups. | No difference of old-new recognition accuracy between active and sham groups. | - |
| Kusztrits et al., 2021 (Experiment 2) | RCT | HP | 27 | 23.44 (3.65) | 16 (59.3) | tDCS | Left STG / Left DLPFC (a: CP5, c: AF3) | 2 mA; 35 cm2; 20 min | 1 | Online (entire task) | No difference of source misattributions (i.e., externalization and internalization biases) between active and sham groups. | No difference of old-new recognition accuracy between active and sham groups. | - |
| Kwon et al., 2022 (Experiment 3) | RCT | HP | 48 | NR [18-35] | nr | cTBS | Left AG (-54, -54, 33)Vertex (0, -15, 74) | 70% MT; 5Hz with 3-pulses bursts at 50Hz; 600 pulses | 1 | Offline (before encoding) | No difference of reality monitoring accuracy and memory precision in the self/experimenter task between left angular gyrus and vertex stimulations. | NR | - |
| Kwon et al., 2022 (Experiment 4) | RCT | HP | 48 | NR [18-35] | nr | cTBS | Left AG (-54, -54, 33)Vertex (0, -15, 74) | 70% MT; 5Hz with 3-pulses bursts at 50Hz; 600 pulses | 1 | Offline (before encoding) | No difference of reality monitoring accuracy and memory precision in the imagined/perceived task between left angular gyrus and vertex stimulations. | NR | - |
| Mammarella et al., 2017 | RCT | HP | 4848 | 22.6 (2.1)69.8 (4.5) | 24 (50)24 (50) | tRNS | mPFC (a: Oz, c: Fp1-Fp2-glabella) | 1 mA; 100-600 Hz; 25 cm2; 22 min | 1 | Online (encoding) | Increase of conditionalized source memory scores (seen and imagined) in older group receiving active stimulation compared to older group receiving sham.No difference in conditionalized source memory scores between active and sham groups for younger participants. | No difference in recognition scores between active and sham groups for both young and old participants. | - |
| Mondino et al., 2016 (Experiment 1) | RCT | HP | 21 | 27.2 (5.2) | 12 (57) | tDCS | Left TPJ (a: T3-P3, c: 02) | 2 mA; a: 35 cm2/c: 100 cm2; 15 min | 1 | Online (entire task) | Reduction of the number of correct responses for imagined items after stimulation in the active group compared to sham group. No difference in the number of correct responses for heard and new items.Increased externalization bias (i.e., imagined items incorrectly recognized as heard) after stimulation in the active group compared to sham group. | No difference in the number of correct new responses after stimulation between active and sham groups. | - |
| Mondino et al., 2016 (Experiment 2) | RCT | HP | 21 | 27.6 (7.1) | 11 (52) | tDCS | Left PFC (a: O2, c: F3-Fp1) | 2 mA; a: 100 cm2/c: 35 cm2; 15 min | 1 | Online (entire task) | No difference in the number of correct source responses for imagined and heard items after stimulation between active and sham groups. | No difference in the number of correct new responses after stimulation between active and sham groups. | - |
| Moseley et al., 2018 (Experiment1) | RCT | HP | 36 | 20.14 (2.5) | 27 (75) | tDCS | Left STG / Right amPFC (a: CP5, c: Fp2)V5-MT / Right amPFC (a: 3cm above inion and 6cm right of midline, c: Fp2) | 1 mA; 25 cm2; 15 min | 1 | Online (encoding) | No difference in reality monitoring accuracy and misattribution bias between fronto-temporal, fronto-occipital and sham groups. | No difference in old-new accuracy between fronto-temporal, fronto-occipital and sham groups. | - |
| Moseley et al., 2018 (Experiment 2) | RCT | HP | 36 | 22.69 (5.7) | 28 (78) | tDCS | Left STG / Right amPFC (a: CP5, c: Fp2)V5-MT / Right amPFC (a: 3cm above inion and 6cm right of midline, c: Fp2) | 1 mA; 25 cm2; 15 min | 1 | Online (retrieval) | No difference in reality monitoring accuracy and misattribution bias between fronto-temporal, fronto-occipital and sham groups. | No difference in old-new accuracy between fronto-temporal, fronto-occipital and sham groups. | - |
| Sidhoumi et al., 2010 | Case report | SCZ | 1 | 52 | 0 (0) | cTBS | Left TPJ (T3-P3) | 80% MT; 5Hz with 3-pulses bursts at 50Hz; 600 pulses | 20 | Offline(pre-post) | No difference of reality monitoring performance between pre- and one month after stimulation. | NR | Reduction of psychotic symptoms (assessed with PSRS but not with PANSS), anxiety, sadness, and hallucinations. |
| Subramaniam et al., 2020 | RCT | HP | 9 | 31.84 (18.95) | 6 (67) | rTMS | Left mPFC (-16, 48, 6) | 110% MT; 10 Hz; 800 pulses | 1 | Offline(pre-post) | Increase of reality monitoring performance (overall, self-generated and externally derived accuracies) after left mPFC stimulation, compared to baseline and to sham. | NA | - |
| External source monitoring (n = 7) | |||||||||||||
| Bonnì et al., 2015 | RCT | HP | 30 | 25 (2) | 16 (53) | cTBS | Medial PC (0, -67, 38)Left PPC (caudal IPS; -46, -66, 47)Vertex (Cz) | 100% MT; 5 Hz with 3-pulses bursts at 50 Hz; 600 pulses | 1 | Offline (before retrieval) | Reduction of source errors scores following medial PC stimulation, compared to both vertex and left PPC stimulations. | No difference of hits, false alarm, omissions, and correct rejection scores between groups receiving medial PC, left PPC or vertex stimulation. | - |
