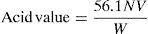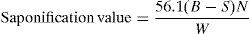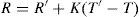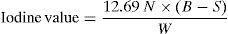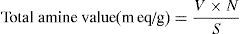Cationic imidazoline surfactants have already gained much interest due to their wide range of industrial applications. In the present scenario on surfactants research, surfactants are being converted into their corresponding gemini surfactants because of their uncommon self-assembling characteristics and unique interfacial activity. Gemini surfactants are showing many times better performance properties than their conventional monomeric counterparts because of their much lesser CMC and far better ability to reduce surface tension. These extraordinary properties make them potential candidates in surfactant industries. Conventional methods used in the synthesis of gemini surfactants are suffering with few disadvantages. Long reaction duration is one of the major issues involved in the synthesis of gemini surfactants. Current research involves the microwave synthesis of C12C18 saturated/unsaturated fatty acids based cationic gemini imidazoline surfactants in much lesser time. An effort has also been made to make them readily degradable through incorporating a carbonate linkage into their spacer moiety. Structural characterization of synthesized gemini surfactants have been achieved through 1H-NMR and FT-IR. Cheaper and easily available plant based materials and microwave assisted organic synthesis of cationic gemini imidazoline surfactants with easily degradable chemical moiety make them to be considered as novel and green surfactants.
Imidazolinium surfactants belong to the class of cationic surfactants which are presently receiving interests in worldwide detergent markets due to their varied range of applications (Bajpai & Tyagi, 2006) (These are comprehensively used as fabric softeners and antistatic agents. Their properties of desirable storage stability, viscosity, dispersability and fabric conditioning make them useful in laundry applications and various industrial applications (Tyagi, Tyagi, & Pandey, 2007).
Conventional imidazolinium surfactant molecules are made up of a long hydrophobic hydrocarbon tail with a polar imidazolinium head group. In comparison to that, their geminis has two hydrocarbon tails and two polar imidazolinium head groups linked by a ‘spacer’ in the sequence: hydrocarbon tail/imidazolinium group/spacer/imidazolinium group/hydrocarbon tail (Dahan & Sundararajan, 2014). These surfactants hold better surface active properties like lower critical micelle concentration (cmc) values, better softening, dispersability and corrosion inhibition properties than their conventional counterparts (Shukla & Tyagi, 2006).
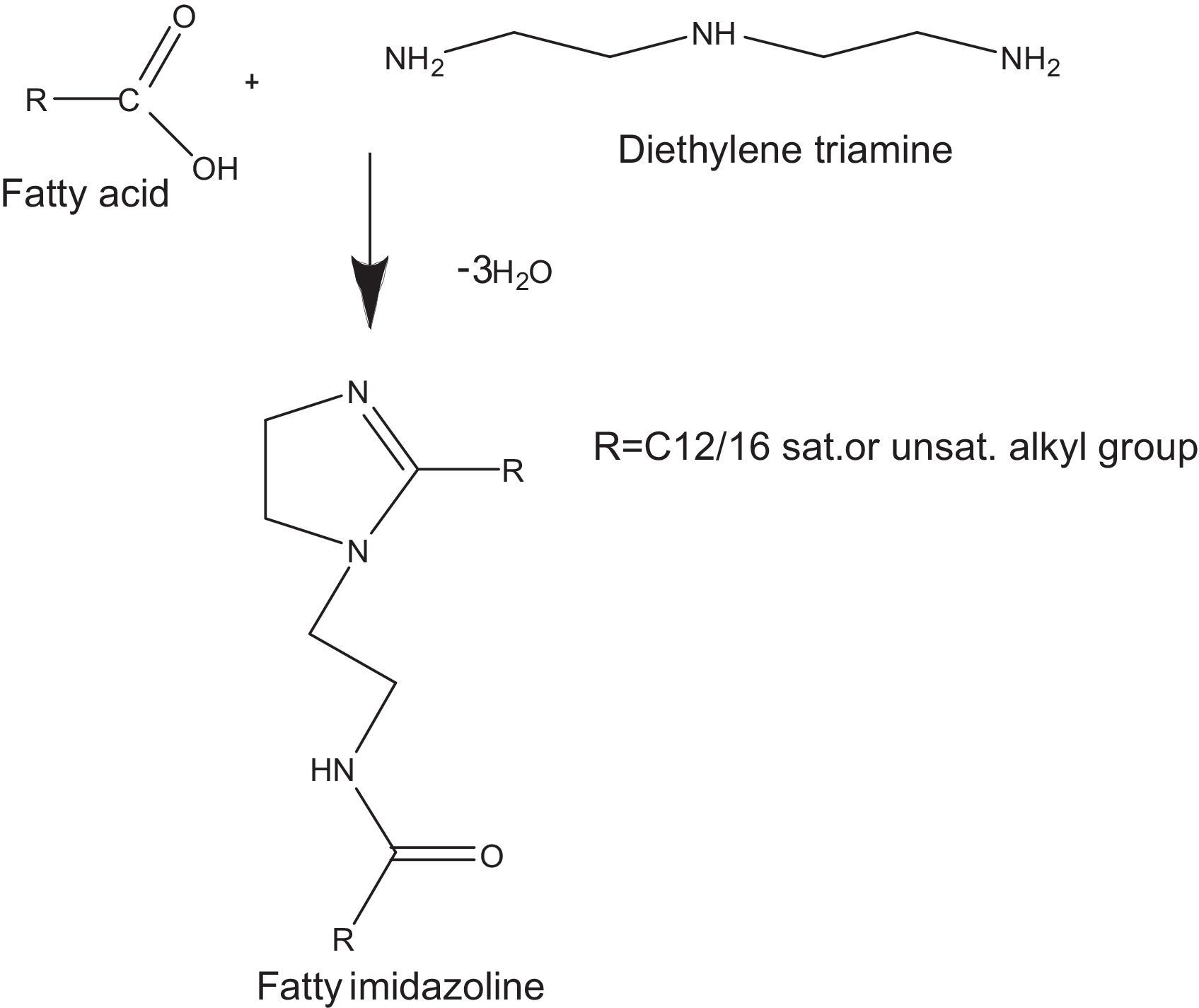
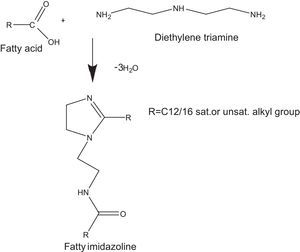
Most often, the imidazolines are prepared by reacting polyamines with a fatty acid, followed by reaction with an alkylating agent to the corresponding quaternary salts (Pracht & Nirschl, 1978). In order to prepare dialkylimidazolines, 2 moles of fatty acid are charged to 1 mole of polyamine and the temperature raised up-to 200°C. These conventional methods suffer from disadvantages such as long times, low yield of the products and tedious work-up.
In the past few years, using microwave energy to heat and drive chemical reactions has become increasingly popular due to generally low reaction time, better yield and high purity of products.
Till date no data available to synthesize cationic gemini imidazoline surfactants using microwave assisted organic synthesis. Present research explored the possibility of synthesizing readily degradable cationic gemini imidazoline surfactants through microwave irradiation in order to reduce long reactions durations from hours to minutes and to avoid tedious work. To enhance the degradability of surfactants easily breakable carbonate linkage has been incorporated in between the spacer moiety (Banno, Kawada, & Matsumura, 2010).
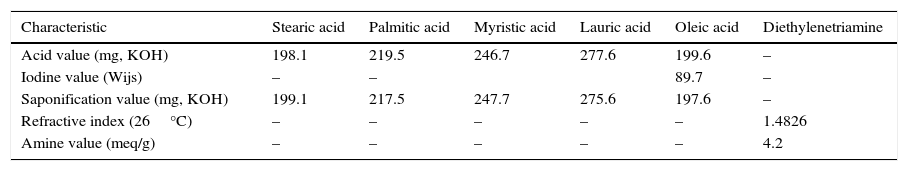
2Experimental2.1MaterialsDifferent fatty acids (Stearic, Plamitic, Myristic, Lauric and Oleic acid), Diethylenetriamine (DETA), diphenylcarbonate, iodoalkanol, acetone and ethyl acetate were of analytical grade, purchased from Sigma Aldrich, Banguluru.
