The present investigation deals with synthesis of new acid dyes from 4-amino-1-(4-sulfophenyl)-3-methyl-5-pyrazolone and phenol derivatives and their metal complexes (Cu (II) and Fe (II)). The phenol derivatives include 4-chlorophenol, 4-nitrophenol, 4-hydroxybenzene sulfonic acid, 2-nitrophenol-4-sulfonic acid, resorcinol and bisphenol A and bisphenol S. The newly synthesized dyes were applied to crust leather to assess their dyeing properties. The fastness properties of unmetallized dyes were less as compared to metal complexes due to the strong interaction of metals with leather protein. The structures of these dyes were also confirmed by UV, FTIR and NMR studies.
Azo compounds are non-naturally occurring nitrogen compounds continuously receiving attention in scientific research (Kirkan & Gup, 2008; Otutu, 2013; Seferoğlu, 2009). Azo dyes constitute the largest group of Azo compounds and the most widely used colorants in the industry. Several derivatives of pyrazole (azo) were the subject of research because of a variety of applications. The applications of the azo dyes include their use in coloring fibers, due to their affinity for wool and silk (Patel & Patel, 2011), photoelectronics (Sekar, 1999), optical storage technology (Wang, Shen, & Xu, 2000), biological reactions (Weglarz-Tomczak & Gorecki, 2012), printing systems (Abe, Mano, Yamaya, & Tomotake, 1999; Dharmalingam, Ramasamy, & Balasuramanian, 2011) as well as in analytical (Abdalla, El-Haty, Adam, & Hassan, 2013; Amin, Mohammed, & Mousa, 2003) and food chemistry (Almeida, Stephani, Dos Santos, & Oliveira, 2009). Beside these many azo compounds have been synthesized with an industrial and medical aim. The coordination complexes of transition metals with azo-ligands are also focus of the current attraction due to the interesting physical, chemical, photophysical and photochemical, catalytic and different material properties. Metal complex dyes play a very important role in the textile industry. Chromium, Cr (III) and cobalt Co (III) complexes are used most frequently for the dyeing of wool and synthetic polyamides (Kocaokutgen, Erdem, & Gümrükçgüoglu, 1998).
Photophysical and coloristic properties of the dyes are modified in the case of aggregate formation (molecular association) in solution. The self-association of dyes in solution is due to the interactions by Van der Waals forces, hydrogen bonds and hydrophobic interactions (Radulescu-Grad, Muntean, Todea, Verdes, & Andelescu, 2015). The dye aggregation is affected by parameters such as dye concentration, dye structure, pH, temperature, solvents and ionic strengths (Goftar, Moradi, & Kor, 2014).
In the present work, two new series of azo-dyes, having Fe+2 and Cu2+ as metalizing ion were synthesized, characterized and applied to leather. At the same time, spectrophotometric analysis was also performed for the qualitative study of ligand acid dyes and their metal complex.
2Experimental2.1Materials and methodsAll commercial products were purchased from Sigma-Aldrich. Solvents were purified and dried by the standard methods. Melting points were determined in open capillary tubes on a Stuart melting point apparatus. The FTIR spectra were run in the single beam Nicolet IR 100 (Fourier-Transform); while UV of all the samples were taken in water using UV-Genesys spectrophotometer. Their mass spectral data were obtained from waters GCT premier spectrometer. The 1H NMR and 13C NMR spectra were recorded in DMSO-d6 using NMR Bruker DPX 400 spectrophotometer operating at 300 and 75MHz for 1H and 13C NMR respectively. TMS was used as internal standard with the deuterium signal of the solvent as the lock and chemical shifts δ recorded in ppm. The elemental analysis (C, H, N, S) of the compounds was performed using Flash EA 1112 elemental analyzer while the pH was monitored using Portable pH Meter Model PHB4. Compounds were routinely checked by TLC on silica gel G plates using three different eluting solvents depending on the polarity disparity. The solvent systems are petroleum ether:chloroform (9:1, v/v), petroleum ether:chloroform (6:4, v/v) and chloroform:methanol (9:1, v/v). Also, the developed plates were visualized using a UV lamp for the presence of spots and Rf values were duly calculated. All of the crude products were isolated as solids and purified. Fastness to light was assessed in accordance with BS 1006-1978. Rubbing fastness was checked with an Atlas Crock meter in accordance with AATCC TM 8-1961 and the wash fastness was determined according to ISO: 765-1979 (Maradiya & Patel, 2002; Sakoma, Bello, & Yakubu, 2012).
2.2General procedure for the synthesis of 1-(p-sulphophenyl)-3-methyl-5-pyrazolone based acid dyesSynthesis of acid dyes and their metal complexes involves three-step procedure which is as follows.