| Burden et al., 2021 | RCT | HP | 28 | 22.8 (3.9) | 18 (64) | tDCS | Dorsal mPFC (a: Fz, c: right upper arm) | 1.5 mA; 3.3×3.3 cm2; 30 min | 1 | Online (encoding) | No difference of context memory accuracy between active and sham group. | No difference of item memory accuracy between active and sham group. | - |
| Chen et al., 2016 | RCT | HP | 36 | 21.2 [20-26] | 14 (39) | tDCS | Left PPC (a: P3, c: right cheek)Left PPC (a: right cheek, c: P3)Right M1 (a: right M1, c: left cheek) | 1.5 mA; 25 cm2; 10 min | 1 | Online (retrieval) | Reduction of source memory accuracy with left PPC cathodal stimulation compared to sham stimulation, right M1 stimulation and all anodal stimulation.No source memory accuracy differences between sham, control and active group for anodal stimulation. | No item recognition accuracy (Pr) and item response bias (Br) differences between sham, control and active group for both anodal and cathodal stimulation. | - |
| Dave et al., 2022 | RCT | HP | 16 | 24.1 [19-31] | 9 (56) | cTBS | Left PPC (-45, -70, 39)Left APC (-62, -50, 28)Vertex (0, -15, 74) | 80% MT; 5Hz with 3-pulses bursts at 50Hz; 600 pulses | 1 | Offline (before encoding) | No difference on source memory accuracy between groups receiving PPC, APC and vertex stimulation.Only a reduction of confident correct item + source responses after PPC stimulation compared to APC stimulation, but not vertex stimulation. Such difference is not found for guessed correct item + source responses or incorrect responses. | No difference on item memory accuracy between groups receiving PPC, APC and vertex stimulation. | - |
| Martin et al., 2019 | RCT | HP | 52 52 (2) | 23.4 (NR)23.2 (NR) | 26 (50)26 (50) | HD-tDCS | Dorsal mPFC (a: 65% of Cz-Fpz)Right TPJ (a: CP6) | 1 mA; concentric electrodes of 2.5cm diameter; 20 min | 1 | Offline (before encoding) | No difference in source memory recognition scores between active and sham groups, for both prefrontal and temporal stimulations. | Reduction of overall memory scores after dorsal mPFC stimulation compared to sham in the south-east Asians group. Only reduction of memory scores for self-encoded items in the Caucasians group.No difference in overall memory scores after right TPJ stimulation compared to sham, in both groups. | - |
| Yazar et al., 2014 | RCT | HP | 69 | 24.57 [19-33] | nr | cTBS | Left AG (-43, -66, 38)Left IPS (-38, -62, 46)Vertex (0, -15, 74) | 70% MT; 5Hz with 3-pulses bursts at 50Hz; 600 pulses | 1 | Offline (before retrieval) | No difference of source recollection accuracy between left AG, left IPS and vertex stimulations.Reduction of source recollection confidence after left AG stimulation compared to vertex stimulation, but not IPS stimulation. | No difference of old-new recognition score and recognition confidence between left angular gyrus and vertex stimulations. | - |
| Yazar et al., 2017 | RCT | HP | 23 | 25.13 (3.88) | 15 (63) | cTBS | Left AG (-43, -66, 38)Vertex (0, -15, 74) | 70% MT; 5Hz with 3-pulses bursts at 50Hz; 600 pulses | 1 | Offline (before retrieval) | Reduction of source memory accuracy in the cross-modal condition following the left AG stimulation compared to vertex stimulation. But no effect on source memory accuracy for single source and within-modality source conditions. | No difference of mean recognition accuracy (hit rate and correct rejection) scores between left angular gyrus and vertex stimulations. | - |
Note: Age is given as mean (SD) or [range].
all coordinates are displayed in the Montreal Neurological Institute (MNI) system, except for the study of Sestieri and collaborators (2013) where the coordinates are displayed in the Talairach system. Stimulation sites in italics are considered as control sites.
in Martin et al. (2019), 104 healthy participants were recruited including 52 Caucasian Australians and 52 South-east Asians, equally distributed in active and sham condition for both stimulation sites.
Abbreviations: a = anode, ac. = active stimulation, AG = angular gyrus, AHRS = Auditory Hallucinations Rating Scale, amPFC = anterior medial prefrontal cortex, APC = anterior parietal cortex, c = cathode, cTBS = continuous theta burst stimulation, DLPFC = dorsolateral prefrontal cortex, HP = healthy participants, IPS = intraparietal sulcus, MT = motor threshold, mPFC = medial prefrontal cortex, NA = not applicable, NR = not reported, PANSS = Positive And Negative Syndrome Scale, PC = precuneus, PPC = posterior parietal cortex, PSRS = Psychotic Symptom Rating Scale, RCT = Randomized controlled trials, rTMS = repetitive transcranial magnetic stimulation, SCZ = patients with schizophrenia, sh. = sham stimulation, SPL = superior parietal lobule, tDCS = transcranial direct current stimulation, TPJ = temporo-parietal junction, tRNS = transcranial random noise stimulation
The majority of the included studies focused on investigating a single subtype of source monitoring, except for four studies that examined both internal source monitoring and reality monitoring processes (Kusztrits et al., 2021; Mondino et al., 2016; Sidhoumi et al., 2010; Jardri et al., 2009). Across these studies, 16 different experimental paradigms were used to measure source monitoring (see Table 1 ).