2.2Methods2.2.1Characterization of raw materialsFatty acids were characterized on the basis of their acid Value (AV), saponification Value (SV) and iodine Value (IV) according to the BIS (Bureau of Indian Standards) methods (Mishra & Tyagi, 2007) (Table 1).
Characterization of fatty acids and DETA.
| Characteristic | Stearic acid | Palmitic acid | Myristic acid | Lauric acid | Oleic acid | Diethylenetriamine |
|---|---|---|---|---|---|---|
| Acid value (mg, KOH) | 198.1 | 219.5 | 246.7 | 277.6 | 199.6 | – |
| Iodine value (Wijs) | – | – | 89.7 | – | ||
| Saponification value (mg, KOH) | 199.1 | 217.5 | 247.7 | 275.6 | 197.6 | – |
| Refractive index (26°C) | – | – | – | – | – | 1.4826 |
| Amine value (meq/g) | – | – | – | – | – | 4.2 |
Amine value of DETA was determined as per BIS standards and refractive index of DETA was checked using refractometer.
2.2.1.1Determination of acid value of fatty acidsSample was mixed thoroughly before weighing. A suitable quantity of the sample was taken in a 200-ml conical flask. 25ml of freshly neutralized hot ethyl alcohol and about one millilitre of phenolphthalein indicator solution were added. Mixture was boiled for about five minutes then titrated while as hot as possible with standard aqueous alkali solution, shaking vigorously during titration.
Calculation:
where V, volume in ml of standard sodium hydroxide solution used, N, normality of standard NaOH solution. W, weight in g of the material taken for the test.2.2.1.2Determination of saponification value of fatty acidsSample was melted and dried completely. The sample was mixed thoroughly, and weighed accurately by a difference of about 1.5–2.0g of the sample in a standard joint conical flask. 25ml of the alcoholic KOH solution was added and the reflux air condenser was connected to the flask. The flask was heated on a hot-plate or on water-bath for not more than one hour. It was boiled gently but steadily until the oil/fat was completely saponified as indicated by absence of any oily matter and appearance of a clear solution. After the flask and condenser cooled somewhat, the inside of the condenser was washed down with about 10ml of hot C2H5OH neutral to phenolphthalein. About one ml. of phenolphthalein indicator solution was added, and titrated with standard HCl. We conducted a blank titration at the same time in identical manner.
Calculation:
where B, volume in ml of standard HCl required for the blank; S, volume in ml of standard HCl required for the sample; N, normality of the standard HCl; W, weight in g of the sample taken for the test.2.2.1.3Determination of refractive index of DETATemperature of the refractometer was adjusted to 40.0±0. After ensuring that the prism was clean and completely dry, a few drops of the sample were placed on the lower prism. The prism was then closed and tightened firmly with the screw-head, and allowed to stand for one or two minutes. The instrument was adjusted to obtain the most distinct reading possible, and the refractive index was determined.
Temperature corrections
where R is the reading of the refractometer reduced to the specified temperature T°C; R′ is the reading at T°C; K is the constant; T′ is the temperature at which the reading is taken; T is the specified temperature.
2.2.1.4Determination of iodine value of fatty acidsThe sample (0.2–0.3g) was weighed accurately into a 500ml iodine value flask. The weighed sample was dissolved in 25ml carbon tetra chloride. Then 25ml of wijs solution (Iodine mono chloride in glacial acetic acid) was added and a glass stopper was replaced after wetting it with potassium iodide solution (freshly prepared by dissolving 10g potassium iodide, free from potassium iodate, in 90ml of water). The flask was swirled and allowed to stand in the dark for about 45min. After taking out the flask, 15ml of potassium iodide and 100ml of distilled water was added, rinsing in the stopper also. The iodine, which liberated, was titrated against a standard sodium thiosulphate solution until the colour of the solution was straw yellow. 1ml of (1%) starch solution was added as an indicator at this stage. The titration was continued until a blue colour formed on addition of starch and disappeared after thorough shaking with the stopper on. A blank determination was also made simultaneously under similar conditions and the iodine value of the sample was calculated using the following relationship:
where, N, normality of sodium thiosulphate solution. B, volume (inml) of standard sodium thiosulphate solution used in blank determination; S, volume (inml) of standard sodium thiosulphate solution used with sample. W, weight of the sample in g.2.2.1.5Determination of amine value of DETAFour gram of DETA was taken in 250ml flask followed by the addition of 50ml of alcohol. The mixture was boiled for 1min to drive off any free ammonia that may be present. The mixture was cooled at room temperature. 5 drops was added of bromophenol blue indicator and titrated, while swirling, with 0.2N HCI to the yellow end point.
Calculation:
where V is the HCI required for titration of the specimen, N is the normality of the HCI solution, S is the specimen weight of sample.2.2.2Synthesis of cationic gemini imidazoline surfactants2.2.2.1Synthesis of imidazolines40mmol of the corresponding fatty acid (Lauric acid, Myristic acid, Palmitic acid, Stearic acid and oleic acid), 20mmol of DETA (diethylenetriamine) and 20g of calcium oxide were taken carefully in the beaker of microwave synthesizer (Saneo uwave-1000). The reaction mixture was irradiated using required power of microwave oven for the required reaction duration and temperature.
The reaction mixture was allowed to cool at room temperature. Ethyl acetate was added (80–100ml), and the mixture was reheated until boiling and filtered off while hot, and the filtrate was concentrated under vacuum to dryness, yielding the corresponding product as a white to yellowish brown, solid to semisolid substances.
Optimization of reaction variables have been made in order to achieve maximum yield of product. Yield of the product was calculated as per stoichiometry of reaction (Fig. 1). Purity of the product has been confirmed through their spectral analysis (Bajpai & Tyagi, 2008a).
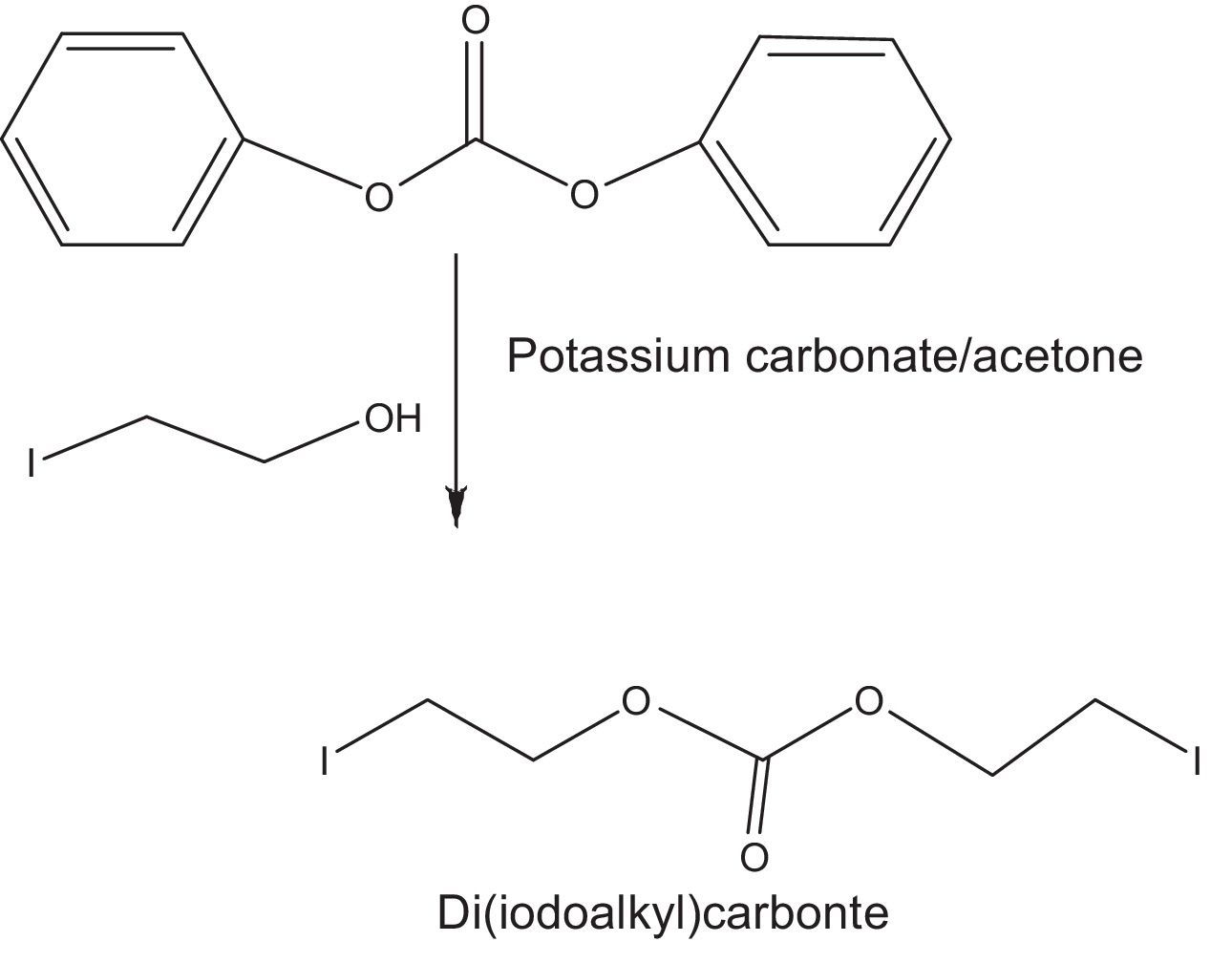
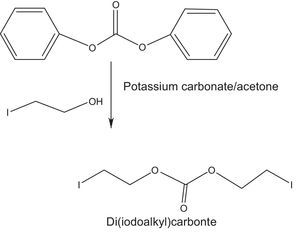
2.2.2.2Synthesis of di(iodoethyl) carbonate as quaternizing agentDi(idodoalkyl) carbonate was prepared by the carbonate exchange reaction of diphenyl carbonate and iodoalkanol using 1:2 molar ratio of both the reactants in acetone (Fig. 2). Reaction was carried out at room temperature through stirring the reaction mixture (Banno et al., 2010).