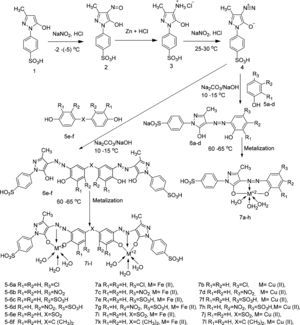
2.2.1Nitrosation of p-sulphophenyl-3-methyl-5-pyrazolone (SPMP)1-(p-sulfophenyl)-3-methyl-5-pyrazolone (1) (25.4g, 0.1mol) was suspended in H2O (250ml). Hydrochloric acid (45ml) was added to this well stirred suspension. The reaction mixture was cooled to 0–5°C in an ice bath. A solution of NaNO2 (6.9g, 0.1mol) in H2O (25ml) previously cooled to 0°C, was then added over a period of 35min with stirring. The stirring was continued for an hour maintaining the same temperature, with a positive test for nitrous acid. Later on the excess of nitrous acid was destroyed with required amount of sulphamic acid. The nitroso (oxime) was filtered after salting out. Then oxime was reduced by stirring in 200ml water containing 85ml HCl and 23g zinc metal at boil for 4h. On completion of reaction, pH of the reaction mixture was raised to 9 with 6N NaOH, and precipitated the 1-(p-methylphenyl)-3-methyl-4-amino pyrazolones.
2.2.2Diazotization and coupling with phenol derivativesTo the well stirred ice jacketed aqueous solution (2.69g) of 1-(p-sulphophenyl)-3-methyl-4-amino pyrazolone (at 0–5°C) was added conc. HCl (3.5ml) and sodium nitrite solution (0.7g in 2ml H2O). The reaction mixture was vigorously stirred for 1h at the above mentioned temperature to obtain the diazonium salt of 1-(p-sulphophenyl)-3-methyl-4-amino pyrazolone. The diazonium compound formed in this way was coupled to various coupler mentioned previously to synthesize our dyes. Thus 1.285g (0.010mol) 4-chlorophenol (5a) was dissolved in 200ml water containing 0.45g NaOH and coupled with prepared diazo. The coupling was facilitated using sodium carbonate as an acid binding agent. The reaction mixture was given 4–5h to complete the coupling at 30–35°C. The dye was cooled to room temperature. Its pH was reduced to 4.5 by HCl and filtered. The cake was dried in oven at 70–75°C till constant weight. By adopting the same procedure other dyes 6b–f were prepared from couplers 5b–f as shown in Scheme 1.
2.2.3Metallization of acid dyesFor the synthesis of metal complexes (iron complex), pH of 25ml (0.005mol) of dye 6a was reduced to 6.5 with HCl. Then it was heated to 70°C and to it 5ml (0.005mol Fe2+) solution of ferrous sulfate (FeSO4·7H2O) was added drop wise. Mixing and heating at this temperature was continued for further 1.0 hour till the metallization was completed, as shown by the comparative TLC. The dye was cooled to room temperature; its pH was reduced to 1.0 with conc. HCl. Then it was salted out with sodium chloride, filtered and dried in oven at 80°C till constant weight.
Similarly, copper (II) complexes of dye 6a were prepared by treating dye with CuSO4·5H2O at 65–70°C with metal to ligand mole ratio 1:1. In this way complexes 7a–l were synthesized from respective dye ligands (see supplementary information).
2.2.3.16a (C16H13ClN4O5S)Orange, (76%). λmax (nm): 460. FTIR (KBr, cm−1) νmax: 3255 (OH str.), 3050 (CCH str.), 2927 (CH2 str.), 1653 (CO str.), 1595, 1541 (CC aromatic, CN), 1498 (NN str.), 1422, 1340 (SO3H str., CH2 bend.), 1236, 1155 (CC, CO str.), 1000 (SO str.), 833 (ArH), 790 (CCl str.). 1H NMR (300MHz, DMSO-d6) δ: 8.07 (1H, d, J=2.35Hz), 7.95 (2H, d, J=8.6Hz), 7.83–7.90 (1H, m), 7.68 (2H, d, J=8.6Hz), 6.65 (1H, d, J=9.1Hz), 2.20 (3H, s). 13C NMR (75MHz DMSO-d6) δ (ppm): 158.54, 156.64, 147.93, 147.37, 143.21, 139.93, 125.43, 124.41, 119.45, 118.06, 116.22 12.45. Anal. Calcd. for C16H13ClN4O5S: C, 47.01; H, 3.21; N, 13.71; S, 7.84. Found: C, 47.05; H, 3.30; N, 13.59; S, 7.79.