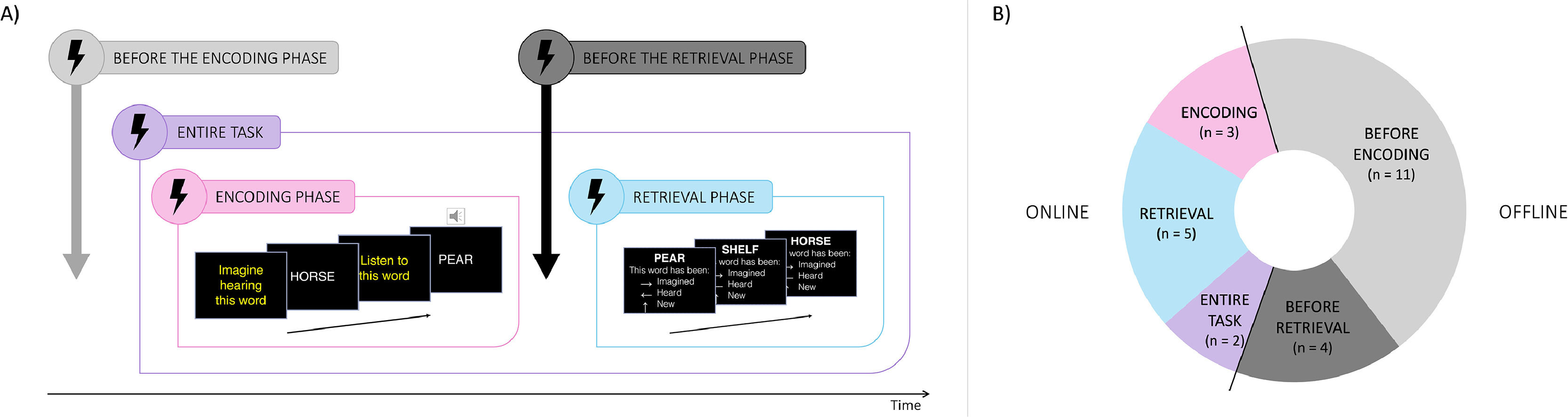
Nine studies used an online approach, applying stimulation during either the encoding (n = 3), the retrieval (n = 5), or both phases of the source monitoring task (n = 2; Fig. 2B). Of note, one study explored both encoding and retrieval phases in two different online experiments (Moseley et al., 2018). Fifteen studies employed an offline approach, administering stimulation either before encoding (n = 11), utilizing both active vs. sham designs (n = 4) and pre- vs. post-cure designs (n = 7), or between encoding and retrieval (n = 4). Additionally, one study investigated the effects of both online and offline stimulation on source monitoring processes (Kusztrits et al., 2021).
Representation of all stimulation timings used in the included studies. (A) Example of possible stimulation timing for one reality monitoring task, summarizing both online (purple, pink and blue frames) and offline (grey frames) approaches. Offline stimulation applied before the encoding includes both pre-post cure and active vs sham designs. (B) Proportion of included studies according to the stimulation timing used. Note that some studies have used both online and offline stimulation in different experiments.
Regarding NIBS techniques, TMS was used in 11 studies, with 5 using rTMS and 6 using cTBS. The other 12 studies employed transcranial electrical stimulation, including tDCS (n = 11) and tRNS (n = 1). The stimulation targets were distributed across various brain regions, with 8 located in the parietal lobe, 5 in the temporoparietal cortex, 9 in the frontal lobe, and 3 using a frontotemporal montage targeting both frontal and temporal cortices.
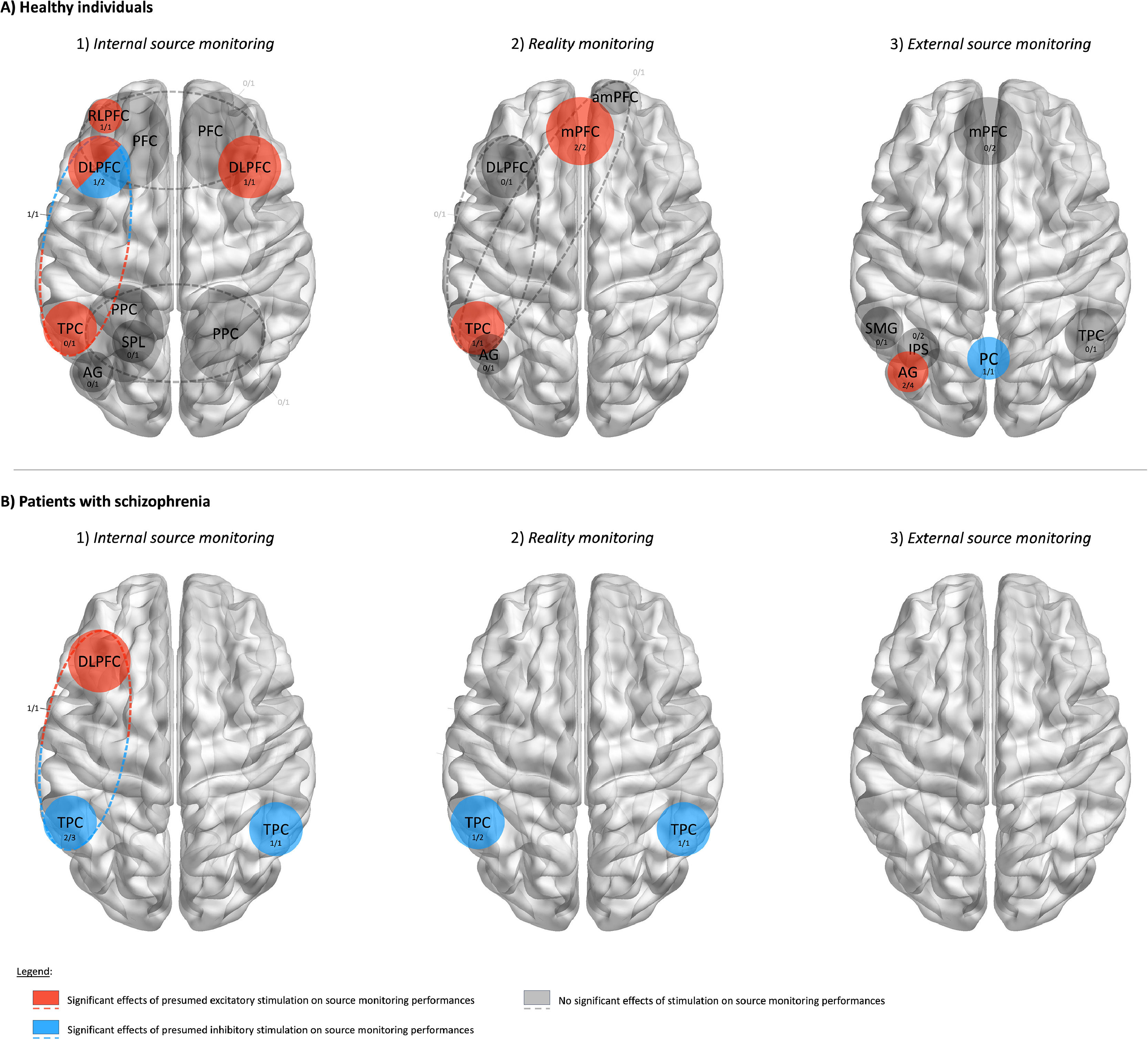
Of the 23 studies, 13 reported a significant effect of NIBS on source monitoring. The results of the individual studies are shown in Table 2 and Fig. 3.
Results of brain regions identified by non-invasive brain stimulation studies as causally involved in each one of the three source monitoring subprocesses for healthy participants (A) and patients with schizophrenia (B). Blue/red colors indicate significant effect of presumed inhibitory/excitatory stimulation applied over a brain region. Dashed lines represent frontotemporal montages of transcranial direct current stimulation. Numbers represent the proportion of studies that found significant effects of stimulation out of all studies that stimulated each brain region.