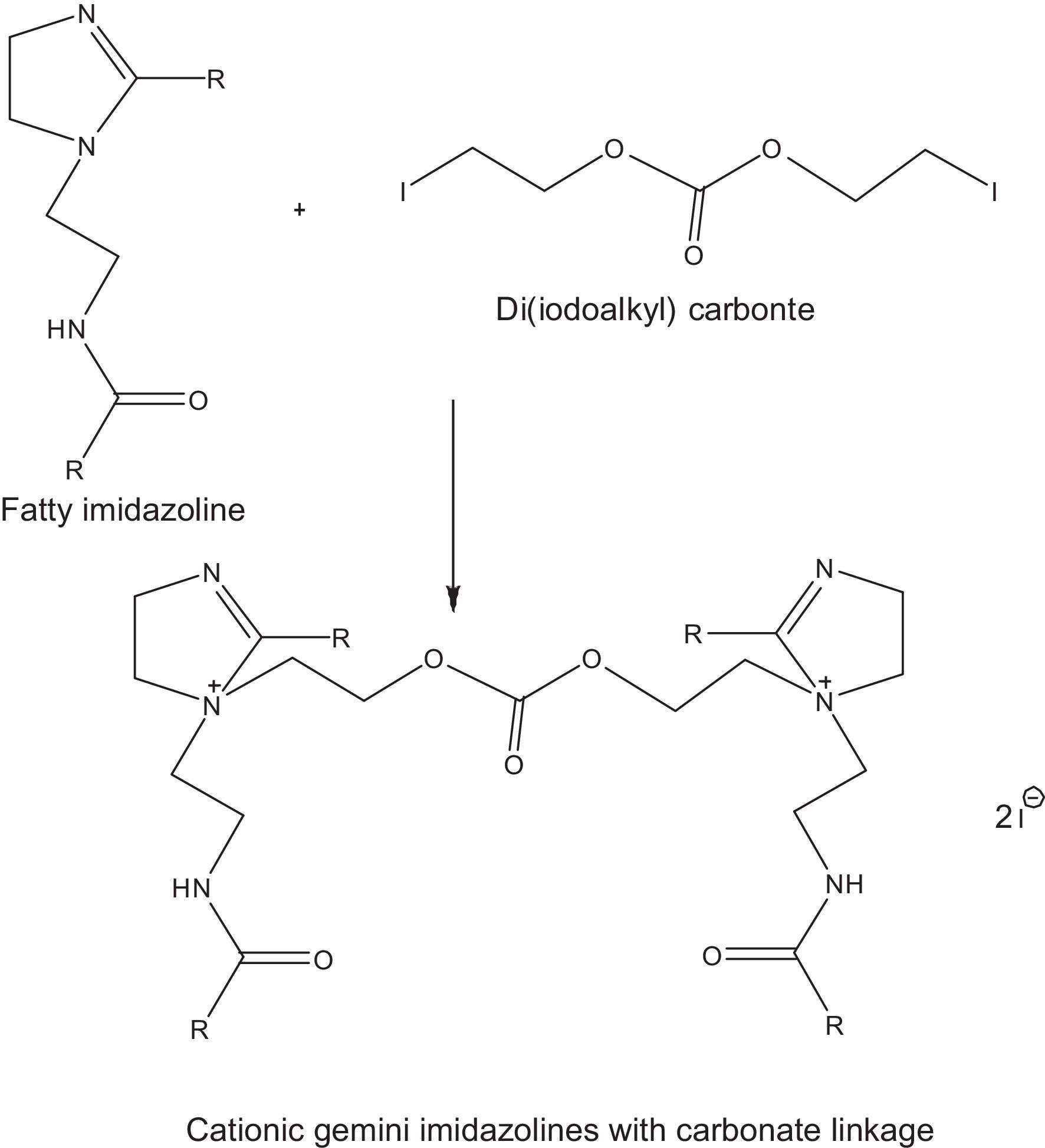
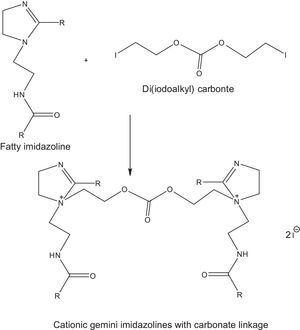
2.2.2.3Synthesis of cationic gemini imidazoline surfactants with carbonate linkageCationic gemini imidazoline surfactants have been synthesized through microwave irradiation of synthesized Di (iodoalkyl) carbonate and synthesized imidazolines. Synthesized gemini imidazolines were recrystallized using 50:50 ethyl acetate/acetone solution. Optimization of reaction variables was made in order to achieve maximum yield of product. Yield of the product was calculated as per stoichiometry of reaction (Fig. 3). Purity of the product was confirmed through their spectral analysis.
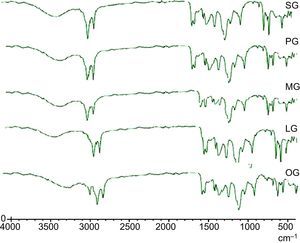
2.2.2.4Spectral characterization of cationic gemini imidazoline surfactantsFT-IR spectra were obtained by using model vector 22, Germany. KBr (Potassium bromide) pellets of the product were subjected to analysis of 20 scans.
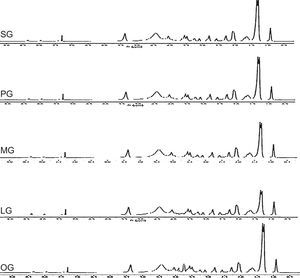
1H NMR and 13C NMR spectra of products were carried out using FT NMR system of A Lambda JEOL JNM-LA 400. Chemical shifts are reported as (ppm) relative to TMS (tetramethyl silane)
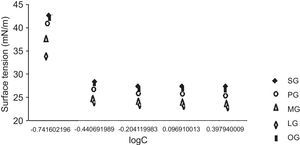
2.2.2.5Surface active properties of cationic gemini imidazoline surfactants.2.2.2.5.1Surface tension and CMCSurface tension and CMC of surfactant solutions were measured by a DCAT tensiometer using the platinum ring detachment technique. Surface tension of different concentrations of surfactant solutions is in Figure 7. All measurements were taken at 298.15K (Bajpai & Tyagi, 2008b).
2.2.2.5.2Cationic contentCationic content of surfactants were determined as per BIS using the following method (Bajpai & Tyagi, 2008a). 10ml of standard Sodium lauryl surfactant were taken in a 100ml stoppered graduated cylinder, to which 15ml of chloroform were added, followed by the addition of 25ml of methylene blue solution.