2.2.3.26b (C16H13N5O7S)Dark Brown, (83%). λmax (nm): 480. FTIR (KBr, cm−1) νmax: 3388 (OH str.), 2924 (CH2 str.), 1666 (CC str.), 1593 (CC aromatic, CC), 1498, 1476 (NN, NO2 str.), 1267, 1172 (CO str.), 1034 (COC str.), 821 (Ar-H). 1H NMR (300MHz, DMSO-d6) δ: 8.15 (1H, d, J=2.4Hz), 7.942 (2H, d, J=8.7Hz), 7.81–7.92 (1H, m), 7.65 (2H, d, J=8.7Hz), 6.38 (1H, d, J=9.3Hz), 2.27 (3H, s). 13C NMR (75MHz DMSO-d6) δ (ppm): 158.0, 155.28, 148.32, 147.57, 144.58, 138.72, 128.18 126.86, 126.24, 118.19, 117.06, 116.17, 12.14. Anal. Calcd. for C16H13N5O7S: C, 45.83; H, 3.12; N, 16.70, S, 7.64. Found: C, 45.78; H, 3.20; N, 16.58, S, 7.57.
2.2.3.36c (C16H14N4O8S2)Orange, (84%). λmax (nm): 460. FTIR (KBr, cm−1) νmax: 3389 (OH str), 3086 (CCH str.), 1638 (NH bend.), 1619, 1597 (CC aromatic), 1541 (NN str.), 1500 (NH bend.), 1340 (SO3H, CH2 str.), 1185 (CO str.), 818 (ArH). 1H NMR (300MHz, DMSO-d6) δ: 11.87 (1H, s, OH), 8.10 (1H, d, J=2.6Hz), 7.93 (2H, d, J=8.6Hz), 7.79–7.90 (1H, m), 7.67 (2H, d, J=8.6Hz), 6.90 (1H, s, SO3H), 6.58 (1H, d, J=9.5Hz), 2.27 (3H, s). 13C NMR (75MHz DMSO-d6) δ (ppm): 159.12, 156.48, 147.72, 147.17, 145.23, 141.33, 138.62, 125.68, 124.42, 117.19, 116.96, 116.43 11.81. Anal. Calcd. for C16H14N4O8S2: C, 42.29; H, 3.11; N, 12.33, S: 14.11. Found: C, 42.24; H, 3.20; N, 12.21, S: 14.04.
2.2.3.46d (C16H13N5O10S2)Orange, (81%). λmax (nm): 520. FTIR (KBr, cm−1) νmax: 3449 (OH str.), 3050 (CCH str.), 1653 (CC str. NH bend), 1619, 1541 (CC aromatic), 1498 (NN str.), 1338 (SO3H, CH2 str.), 1183 (CO str.), 1008 (SO), 840 (ArH). 1H NMR (300MHz, DMSO-d6) δ: 11.33 (1H, s, OH), 8.15 (1H, d, J=2.4Hz), 7.99 (1H, d, J=2.4Hz), 7.922 (2H, d, J=8.7Hz), 7.63 (2H, d, J=8.7Hz), 2.23 (3H, s). 13C NMR (75MHz DMSO-d6) δ (ppm): 157.05, 156.28, 149.25, 147.63, 144.28, 142.76, 140.92, 127.16, 125.54, 119.19, 116.56, 115.20 12.14. Anal. Calcd. for C16H13N5O10S2: C, 38.48; H, 2.62; N, 14.02; S, 12.84. Found: C, 38.37; H, 2.69; N, 13.96; S, 12.88.
2.2.3.56e (C32H26N8O12S3)Orange, (80%). λmax (nm): 420. FTIR (KBr, cm−1) νmax: 3395 (OH, NH str.), 3050 (CCH str.), 2926 (CH2 str.), 1619, 1586 (CC aromatic, CN), 1498 (NN str.), 1125 (CO str.), 1008 (SO), 872 (ArH). 1H NMR (300MHz, DMSO-d6) δ: 11.67 (1H, s, OH), 7.91 (2H, d, J=8.7Hz), 7.81–7.92 (1H, m), 7.69 (2H, d, J=8.7Hz), 6.90 (1H, d, J=9.4Hz), 6.78 (1H, s), 6.62 (1H, s, SO3H), 6.59 (1H, d, J=9.4Hz), 2.51(3H, s), 1.469 (6H, s). 13C NMR (75MHz, DMSO-d6) δ (ppm): 157.56, 154.89, 148.97, 154.64, 144.47, 143.44, 142.29, 141.31, 138.88, 137.90, 129.96, 128.05, 127.74, 126.78, 119.85, 118.63, 117.64, 115.79, 115.64, 115.13, 115.02, 41.19, 30.83, 11.59. Anal. Calcd. for C32H26N8O12S3: C, 47.40; H, 3.23; N, 13.82; S, 11.86. Found: C, 47.46; H, 3.31; N, 13.56; S, 11.80.