Individual quality scores of studies ranged from 0.64 to 0.89 (average quality score of 0.79, SD = 0.08) for RCTs and from 0.33 to 0.75 (average quality score of 0.54, SD = 0.29) for case reports (Supplementary material, Table S2). Items with the lowest ratings pertained to randomization and investigator blinding (criteria 5 and 6). Pre-consensus interrater agreement on total scores was excellent (ICC = 0.961, [95 % confidence interval: 0.912–0.983]).
Effects of NIBS on internal source monitoringWe identified 12 studies that investigated the effects of NIBS on the ability to discriminate between different internal sources. Half of them focused on discriminating between memories of covert and overt speech (Brunelin et al., 2006; Jardri et al., 2009; Kusztrits et al., 2021; Mondino et al., 2015, 2016; Sidhoumi et al., 2010), and the other half focused on distinguishing memories from two internal and inner sources, such as two different types of cognitive operations performed on the items (Cui et al., 2020; Huo et al., 2020; Mizrak et al., 2018; Pergolizzi & Chua, 2016; Sestieri et al., 2013; Westphal et al., 2019).
Temporoparietal and parietal lobe stimulationTwo offline clinical studies involving patients with schizophrenia reported significant effects of repeated NIBS sessions applied over the temporoparietal cortex on internal source monitoring. Specifically, 10 sessions of LF-rTMS applied over the left (Brunelin et al., 2006; Jardri et al., 2009) or right (Jardri et al., 2009) temporoparietal cortex reduced the number of source inversions between overt and covert speech. However, in a single-case study, Sidhoumi et al. (2010) did not report any significant effects of 20 sessions of cTBS over the left temporoparietal cortex on internal source monitoring.
In another study using a single session of online anodal tDCS over the left temporoparietal cortex during both the encoding and retrieval phases in healthy individuals, Mondino et al. (2016) reported no significant effect on internal source monitoring performance or recognition of new items.
Furthermore, two studies used online approaches to target the parietal cortex during the retrieval phase of the internal source monitoring task. One applied a single session of tDCS with the anode placed over the left and the cathode over the right posterior parietal cortex (Pergolizzi & Chua, 2016). The other used a single session of HF-rTMS over either the left angular gyrus or the left superior parietal lobule (Sestieri et al., 2013). However, neither study found a significant effect on internal source monitoring or old/new recognition. Nevertheless, they observed that stimulating either the bilateral posterior parietal cortex or the left angular gyrus could increase the response bias towards one specific internal source relative to the other.