This solution was titrated against a known concentration of cationic imidazoline surfactants solution.
The end point was the reading of burette at which, intensity of blue colour was found same in both the chloroform layer and the aqueous layer.
2.2.2.5.3DispersabilityDispersing ability of surfactants was measured by adding carbon black to the 1ml of 1% surfactant solution. After vigorous shaking, surfactant solution was allowed to stand. Reading was taken by observing the cloudiness and height of solution (Bajpai & Tyagi, 2008b).
2.2.2.5.4SofteningSoftening of surfactant solution was determined using a panel ‘feel test’ on a 100% cotton fabric (Egan, 1978). Ranking of softening is assigned as 1 for worst and 17 for best.
2.2.2.5.5RewettabilityRewettability of surfactant solutions was determined using dye wick up test. In a measuring cylinder, 1% aqueous solution of acid blue dye was taken. Cationic gemini surfactant treated 4×4cm 100% cotton swatches suspended into the dye solution. Wetting time is the time noted down from the second the fabric was kept into the solution until the moment it started going down (Ginn, Schenach, & Jungermann, 1965).
3Results and discussion3.1Synthesis of fatty imidazolines3.1.1Effect of molar ratio on the synthesis of imidazolines at constant temperature and reaction durationIn the current research, reactions were carried out by irradiating different molar ratios of fatty acids and DETA at constant reaction durations. In the cases of all the fatty acids, maximum yield of imidazolines were obtained by reacting 2mol. of fatty acid with one mol. of DETA. These results were also supported by the stoichiometry of reaction (Fig. 1).
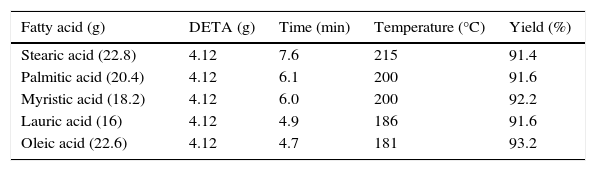
3.1.2Effect of time on the synthesis of imidazolines at constant molar ratio of reactants and reaction durationEffect of time on the synthesis of imidazolines was studied using constant molar ratio of fatty acids and DETA (2:1). Results revealed that optimum reaction duration was different for different fatty acids. This was due to the different fatty alkyl chain and unsaturation present in different fatty acids. Maximum yield of stearic acid based imidazoline was obtained at 9min of reaction duration. Decrease in the chain length of fatty alkyl chain gradually decreased the optimum temperature required to get maximum yield of products. Maximum yield of Lauric acid based imidazolines were obtained at 6min of reaction duration. In case of Oleic acid based imidazolined optimum temperature found was again 6min, which may be due to the unsaturation and liquid state of Oleic acid (Table 2).
Synthesis of fatty imidazolines using different fatty acids under optimum reaction conditions.
| Fatty acid (g) | DETA (g) | Time (min) | Temperature (°C) | Yield (%) |
|---|---|---|---|---|
| Stearic acid (22.8) | 4.12 | 7.6 | 215 | 91.4 |
| Palmitic acid (20.4) | 4.12 | 6.1 | 200 | 91.6 |
| Myristic acid (18.2) | 4.12 | 6.0 | 200 | 92.2 |
| Lauric acid (16) | 4.12 | 4.9 | 186 | 91.6 |
| Oleic acid (22.6) | 4.12 | 4.7 | 181 | 93.2 |