2.2.3.66f (C35H32N8O10S2)Brown, (83%). λmax (nm): 450. FTIR (KBr, cm−1) νmax: 3464 (OH, NH str.), 2963 (CH2 str.), 1653, 1593 (CC aromatic), 1490 (NN str.), 1338 (SO3H str.), 1213, 1153, 1120 (CC str.), 1002 (SO), 872 (ArH). 1H NMR (300MHz, DMSO-d6) δ: 11.30 (1H, s, OH), 7.94 (2H, d, J=8.7Hz), 7.82–7.93 (1H, m), 7.67 (2H, d, J=8.7Hz), 6.98 (1H, d, J=9.4Hz), 6.81 (1H, s), 6.58 (1H, d, J=9.4Hz), 6.42 (1H, s, SO3H), 2.47 (3H, s). 13C NMR (75MHz, DMSO-d6) δ (ppm): 158.77, 155.9, 147.61, 145.61, 143.86, 143.25, 141.63, 139.81, 136.75, 130.25, 127.91, 125.78, 119.80, 118.31, 116.73, 115.94, 115.23, 11.59. Anal. Calcd. for C35H32N8O10S2: C, 53.29; H, 4.09; N, 14.21; S, 8.13. Found: C, 53.35; H, 4.13; N, 14.04; S, 8.20.
2.3Dyeing methodDye solution (10ml, 0.4%, w/v) was taken in a dye-bath. The pH of the dye-bath was adjusted to 6.5 by adding acetic acid solution (1.0ml, 10%, w/v) solution. The total volume of the dye-bath was adjusted to 100ml by adding required amount of water. The leather swatches were introduced into the dye-bath with stirring. The content of the dye-bath was stirred for 1h at 25–30°C. The temperature was then gradually raised to 55°C over period of half hour and maintained for one hour. The dye-bath was kept rotating during the process of dyeing. Then 2.0ml formic acid was added to adjust pH to 2.0 for dye fixation. After this, the spent dye liquor was taken in 250ml