Frontal lobe stimulationThe PFC, whether on the left or right hemisphere, has also been a highly targeted brain region in studies investigating the effects of NIBS on internal source monitoring. One study reported improved internal source monitoring performance following 10 sessions of HF-rTMS applied over the right dorsolateral PFC before the task (Cui et al., 2020). However, another offline approach involving 10 sessions of anodal tDCS targeting the left dorsolateral PFC showed no significant effect on internal source monitoring performance, but improved old/new recognition in older adults (Huo et al., 2020).
Westphal et al. (2019) reported that a single session of online anodal tDCS applied to the left rostrolateral PFC during retrieval improved internal source monitoring while cathodal tDCS had no effect on source monitoring or old/new recognition. Similarly, Mondino et al. (2016) did not find any significant effect of online cathodal tDCS applied over the left dorsolateral PFC during the entire task on internal source monitoring. Online tDCS applied over the bilateral dorsolateral PFC, with the anode over the left and the cathode over the right dorsolateral PFC, during the retrieval phase had no effect on internal source monitoring or old/new recognition (Pergolizzi & Chua, 2016).
Finally, Mizrak et al. (2018) proposed that inducing frontal theta oscillations with theta oscillatory-tDCS prior to retrieval could have beneficial effects on source monitoring. They selected the theta frequency range (4–7 Hz) based on previous observations of enhanced theta activity during successful item and source retrieval, compared to successful item recognition without source retrieval (Addante et al., 2011). They reported a reduction in internal source monitoring performance after a single session of anodal oscillatory-tDCS (at 5.5 Hz) applied over the left dorsolateral PFC offline, between the encoding and retrieval phases, with no significant effect on old/new recognition.
Frontotemporal stimulationTwo studies explored the involvement of the left frontotemporal network in internal source monitoring using a paradigm in which participants had to discriminate between words they imagined saying (covert speech) and words they actually said (overt speech). One of these studies, conducted in healthy participants, found that online frontotemporal tDCS with the anode placed over the left superior temporal gyrus and the cathode over the left dorsolateral PFC improved internal source monitoring (Kusztrits et al., 2021). Specifically, it resulted in a reduction in both externalization and internalization errors, which refer to the tendency to misattribute imagined items as said items and vice versa. The authors also reported an improvement in old/new recognition. However, when the same stimulation was applied offline, before the internal source monitoring task, no effects on internal source monitoring or old/new recognition were observed.
In the second study, Mondino et al. (2015) investigated the effects of 10 sessions of tDCS applied with the opposite frontotemporal montage (anode over the left dorsolateral PFC and cathode over the left temporoparietal cortex) in patients with schizophrenia and reported an improvement in source monitoring, specifically a reduction in externalization bias.
Summary of findings on internal source monitoringAmong the 12 studies that investigated internal source monitoring, only 7 reported significant effects of NIBS. Despite some inconsistent findings, these studies highlighted that presumed excitatory stimulation over the left rostrolateral and dorsolateral PFC enhanced internal source monitoring in healthy individuals. Clinical studies showed that presumed inhibitory stimulation applied over either the left or the right temporoparietal cortices improved internal source monitoring in patients with schizophrenia. Results are summarized in Fig. 3A-1 and 3B-1.
Effects of NIBS on reality monitoringWe identified 8 studies that investigated the effects of NIBS on reality monitoring using two types of paradigms in which participants had to discriminate the source of memories: either between self-generated and externally derived items, or between items they had imagined and items they had perceived (saw or heard).
Temporoparietal and parietal lobe stimulationThree studies investigated the effect of applying NIBS with presumed inhibitory effects over the temporoparietal or parietal areas on reality monitoring. In a case report, Jardri et al. (2009) observed improved reality monitoring in a young patient with schizophrenia after 10 sessions of LF-rTMS applied over the left or the right temporoparietal cortices. However, in another case report, Sidhoumi et al. (2010) found no effect of 20 cTBS sessions on reality monitoring in a patient with schizophrenia. Additionally, Kwon et al. (2022) reported no effect of a single session of offline cTBS over the left angular gyrus on healthy participants' reality monitoring performance.
In contrast, a single session of online anodal tDCS over the left temporoparietal cortex during the entire task reduced reality monitoring performance and specifically increased externalization bias (i.e., misattribution of imagined items as being perceived externally) in healthy participants (Mondino et al., 2016).
Frontal lobe stimulationTwo studies targeted the medial PFC with NIBS and reported improved reality monitoring performance: one after a single session of offline HF-rTMS applied over the left medial PFC in healthy participants (Subramaniam et al., 2020), and the other when online tRNS was applied over the bilateral medial PFC during the encoding phase of the task in older but not in younger healthy participants, with no effect on old/new recognition (Mammarella et al., 2017).
One study targeted the left dorsolateral PFC with online cathodal tDCS during the entire task and found no effect on reality monitoring or old/new recognition in healthy participants (Mondino et al., 2016).
Frontotemporal stimulationTwo studies using frontotemporal montage reported no significant effect of tDCS on reality monitoring or old/new recognition. In one study, an online approach was employed, stimulating either during encoding or retrieval, with the anode over the left superior temporal gyrus and the cathode over the right anterior medial PFC (Moseley et al., 2018). In the second study, both online (during the entire task) and offline (before encoding) approaches were used with the anode over the temporoparietal cortex and the cathode over the left dorsolateral PFC (Kusztrits et al., 2021).
Summary of findings on reality monitoringAmong the 8 studies investigating reality monitoring, only 4 reported significant effects of NIBS. These studies emphasized that presumed excitatory stimulation applied over the medial PFC during encoding improved reality monitoring. Additionally, presumed inhibitory stimulation applied over the left or right temporoparietal cortex improved reality monitoring in patients with schizophrenia, whereas presumed excitatory stimulation over the left temporoparietal cortex reduced reality monitoring in healthy participants. Results are summarized in Fig. 3A-2 and 3B-2.