(Fatty acid: DETA=2:1).
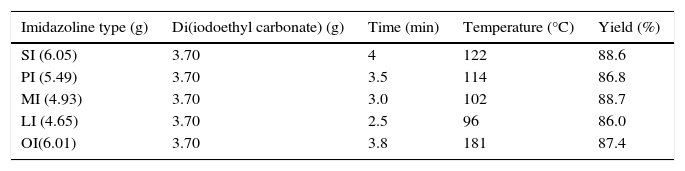
In the quaternization of imidazolines, reactions were carried out using different molar ratios of reactants at a constant temperature of 80°C using a reaction duration of 2min. Maximum yield was obtained at 2:1 molar ratio of imidazoline and di (iodoethyl) carbonate (Table 3).
Synthesis of cationic imidazoline surfactants using different imidazolines under optimum reaction conditions.
| Imidazoline type (g) | Di(iodoethyl carbonate) (g) | Time (min) | Temperature (°C) | Yield (%) |
|---|---|---|---|---|
| SI (6.05) | 3.70 | 4 | 122 | 88.6 |
| PI (5.49) | 3.70 | 3.5 | 114 | 86.8 |
| MI (4.93) | 3.70 | 3.0 | 102 | 88.7 |
| LI (4.65) | 3.70 | 2.5 | 96 | 86.0 |
| OI(6.01) | 3.70 | 3.8 | 181 | 87.4 |
SI, PI, MI, LI and OI are the imidazolines based on stearic acid, palmitic acid, myristic acid, lauric acid and oleic acid, respectively.
This molar ratio also confirms the stoichiometry of reaction (Fig. 2).
3.2.2Effect of time on the quaternization of imidazolinesAt constant 1:1 molar ratio of Imidazolines and Di(iodoethyl)carbonate, maximum yield of cationic gemini imidazoline surfactants were obtained in between 2 and 4min of reaction duration, depending upon the chain length and unsaturation present in the fatty alkyl tail. In case of stearic acid based imidaolines, maximum yield of their corresponding gemini surfactant was obtained at 4min of reaction duration. Decrease in the chain length of imidazolines taken for quaternization gradually decreased the optimum temperature required to get maximum yield of gemini surfactant. In case of oleic acid based imidazoline, although the fatty alkyl chain length was similar to that of stearic acid based imidazolines, unsaturation present in alkyl chain decrease the optimum temperature required to get its geminis (Table 3).
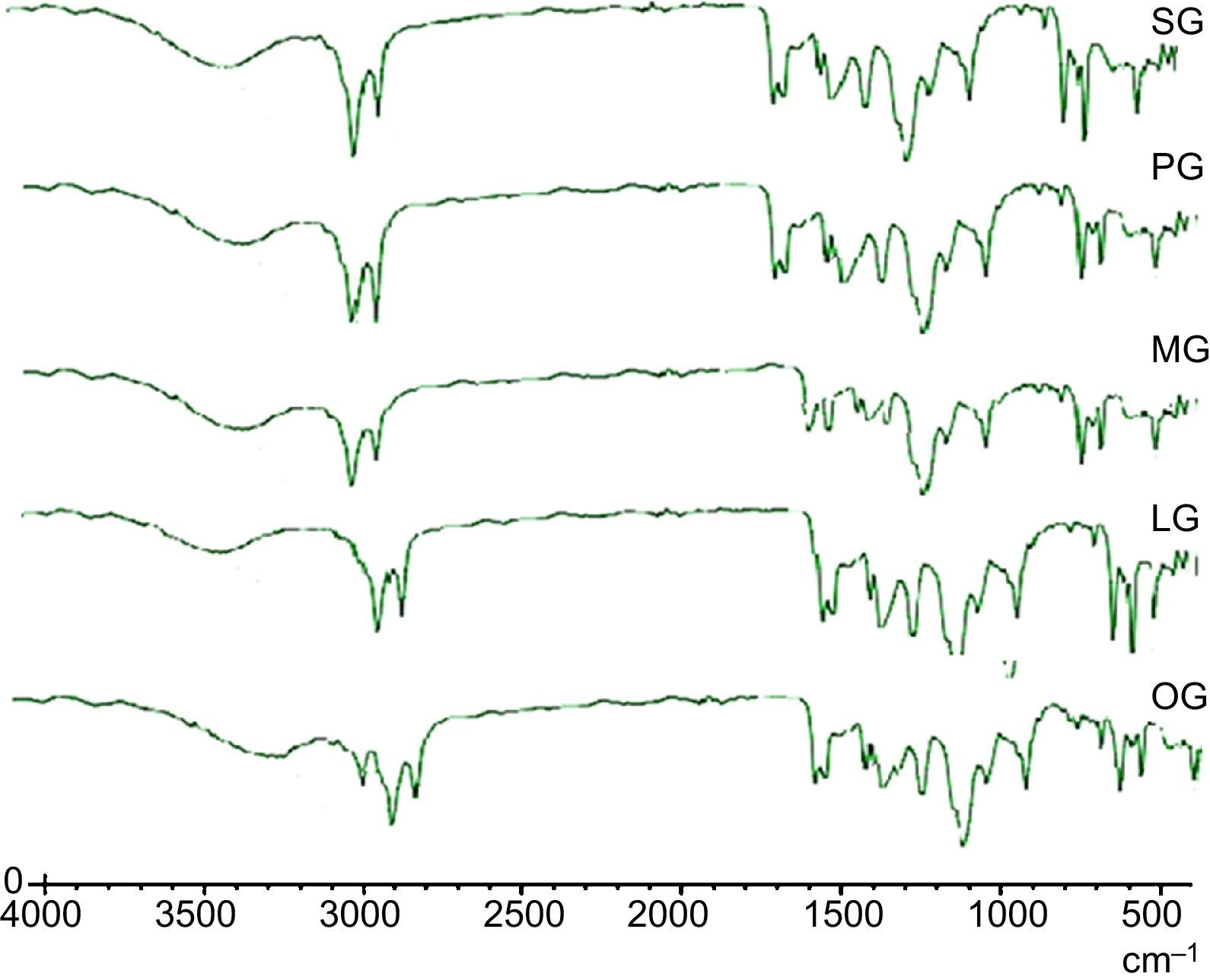
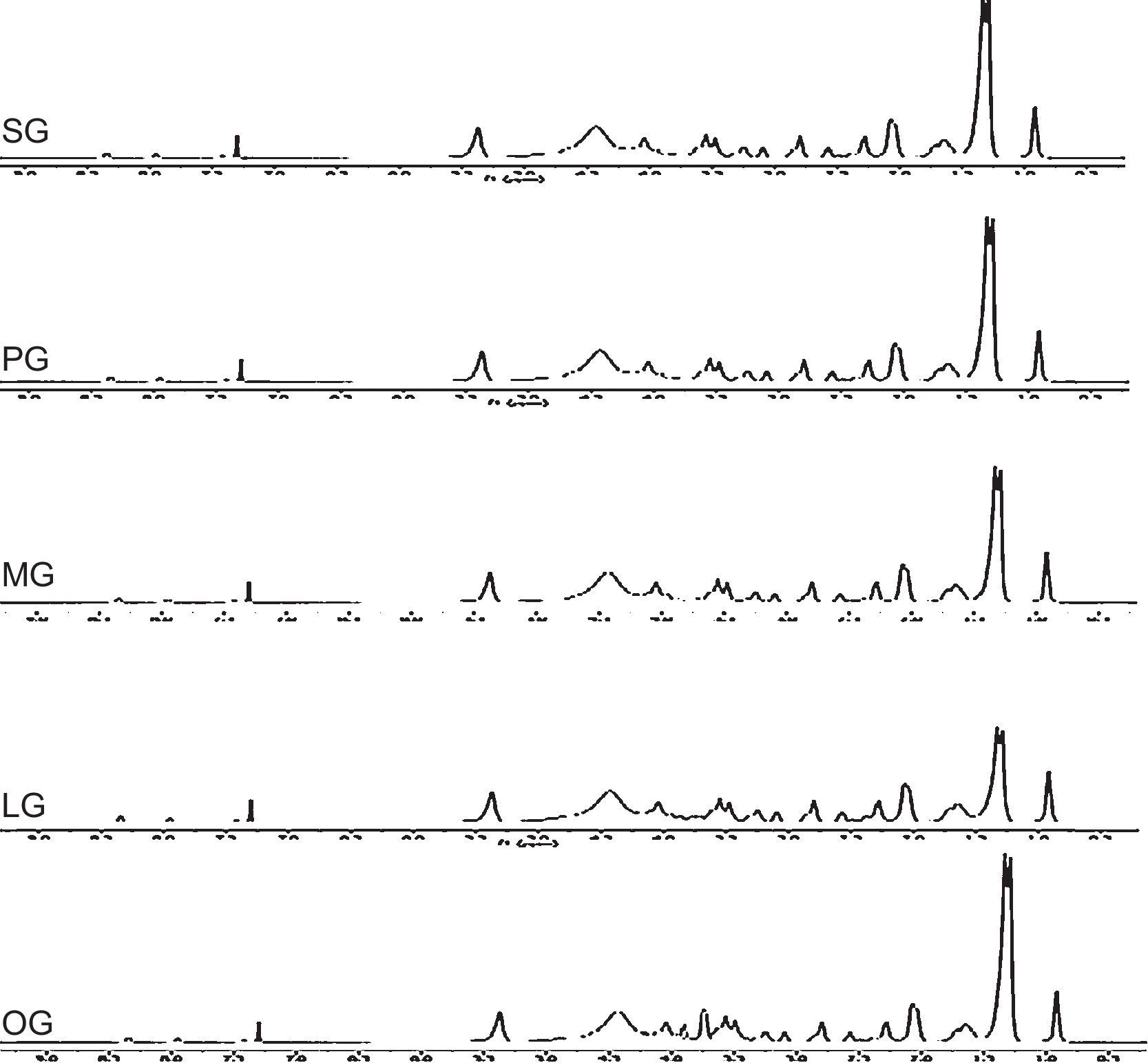
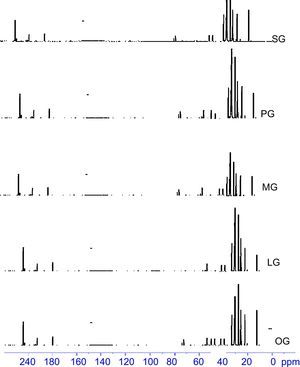
3.3Spectral characterizations of products3.3.1Stearic acid based cationic gemini imidazoline surfactants (SG)FT-IR-Peaks at 1651.22cm−1 confirm the CO str- amide linkage, peak at 1604.42cm−1 confirms the preparation of imidazoline ring. Presence of peaks at 695.55cm−1, 726.54cm−1 and 758.94 confirms the quaternization of ring. Peaks at 3381.52cm−1 corresponds to NH str- of amide linkage. FTIR spectra of synthesized cationic gemini imidazoline surfactants are given in Figure 4.
1H-NMR-peaks at 2.74ppm and 3.5ppm correspond to NCH2CH2N methylene group of imidazoline ring. Peak at 7.27ppm corresponds to the proton of CONH group whereas signal at 4.2ppm confirms the presence of CH2 group adjacent to amide linkage. Quaternized product shows four peaks for two CH2 groups of rings at 3.5ppm, 4.1ppm and 2.7ppm, 2.9ppm which is due to the resonance in between two nitrogen of imidazolinium ring. The peak at 4.3ppm shows the presence of CH2 adjacent to carbonate linkage, present in the spacer group. 1H-NMR spectra of synthesized cationic gemini imidazoline surfactants are given in Figure 5.