volumetric flask. The swatches were washed with cold water and the combined solution of dye liquor and washings was then further diluted to 250ml with water. The absorbance of this solution was measured to find out the exhaustion of dye on leather. The dyed swatches were dried and mounted on shade card. A weighed amount of leather swatch was stirred in boiling acidified pyridine which dissolves the unfixed dye from swatch and from the absorbance of this solution percentage fixation was checked.
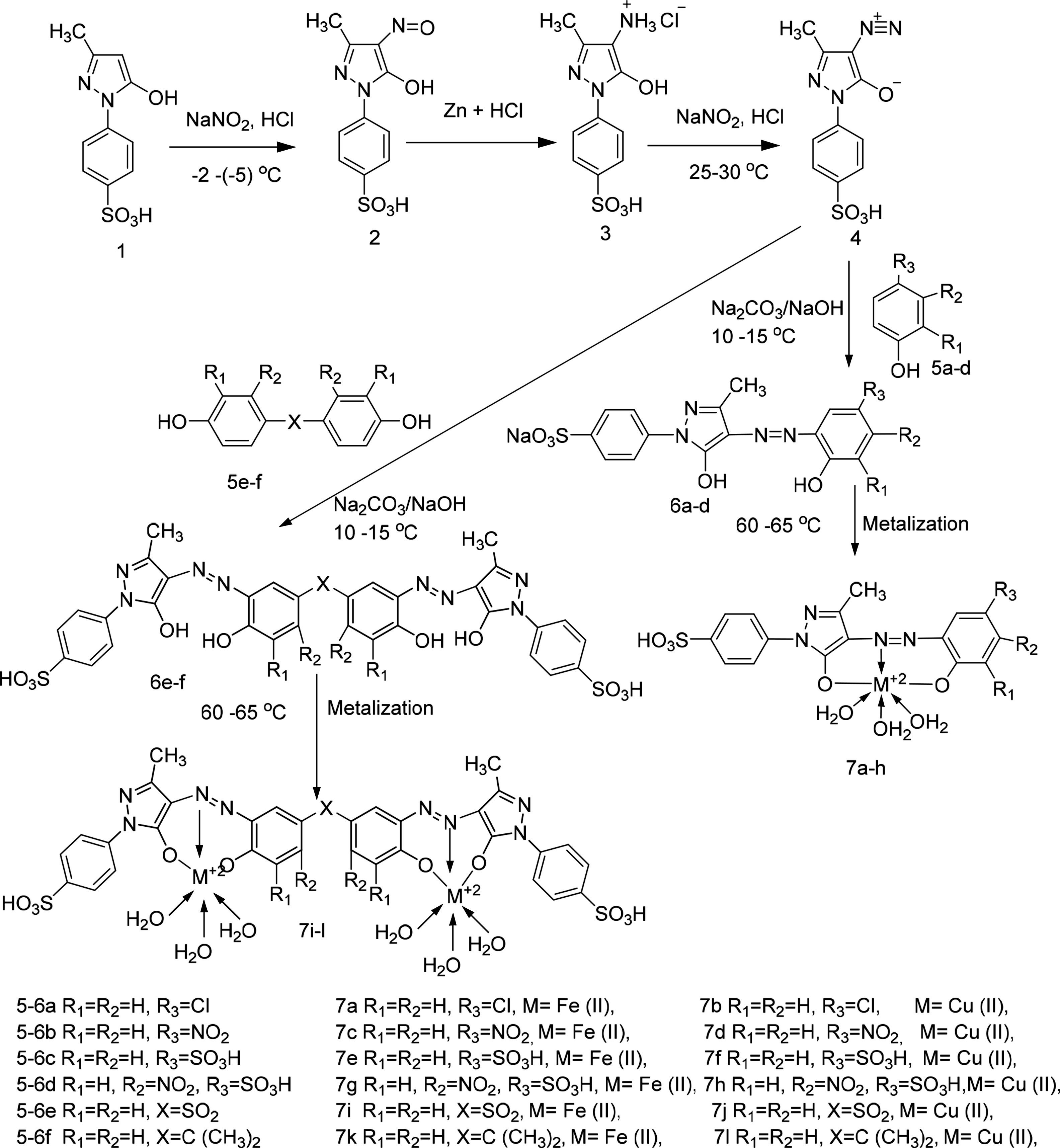
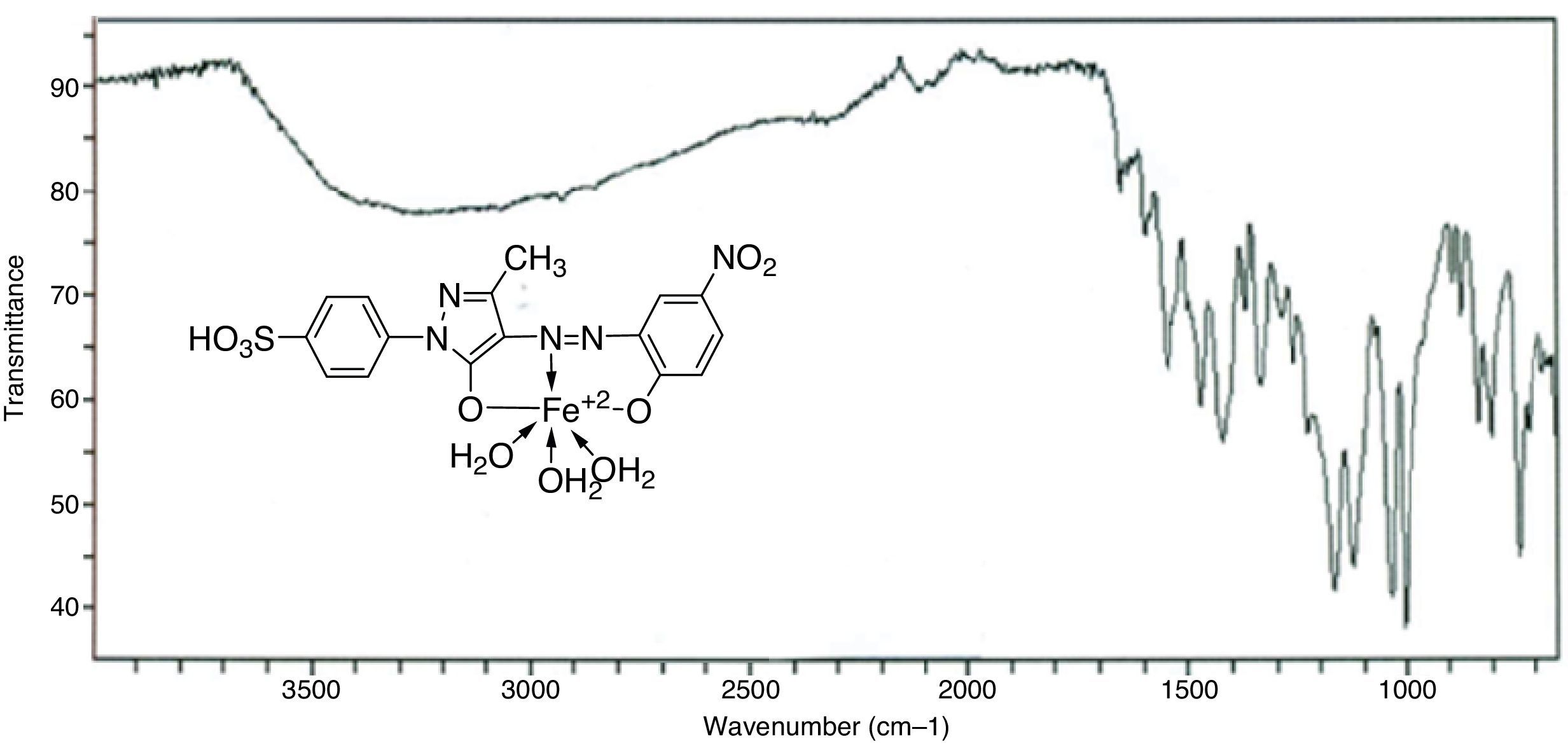
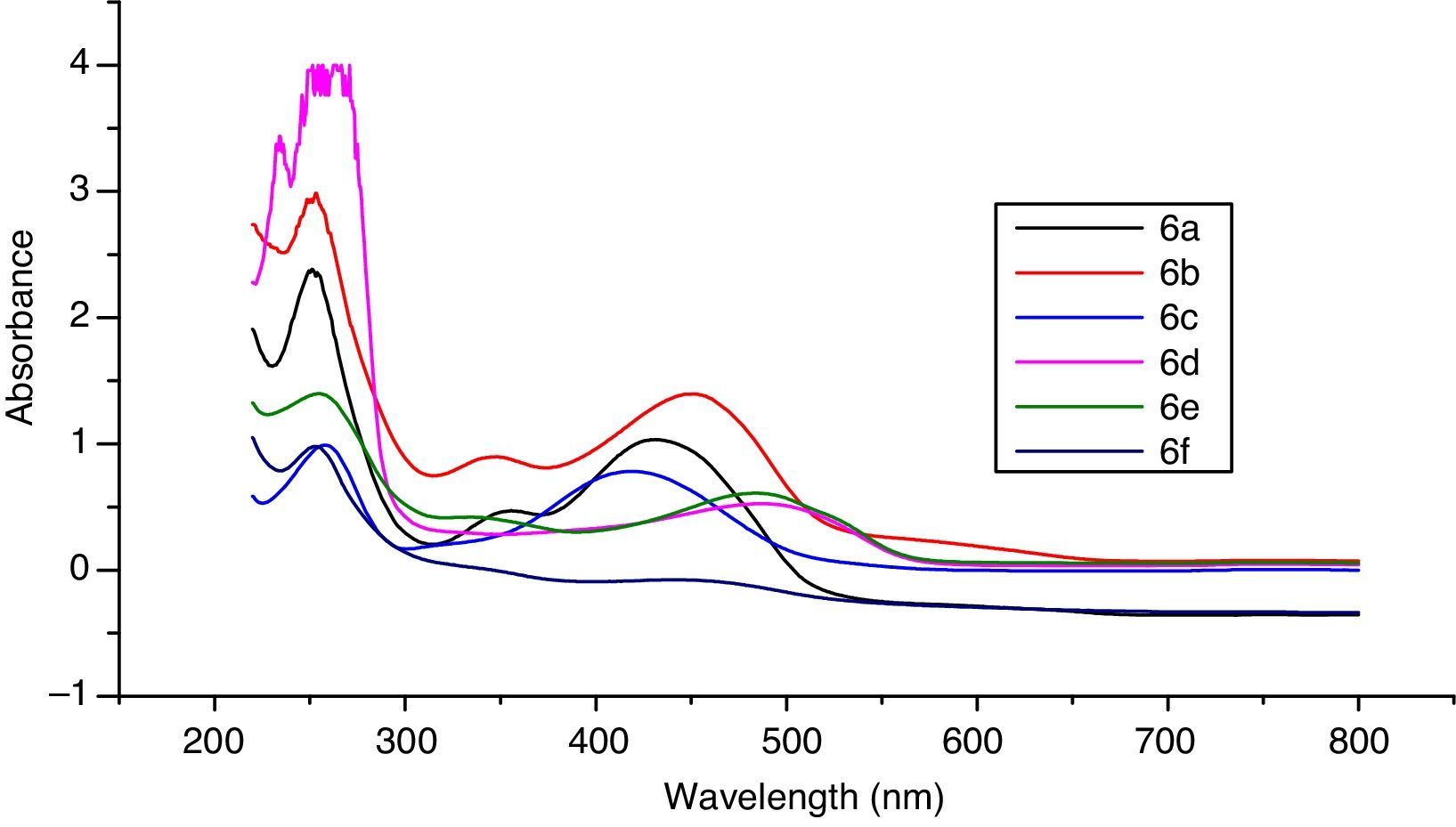
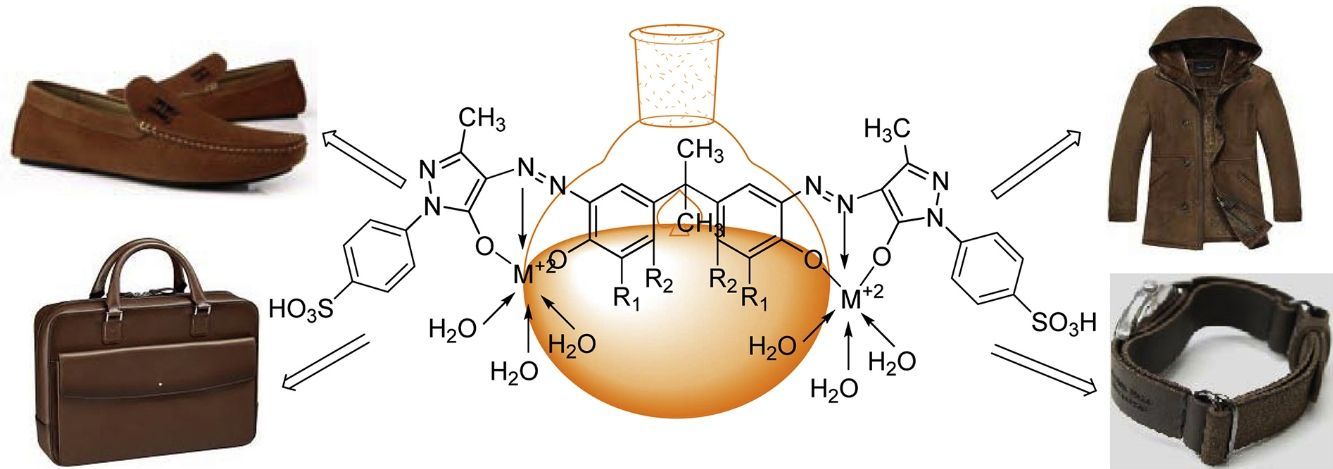
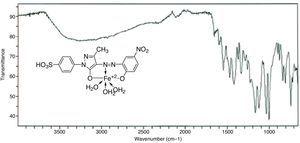
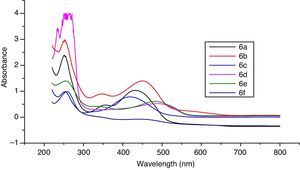
3Results and discussion3.1Synthesis and spectral characterization of ligand acid dyes and their metal complexesSynthesis of acid dyes and their iron (Fe, II) and copper (Cu, II) complexes were prepared in five step procedure as shown in Scheme 1. SPMP [1-(4-sulphophenyl)3-methyl-2-pyrazolin-5-one] was nitrosated at 0–5°C using NaNO2 and HCl as described by Knorr. The nitroso compound was filtered to remove some terry material. The clarified nitroso derivative [that usually exists in an oxime form (as indicated by its FTIR)] was salted out by common salt. It was dried after filtration. Reduction of oxime was carried at 100–105°C using zinc and HCl. The oxime