Effects of NIBS on external source monitoringWe identified 7 studies that investigated the effects of NIBS on the ability of healthy participants to discriminate between different external sources.
Parietal lobe stimulationFive studies investigated the effects of NIBS applied over the parietal cortex on external source monitoring in healthy participants. Chen et al. (2016) reported that a single online session of cathodal, but not anodal, tDCS applied over the left posterior parietal cortex during retrieval reduced external source monitoring, with no significant effect on old/new recognition. In contrast, Bonnì et al. (2015) reported that a single offline session of cTBS applied over the medial part of the posterior parietal lobule (the precuneus) between encoding and retrieval improved external source monitoring, but not old/new recognition. However, no significant effects on source monitoring or old/new recognition were found after a single offline session of cTBS delivered over either the left posterior or the left anterior parietal cortices before encoding (Dave et al., 2022).
In two different studies, Yazar et al. (2014, 2017) targeted the left angular gyrus with a single offline session of cTBS delivered between encoding and retrieval. They reported reduced performance when participants had to discriminate between external sources composed of different perceptual modalities (i.e., auditory and visual; cross-modal condition), but not when participants had to discriminate between external sources derived from only one perceptual modality or for stimuli composed of single or multiple features (e.g., in the visual condition, discrimination of left versus right and/or top versus down position).
Two of the aforementioned studies also tested the effects of offline cTBS over the left intraparietal sulcus between encoding and retrieval, reporting no significant effects on external source monitoring (Bonnì et al., 2015; Yazar et al., 2014).
Frontal lobe stimulationTwo studies investigated the effects of anodal tDCS applied over the dorsomedial PFC on external source monitoring, and both reported no significant effect on performance. In one study, stimulation was applied online during encoding (Burden et al., 2021), whereas in the other study, stimulation was applied offline before encoding (Martin et al., 2019). The latter study showed that offline stimulation of the dorsomedial PFC reduced overall memory in the South-East Asian population, but not in the Caucasian population.
Summary of findings on external source monitoringOf the 7 studies investigating external source monitoring, only 3 reported significant effects of NIBS. These studies highlighted that presumed inhibitory stimulation reduces external source monitoring when applied over the left posterior parietal cortex and the left angular gyrus, but improves performance when applied over the precuneus. Results are summarized in Fig. 3A-3 and 3B-3.
DiscussionThe present systematic review provided an overview of the available literature on the effect of NIBS on source monitoring and refined the brain mechanisms underlying source monitoring processes. First, our review found that monitoring the source of internally generated information is supported by the lateral prefrontal regions including the left rostrolateral PFC and the dorsolateral PFC, along with the bilateral temporoparietal cortices, at both encoding and retrieval. These results are consistent with previous neuroimaging results reporting greater activity for both lateral prefrontal and temporoparietal regions during correct internal source recognition (Spaniol & Grady, 2012; Diana, 2017). Interestingly, these prefrontal regions were previously found to support cognitive functions associated with internal processing of information like abstract thinking, mind wandering and spontaneous self-generated thoughts (Dumontheil, 2014; Fox et al., 2016) and intentional decisions in humans (Si et al., 2021). It is worth mentioning that Kusztrits et al. (2021) found counterintuitive results regarding the role of the left dorsolateral PFC and the left temporoparietal cortex in internal source monitoring. In their study, frontotemporal tDCS was applied with the anode placed over the left temporoparietal junction and the cathode over the left PFC to mimic the altered activity pattern observed in patients with schizophrenia, characterized by increased temporoparietal junction activity and reduced PFC activity. They expected to induce internal source monitoring impairments similar to that observed in patients. Notably, the temporoparietal cortices have been associated with mental imagery of the speech (Tian et al., 2016). Given the verbal nature of the internal source monitoring task used in this study, the excitatory stimulation applied over the left temporoparietal junction was expected to enhance brain activity during imagine trials, making it more difficult to differentiate the memories of the two internal sources, leading to a decrease in internal source monitoring performance. However, contrary to these expectations, Kusztrits et al. observed an improvement in internal source monitoring, a finding that contrasts with the findings of Mondino et al. (2015), who reported that the opposite frontotemporal montage could alleviate internal source monitoring deficits in patients. Overall, these inconsistent findings highlight the need for further research to clarify the causal role of the left dorsolateral PFC and the left temporoparietal cortex in internal source monitoring in both healthy and pathological conditions.
Second, we found that reality monitoring is supported by the medial PFC, along with the bilateral temporoparietal cortices. These findings are consistent with a recent meta-analysis that reported greater activation within the right superior/medial frontal gyri and the left supramarginal/superior temporal gyri during retrieval of internally generated items compared to externally perceived items (Lavallé et al., 2023). Our results provide further evidence for the predominant role of the medial PFC in reality monitoring, which has been associated with the ability to correctly discriminate memories of internally from externally derived information (Simons et al., 2017). Specifically, reduced activity in the medial PFC has been correlated with externalization bias (i.e., misattribution of internal information as arising from an external source) in healthy participants, during both reality monitoring encoding and retrieval (Simons et al., 2006; Sugimori et al., 2014). Our results also complement the study of Garrison et al. who investigated the causal involvement of the medial PFC in reality monitoring using fMRI neurofeedback and reported a trend effect of the neurofeedback-induced modulation of the medial PFC activity on reality monitoring performance (Garrison et al., 2021). Future studies should replicate this finding using causal approaches to confirm a causal relationship between medial PFC activity and reality monitoring.