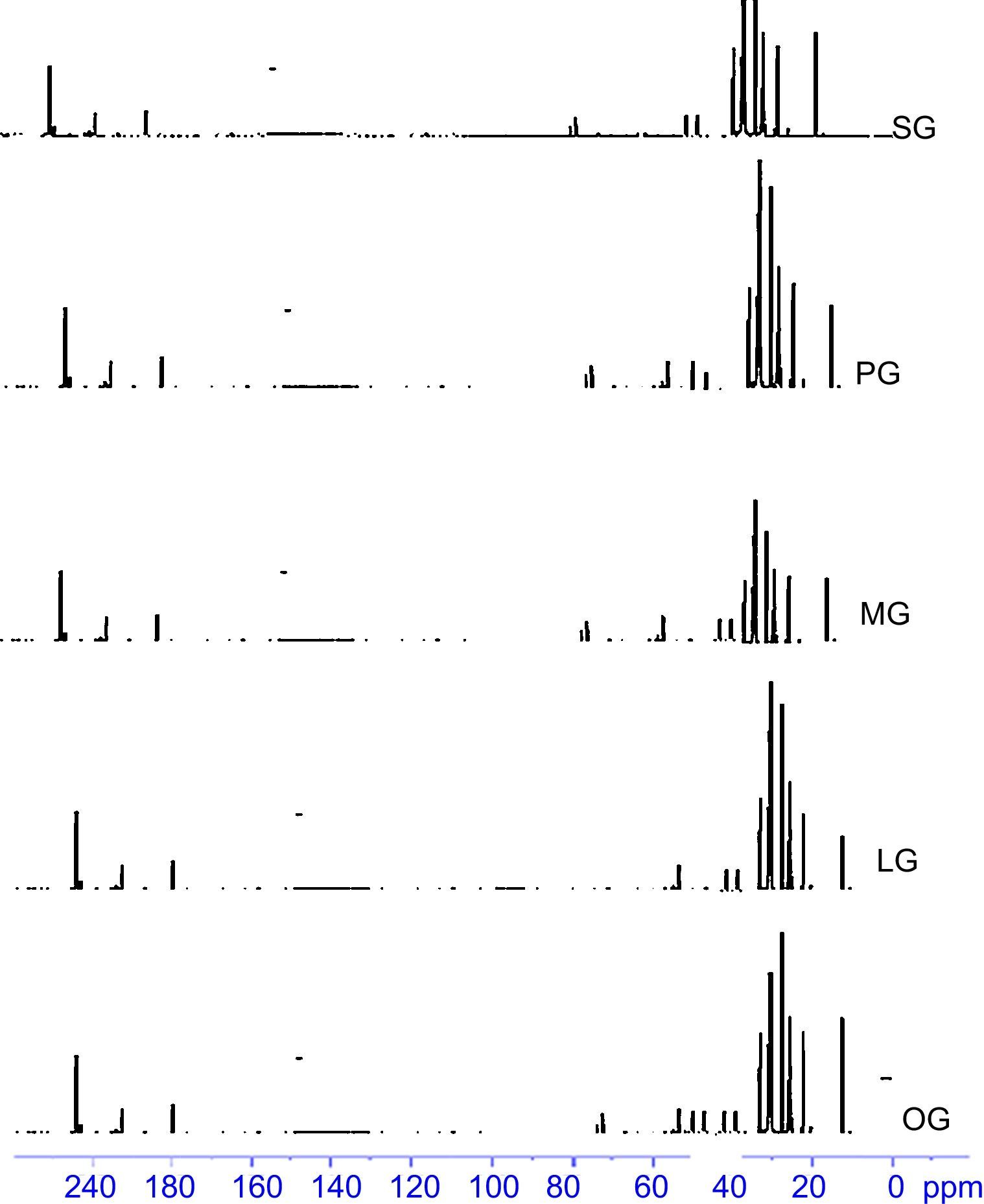
13C-NMR-Peaks at 48.22ppm, 53.04ppm correspond to NCH2CH2N methylene groups of imidazoline ring. A peak at 173.85ppm corresponds to the carbon of CONH group whereas signal at 164.48ppm corresponds to the carbon of imidazoline ring. Peaks at 45.21ppm, 54.11ppm, 50.48ppm and 37.48ppm correspond to NCH2CH2N methylene groups of gemini imidazoline ring. Peaks at 77.69, 77.38 and 155.14ppm shows the carbons of carbonate linkage. 13 C-NMR spectra of synthesized cationic gemini imidazoline surfactants are given in Figure 6.
3.3.2Palmitic acid based cationic gemini imidazoline surfactants (PG)FT-IR-Peaks at 1653.14cm−1 confirm the CO str- amide linkage, peak at 1602.66cm−1 confirms the preparation of imidazoline ring. Presence of peaks at 698.58cm−1, 727.51cm−1 and 759.92 confirms the quaternization of ring. Peaks at 3382.12cm−1 corresponds to NH str- amide linkage.
1H-NMR-peaks at 2.72ppm and 3.45ppm correspond to NCH2CH2N methylene group of imidazoline ring. Peak at 7.28ppm corresponds to the proton of CONH group whereas signal at 4.3ppm confirms the presence of CH2 group adjacent to amide linkage. Quaternized product shows four peaks for two CH2 groups of rings at 3.4ppm, 4.1ppm and 2.6ppm, 2.8ppm which is due to the resonance in between two nitrogen of imidazolinium ring. The peak at 4.4ppm shows the presence of CH2 adjacent to carbonate linkage, present in the spacer group.
13C-NMR Peaks at 49.22ppm, 53.08ppm correspond to NCH2CH2N methylene groups of imidazoline ring. A peak at 173.87ppm corresponds to the carbon of CONH group whereas signal at 164.52ppm corresponds to the carbon of imidazoline ring. Peaks at 45.28ppm, 54.16ppm, 50.54ppm and 37.52ppm correspond to NCH2CH2N methylene groups of gemini imidazoline ring. Peaks at 78.29, 78.38 and 156.14ppm shows the carbons of carbonate linkage.
3.3.3Myristic acid based cationic gemini imidazoline surfactants (MG)FT-IR-Peaks at 1649.64cm−1 confirm the CO str- amide linkage, peak at 1602.32cm−1 confirms the preparation of imidazoline ring. Presence of peaks at 696.55cm−1, 727.54cm−1 and 759.24 confirms the quaternization of ring. Peaks at 3381.32cm−1 corresponds to NH str- of amide linkage.
1H-NMR-peaks at 2.72ppm and 3.3ppm correspond to NCH2CH2N methylene group of imidazoline ring. Peak at 7.25ppm corresponds to the proton of CONH group whereas signal at 4.2ppm confirms the presence of CH2 group adjacent to amide linkage. Quaternized product shows four peaks for two CH2 groups of rings at 3.7ppm, 4.4ppm and 2.9ppm, 3.0ppm which is due to the resonance in between two nitrogen of imidazolinium ring. The peak at 4.1ppm shows the presence of CH2 adjacent to carbonate linkage, present in the spacer group.
13C-NMR-peaks at 49.22ppm, 54.04ppm correspond to NCH2CH2N methylene groups of imidazoline ring. A peak at 175.85ppm corresponds to the carbon of CONH group whereas signal at 166.48ppm corresponds to the carbon of imidazoline ring. Peaks at 47.24ppm, 56.16ppm, 53.42ppm and 38.44ppm correspond to NCH2CH2N methylene groups of gemini imidazoline ring. Peaks at 77.59, 77.42 and 156.14ppm shows the carbons of carbonate linkage.
3.3.4Lauric acid based cationic gemini imidazoline surfactants (LG)FT-IR-Peaks at 1645.72cm−1 confirm the CO str- amide linkage, peak at 1602.24cm−1 confirms the preparation of imidazoline ring. Presence of peaks at 696.54cm−1, 724.58cm−1 and 762.96 confirms the quaternization of ring. Peaks at 3379.32cm−1 corresponds to NH str- of amide linkage.
1H-NMR-peaks at 2.72ppm and 3.5ppm correspond to NCH2CH2N methylene group of imidazoline ring. Peak at 7.29ppm corresponds to the proton of CONH group whereas signal at 4.3ppm confirms the presence of CH2 group adjacent to amide linkage. Quaternized product shows four peaks for two CH2 groups of rings at 3.5ppm, 4.1ppm and 2.7ppm, 2.9ppm which is due to the resonance in between two nitrogen of imidazolinium ring. The peak at 4.4ppm shows the presence of CH2 adjacent to carbonate linkage, present in the spacer group.
13C-NMR-peaks at 48.22ppm, 53.04ppm correspond to NCH2CH2N methylene groups of imidazoline ring. A peak at 173.85ppm corresponds to the carbon of CONH group whereas signal at 164.48ppm corresponds to the carbon of imidazoline ring. Peaks at 45.41ppm, 54.21ppm, 50.48ppm and 37.46ppm correspond to NCH2CH2N methylene groups of gemini imidazoline ring. Peaks at 77.68, 77.38 and 155.16ppm shows the carbons of carbonate linkage.
3.3.5Oleic acid based cationic gemini imidazoline surfactants (OG)FT-IR-Peaks at 1651cm−1 confirm the CO str- amide linkage, peak at 1604cm−1 confirms the preparation of imidazoline ring. (CH2)HCCH(CH2) stretching of unsaturation in alkyl chain was confirmed by the peak present at 898.44cm−1, Presence of peaks at 695.55cm−1, 726.54cm−1 and 758.94 confirms the quaternization of ring. Peaks at 3381cm−1 corresponds to NH str- of amide linkage.