and zinc were alternatively added in small portions in boiling HCl solution. The reduction was completed as the solution became colorless. A small amount of additional zinc dust was also added to prevent aerial oxidation on cooling. The resultant amine hydrochloride was quenched to −7°C. The excessive un-reacted zinc was removed by filtration. This amine hydrochloride was diazotized using an aqueous solution of NaNO2 (6.9g dissolved in 250ml of solution) and HCl at −5 to −2°C to avoid the formation of Rubazoic acid, which is automatically formed during this reaction with increasing temperature due to oxidizing action of nitrous acid, formed in situ. The diazonium salt prepared in this way was coupled with different para substituted phenols, like, p-chlorophenol, p-nitrophenol, phenol-4-suphonic acid, 2-nitrophenol-4-suphonic acid and bisphenols (bisphenol S and bisphenol A). The coupling was carried out in alkaline medium at pH 8–9. The synthesis of this diazo has been confirmed from X-ray structure of its crystal. Coupling in alkaline medium occurred at ortho position to the hydroxyl groups of phenol and bisphenol derivatives, as para position was blocked. The synthesized dyes 6a–f were precipitated on completion of reaction by reducing the pH of solution to 1.0 with HCl. The filtered dyes were dried and purified in ethanol. Metallization of these dyes was done by treating their alkaline