Thirdly, our review highlighted the role of parietal regions, including the left angular gyrus and the precuneus bilaterally in monitoring the source of externally generated information. Namely, targeting the precuneus with presumed inhibitory stimulation enhanced external source monitoring. The precuneus is one of the associative cortices that integrate both external and self-generated information and support a wide range of higher-order cognitive functions, including self-referential processing, mental imagery, and episodic memory (Cavanna & Trimble, 2006). One fMRI study has reported that activation of the left posterior precuneus was greater at retrieval for correct source recognition of imagined stimuli compared to perceived stimuli (Lundstrom et al., 2003). However, a meta-analysis of fMRI studies reported activation in the precuneus for correct external source recognition (Spaniol et al., 2009). These findings suggest that the precuneus plays a role in source monitoring, but further studies are needed to understand how. In contrast, the results of our review suggest that activity within the left posterior parietal cortex (i.e., the inferior parietal lobule) or the left angular gyrus supports external source monitoring. Interestingly, these regions have been associated with numerous cognitive processes that involve the processing of external information and cognitive states that give the illusion of an external perspective, such as theory of mind and out-of-body experiences (Seghier, 2013). In addition, activation in the left inferior parietal lobule and the left intraparietal sulcus has been associated with correct external source recognition (Spaniol et al., 2009). Although some evidence suggests that the left angular gyrus and the left inferior parietal lobule are involved in the processing of external information, some studies included in this review failed to find a significant effect of NIBS applied over these regions on external source monitoring. Further studies will help to understand the involvement of parietal regions in external source monitoring, possibly by homogenizing both the stimulation procedure and the external source monitoring paradigms.
In this review, we have summarized the brain bases of source monitoring by examining the effects of NIBS on the three source-monitoring sub-processes separately. However, it is unlikely that the cerebral bases of these three sub-processes are completely distinct. In fact, they overlap in the mechanisms they involve, e.g., the self-generation/imagination that is common to internal source monitoring and reality monitoring, and the perceptual processes that are common to reality monitoring, external source monitoring, and even internal source monitoring when speech production, and hence auditory perception, is involved. And indeed, we have reported that some regions, such as the temporoparietal regions, are involved in both reality monitoring and internal source monitoring. Interestingly, in the light of the classification proposed by Damiani et al. (2022), these temporoparietal regions are particularly involved in paradigms that distinguish between imagined information and information in the auditory modality (heard or performed speech). Specifically, presumed inhibitory NIBS over the temporoparietal regions reduced imagined-heard/performed speech confusion in patients with schizophrenia, whereas presumed excitatory NIBS over these brain regions increased imagined-heard confusion in healthy participants. These findings suggest that targeting temporoparietal regions with NIBS modulates the sensory signals associated with the information and drives the source attribution towards either the imagined source (by attenuating sensory signals) or the auditory source (by increasing signal in temporoparietal regions). Similarly, according to this alternative classification, the dorsolateral and rostrolateral PFC appear to be involved in distinguishing between internal imagined sources, and the precuneus and the left angular gyrus in distinguishing between visual sources. In contrast, the mPFC seems to be involved in distinguishing between internal and external sources, regardless of the modality (imagined or performed for internal sources, visual or auditory for external sources). These brain mechanisms are consistent with those recently proposed by Dijkstra et al. (2022), who suggest that source attribution is based on first-order perceptual and cognitive processes that distinguish perception from imagination (here, for example, supported by the DLPFC for imagination and the temporoparietal region for perception), and on a second-order process that evaluates these sensory and cognitive control aspects to make a source attribution, which would be based in the mPFC.
Several of the studies reviewed reported that stimulation selectively modulated source monitoring or old/new recognition as a measure of item memory. These findings are consistent with a large body of evidence suggesting that source and item memory processes can be dissociated and would rely, at least in part, on separate brain networks (Mitchell & Johnson, 2009), although the exact relationship between these processes is still being investigated and questioned (Guo et al., 2021). It is worth mentioning some nuances regarding the specificity of the role of the aforementioned brain regions in source monitoring process and/or item memory. Indeed, two studies reported a significant modulation of item memory with no effect on internal source monitoring after stimulation of the left dorsolateral PFC (Huo et al., 2020) or the dorsal medial PFC (Martin et al., 2019). However, several studies reported the opposite pattern, i.e., that stimulation of prefrontal regions modified source monitoring performance but not item memory (Mammarella et al., 2017; Mizrak et al., 2018; Westphal et al., 2019). Similarly, one study reported significant simultaneous modulations of source monitoring performance and item memory with frontotemporal stimulation (Kusztrits et al., 2021). The conceptual distinction between item memory and source monitoring lies in the distinction between what is detected as central in the information and what is detected as contextual. The dual-process model proposed that source monitoring relies on conscious recollection of the encoded information, whereas item memory rather relies on familiarity, then on conscious recollection if necessary, making recollection demand greater for source monitoring than for item memory (Yonelinas, 1999). In some cases, however, the source can be intrinsic to the item, and remembering the source can be comprised in the item memory process and also relies on familiarity (Diana et al., 2008). Thus, the aforementioned brain network overlap between source monitoring and item memory reported in some studies can be due to the nature of stimulus used in the experimental paradigms. Further studies are needed to elucidate the differences and/or similarities between these two processes and their neural bases.