1H-NMR-peaks at 2.74ppm and 3.5ppm correspond to NCH2CH2N methylene group of imidazoline ring. Peak at 2.02 confirm the presence of olefinic hydrogen of alkyl chain. Peak at 7.27ppm corresponds to the proton of CONH group whereas signal at 4.2ppm confirms the presence of CH2 group adjacent to amide linkage. Quaternized product shows four peaks for two CH2 groups of rings at 3.5ppm, 4.1ppm and 2.7ppm, 2.9ppm which is due to the resonance in between two nitrogen of imidazolinium ring. The peak at 4.3ppm shows the presence of CH2 adjacent to carbonate linkage, present in the spacer group.
13C-NMR-Peaks at 48.22ppm, 53.04ppm correspond to NCH2CH2N methylene groups of imidazoline ring. A peak at 173.85ppm corresponds to the carbon of CONH group whereas signal at 164.48ppm corresponds to the carbon of imidazoline ring. Peak at 131.02 confirm the presence of olefinic carbon of alkyl chain. Peaks at 45.21ppm, 54.11ppm, 50.48ppm and 37.48ppm correspond to NCH2CH2N methylene groups of gemini imidazoline ring. Peaks at 77.69, 77.38 and 155.14ppm shows the carbons of carbonate linkage.
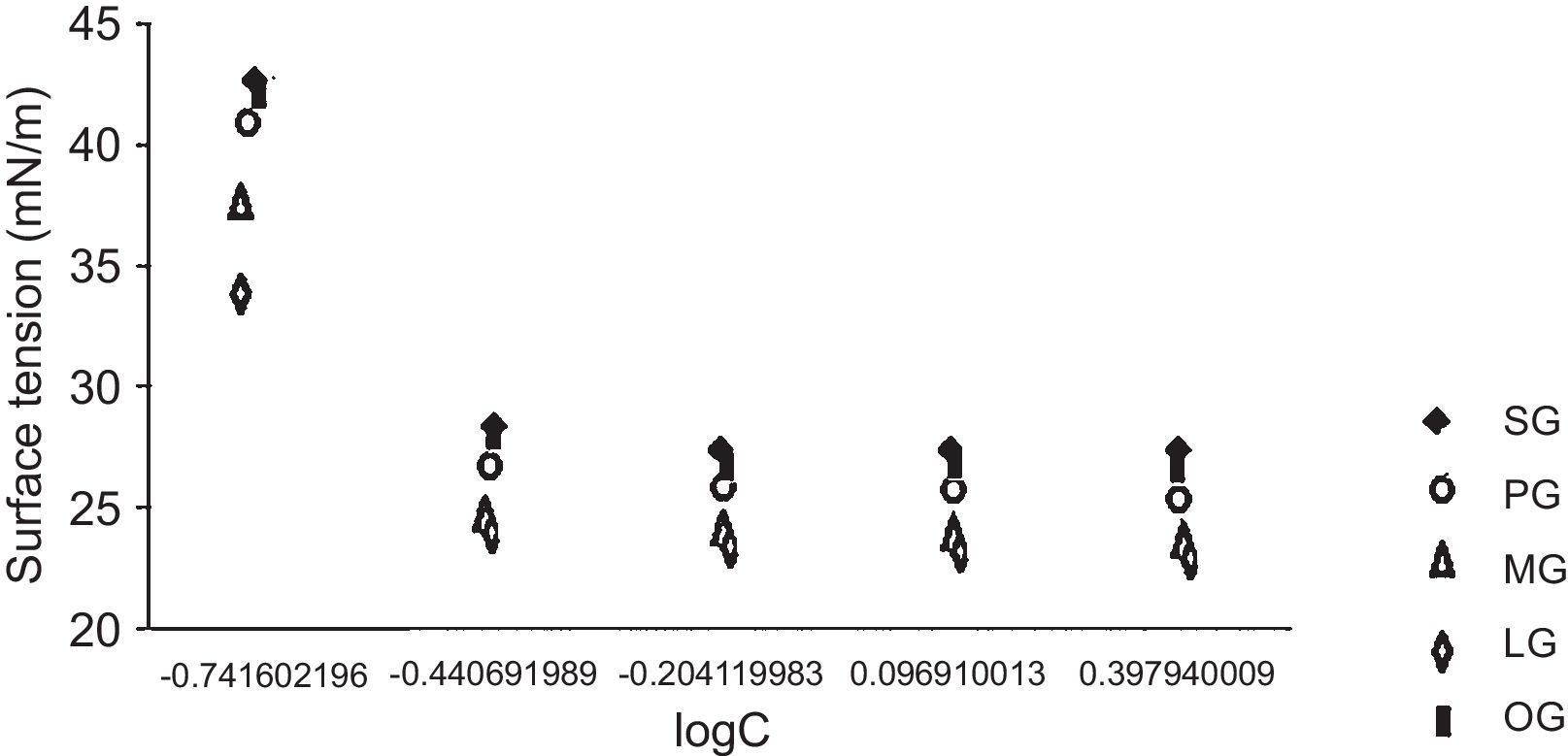
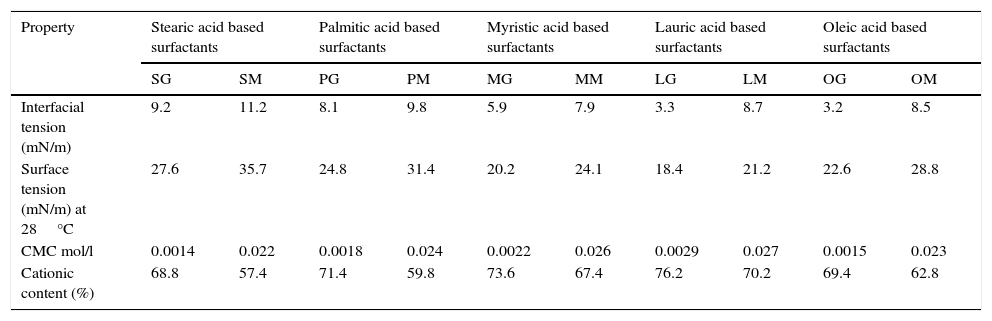
3.4Surface active properties of cationic gemini imidazoline surfactants3.4.1Surface tensionIn case of aqueous solutions of stearic acid based surfactant, at 0.1813g/l of concentration the surface tension was found to be 42.8mN/m. An increase in the concentration of surfactant from 0.1813 to 0.3626g/l resulted in the decrease of the surface tension to 28.6mN/m, while an increase in concentration of surfactant from 0.3626 to 0.6250g/l results in decrease in surface tension to 27.6mN/m. Further an increase up to 1.25g/l and 2.5g/l did not show any decrease in surface tension (Fig. 1). Same trends were found in case of all synthesized gemini surfactants but values were shifted to their lower end with decrease in hydrophobic groups of surfactants. If we compare the surface tension of 1% aqueous of synthesized Gemini surfactants with the surface tensions of their monomeric counterparts given in literature, it was found that Gemini surfactants have greater ability to reduce the surface tension than their monomeric cationic imidazolines shows surface tension in the range of 24.1–35.7mN/m. Same pattern was obtained for the interfacial tension of 1% aqueous solutions of gemini surfactants (Table 4).
Surface active properties of synthesized cationic imidazoline surfactants and their corresponding monomers.
| Property | Stearic acid based surfactants | Palmitic acid based surfactants | Myristic acid based surfactants | Lauric acid based surfactants | Oleic acid based surfactants | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SG | SM | PG | PM | MG | MM | LG | LM | OG | OM | |
| Interfacial tension (mN/m) | 9.2 | 11.2 | 8.1 | 9.8 | 5.9 | 7.9 | 3.3 | 8.7 | 3.2 | 8.5 |
| Surface tension (mN/m) at 28°C | 27.6 | 35.7 | 24.8 | 31.4 | 20.2 | 24.1 | 18.4 | 21.2 | 22.6 | 28.8 |
| CMC mol/l | 0.0014 | 0.022 | 0.0018 | 0.024 | 0.0022 | 0.026 | 0.0029 | 0.027 | 0.0015 | 0.023 |
| Cationic content (%) | 68.8 | 57.4 | 71.4 | 59.8 | 73.6 | 67.4 | 76.2 | 70.2 | 69.4 | 62.8 |
SG, PG, MG, LG and OG are the cationic imidazoline surfactants based on stearic acid, palmitic acid, myristic acid, lauric acid and oleic acid, respectively. SM, PM, MM, LM and OM are the cationic monomeric surfactants based on stearic acid, palmitic acid, myristic acid, lauric acid and oleic acid, respectively.