solution with FeSO4·7H2O and CuSO4·5H2O at 65–70°C. It took about 4–5h to complete the metallization as was observed by taking the TLC of reaction mixture in 9:1 chloroform and methanol. Dyes (7a–l) were precipitated with addition of HCl, filtered and dried in oven at 80°C. These dyes were again purified from ethanol, dried, weighed and determined the percentage yield. These unmetallized dyes 6a–f are tridentate ligands which form complexes with iron (Fe, II) and copper (Cu, II) through 1:1 metal and ligand stoichiometric ratio (Fig. 2). In case of Fe2+ and Cu2+ complexes lone pairs of electrons are donated by two oxygen atoms and one nitrogen atom of the diazo linkage, while the other three coordination numbers of these metals have been satisfied by three water molecules. The complex formation pattern has been verified by the UV–vis spectrophotometric studies of these dyes 7a–l. In case of IR spectra of compounds different moieties showed stretching and bending bands characteristic of the synthesized compounds. The infrared spectra of the synthesized acid dyes and their metal complexes exhibited absorption peaks due to OH, ArH, CH, CO, CC, NN, SO3H, CO and OM stretching and bending vibrations at 3399, 3050, 2926, 1550, 1472, 1272, 1164, 1004, 834,610cm−1 as depicted from their FTIR spectra (Fig. 1). Similarly other metal complexes have been confirmed from their respective IR spectra.