Our review benefits from the inclusion of several studies in patients with schizophrenia, a population that has been reported to have deficits in source monitoring (Damiani et al., 2022). The results of these studies are relevant to a better understanding of the brain basis of source monitoring, as they highlight a beneficial effect of presumed inhibitory NIBS applied over the temporoparietal region on source monitoring deficits in schizophrenia, thus providing additional and causal evidence for the involvement of this brain region in source monitoring. Indeed, fMRI studies have already reported abnormal brain activations during source monitoring processes in patients with schizophrenia, namely decreased activity in the mPFC and increased activity in the superior temporal regions compared to healthy controls (for review see Kowalski et al., 2021). Thus, the beneficial effects of NIBS on source monitoring may occur by rebalancing the activity pattern in prefrontal and temporoparietal cortices, which are disrupted during source monitoring in patients with schizophrenia (Wang et al., 2011). Considering the multi-level system proposed by Dijkstra and collaborators (2022), our results suggest that source monitoring deficits in patients with schizophrenia are, at least, caused by impaired bottom-up sensory processing. However, a recent meta-analysis has found that patients with schizophrenia rather have alterations in the top-down cognitive control and the source attribution levels, but not in the bottom-up sensory processing (Damiani et al., 2024). Further studies are needed to gain a more complete understanding of the brain mechanisms underlying source monitoring deficits in patients with schizophrenia. Nevertheless, by identifying brain circuits involved in source monitoring, our review may provide relevant targets for neuromodulation, offering therapeutic options for populations with source monitoring deficits, including psychiatric populations such as patients with schizophrenia (Damiani et al., 2022) patients with obsessive-compulsive disorder (Lavallé et al., 2020a; 2020b), and patients with Alzheimer's disease (El Haj et al., 2020).
Our review has some limitations that should be acknowledged. First, the reviewed studies included a large number of source monitoring paradigms, differing in the nature of stimuli used, task instructions, and outcomes calculated to assess source monitoring and/or old/new recognition performance. However, this heterogeneity makes it possible to be more representative of all situations in which source monitoring processes can be used. Second, there was great variability in the type of NIBS (i.e., magnetic vs. electrical, excitatory vs. inhibitory), but also in the parameters used, such as duration, intensity, frequency, current density, number of sessions and stimulation timing with respect to the source monitoring task. In addition, the choice of the control condition may substantially impact the results of a study, due to non-optimal blinding efficacy or to variable biological effects underpinned by the different sham stimulation protocols (Fonteneau et al., 2019). These parameters, related to the stimulation procedure and its application, may constitute confounding factors that influence the effects of NIBS on cortical excitability and underlying cognitive functions. Nevertheless, the inclusion of all types of NIBS provides the most comprehensive view of source monitoring brain networks. When investigating brain mechanisms with NIBS, other confounding factors inherent to the participants may be taken into account. Indeed, the participant's age, brain anatomy, arousal and brain states (e.g., cognitive task or symptom expression during stimulation), beliefs and expectations about NIBS efficacy or side effects, can influence the effects of NIBS on cortical excitability (Bergmann & Hartwigsen, 2021). Several factors are already controlled in NIBS studies, but some are more difficult to control, namely those related to the history of neuron's postsynaptic plasticity (i.e., metaplasticity). Because of these factors, there are some controversies about the exact physiological mechanisms of NIBS techniques on cortical excitability and their behavioral effects. Indeed, the actual effects of stimulation as a function of stimulation polarity are not fully understood, and it is not clear whether behavioral improvements are associated with greater brain activity (Polanía et al., 2018). Furthermore, only a very limited number of studies have combined the stimulation procedure with neuroimaging techniques (Cui et al., 2020; Dave et al., 2022; Huo et al., 2020; Jardri et al., 2009; Mizrak et al., 2018), making it impossible to validate that the behavioural effects reported in the other studies are actually due to a change in brain activity at the stimulation site. A recent review of TMS-fMRI studies suggests that stimulation does not modulate brain activity at the target site but rather has remote effects within neural networks associated with the targeted brain region (Rafiei & Rahnev, 2022). NIBS would seem to be a more appropriate tool for testing causality at the level of a brain network or circuit rather than at the level of a brain region. Moreover, only few studies have included a control condition consisting of NIBS applied over an active control site (i.e., a brain region not involved in source monitoring), in addition to a sham condition, to test whether the effects of NIBS on source monitoring are specific to the targeted brain region. With this in mind, future stimulation studies should control for many potentially confounding parameters as possible, could personalize the stimulation protocol to use more accurate target, and should be combined with appropriate neuroimaging techniques to capture stimulation-induced neuronal changes to better understand the physiological and behavioral effects of NIBS and their causal relationship.
ConclusionsThe results of the current systematic review deepen the understanding of the brain mechanisms of source monitoring and describe the involvement of a lateral prefrontal-temporoparietal circuit in internal source monitoring, a medial prefrontal-temporoparietal circuit in reality monitoring, and a parietal circuit including the precuneus and the left angular gyrus in external source monitoring. These findings provide relevant therapeutic options for populations with source monitoring deficits including psychiatric populations. Further randomized controlled studies are now needed to confirm the role of these regions in source monitoring using causal approaches such as stimulation, but also neurofeedback or other data processing methods for fMRI. In any case, standardized procedures controlled for maximum potential bias should be used to provide stronger evidence for the brain mechanisms underlying source monitoring.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.