CMC values of 1% aqueous solution of gemini surfactants were found to be in the range of 0.0014–0.0029m/l depending upon the chain length. Greater the chain length of hydrophobic moiety, greater the CMC values of surfactant solutions (Table 4).
3.4.2Cationic contentCationic content of synthesized cationic gemini imidazoline surfactants is given in Table 4. Cationic contents of synthesized gemini surfactant showed decreasing trend with decreasing length of alkyl chain. Stearic acid based cationic gemini showed 68.8% whereas Lauric acid based surfactant showed 76.2% of cationic content. When comparison had been made with the data present in literature, all the values of cationic contents were found much higher than their monomeric counterparts.
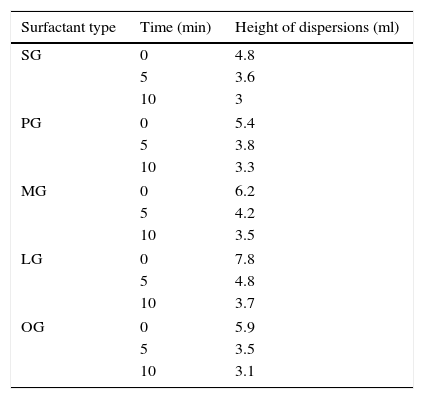
3.4.3DispersabilitySynthesized cationic gemini imidazoline surfactants was found to have good as well as stable dispersing ability. In case of stearic acid based cationic geminis, the height of the cloudiness observed immediately was 4.8ml. After 5min and 10min the heights observed were 3.6ml and 3.0ml, respectively. Results confirm the formation of good and stable dispersions. Decrease in the chain length of alkyl group resulted in the increase in the height of cloudiness but stability of dispersion gradually decreased. In case of oleic acid based gemini surfactants, height of dispersions found immediately, after 5min and 10min were 13ml, 3.8ml and 3.3ml respectively which may be due to the presence of unsaturation in alkyl group (Table 5).
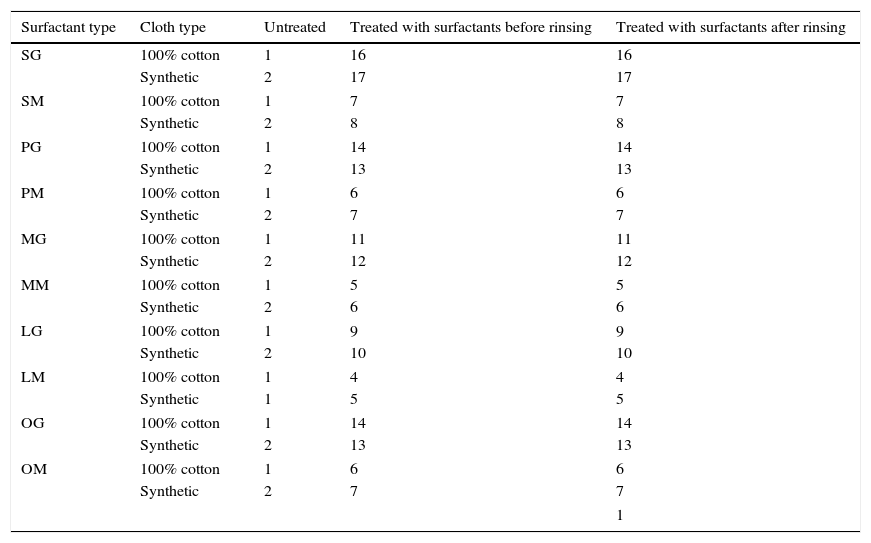
3.4.4SofteningThe softening property of synthesized gemini surfactants is presented in Table 6. Results revealed that decrease in the chain length of alkyl group gradually decrease the softening ability of surfactants. Oleic acid based surfactants showed moderate softening ability when compared with highest alkyl chain containing surfactants and smallest fatty alkyl chain containing surfactants. The untreated fabric was found to have poor softening, whereas fabric treated with surfactant showed very good softening even after rinsing.
Softening properties of synthesized cationic imidazoline surfactants.
| Surfactant type | Cloth type | Untreated | Treated with surfactants before rinsing | Treated with surfactants after rinsing |
|---|---|---|---|---|
| SG | 100% cotton | 1 | 16 | 16 |
| Synthetic | 2 | 17 | 17 | |
| SM | 100% cotton | 1 | 7 | 7 |
| Synthetic | 2 | 8 | 8 | |
| PG | 100% cotton | 1 | 14 | 14 |
| Synthetic | 2 | 13 | 13 | |
| PM | 100% cotton | 1 | 6 | 6 |
| Synthetic | 2 | 7 | 7 | |
| MG | 100% cotton | 1 | 11 | 11 |
| Synthetic | 2 | 12 | 12 | |
| MM | 100% cotton | 1 | 5 | 5 |
| Synthetic | 2 | 6 | 6 | |
| LG | 100% cotton | 1 | 9 | 9 |
| Synthetic | 2 | 10 | 10 | |
| LM | 100% cotton | 1 | 4 | 4 |
| Synthetic | 1 | 5 | 5 | |
| OG | 100% cotton | 1 | 14 | 14 |
| Synthetic | 2 | 13 | 13 | |
| OM | 100% cotton | 1 | 6 | 6 |
| Synthetic | 2 | 7 | 7 | |
| 1 | ||||
1 for worse and 17 for best softening.
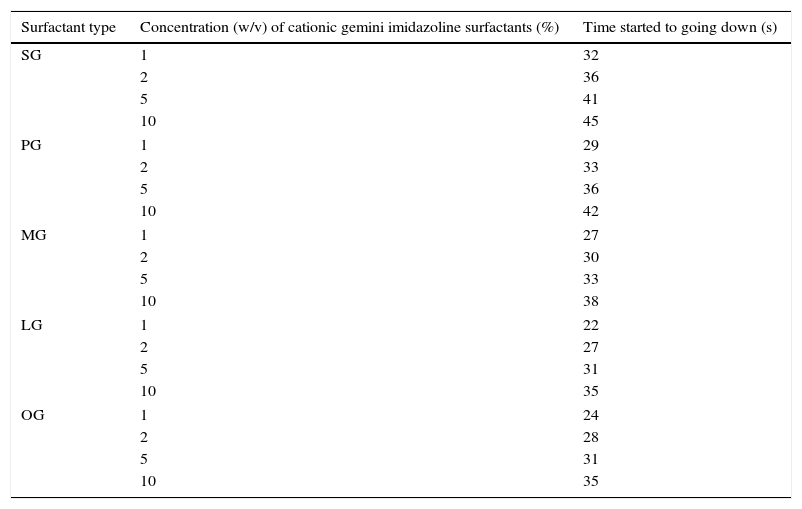
Results of rewettability revealed that decrease in the chain length hydrophobic group gradually increased the rewettability of surfactants. Whereas, increase in the concentration of gemini surfactants showed decreased in their rewettability (Table 7).
Rewettability of synthesized cationic gemini imidazoline surfactants.
| Surfactant type | Concentration (w/v) of cationic gemini imidazoline surfactants (%) | Time started to going down (s) |
|---|---|---|
| SG | 1 | 32 |
| 2 | 36 | |
| 5 | 41 | |
| 10 | 45 | |
| PG | 1 | 29 |
| 2 | 33 | |
| 5 | 36 | |
| 10 | 42 | |
| MG | 1 | 27 |
| 2 | 30 | |
| 5 | 33 | |
| 10 | 38 | |
| LG | 1 | 22 |
| 2 | 27 | |
| 5 | 31 | |
| 10 | 35 | |
| OG | 1 | 24 |
| 2 | 28 | |
| 5 | 31 | |
| 10 | 35 | |
Synthesis of cationic gemini imidazoline surfactants using cheaper and easily available fatty acids is an effort to synthesize cost effective surfactants. Incorporation of carbonate linkage in between the spacer group provides novelty to the compound and makes them easily biodegradable. Surface active and performance properties of synthesized gemini surfactant establish its better efficiency over its monomeric counterparts.
Conflict of interestThe authors have no conflicts of interest to declare.
Divya Bajpai Tripathy acknowledges the Department of Science and Technology, Government of India, for financial support through grant no. SR/WOS-A/CS-1056/2014 (G).
Peer Review under the responsibility of Universidad Nacional Autónoma de México.