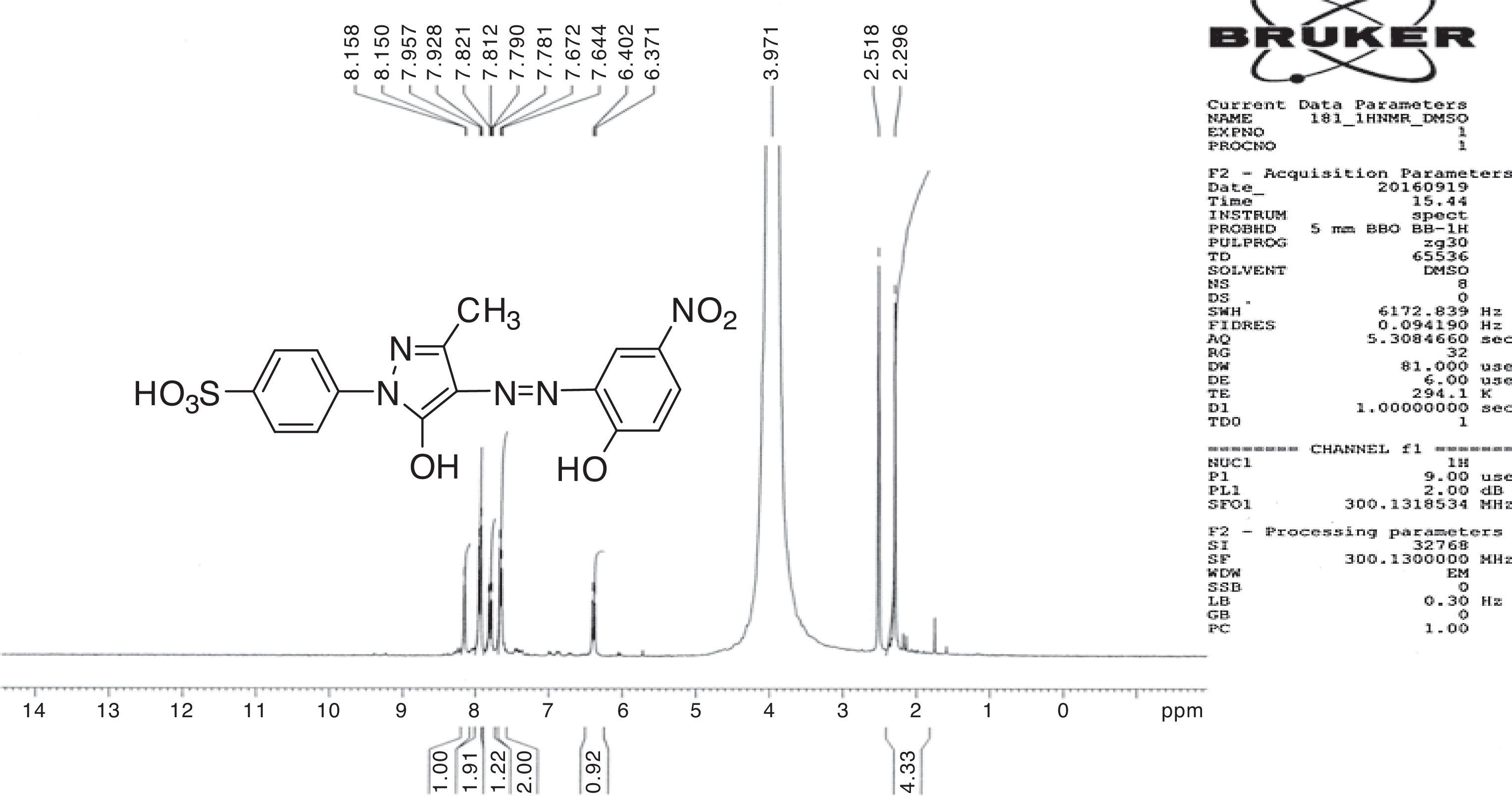
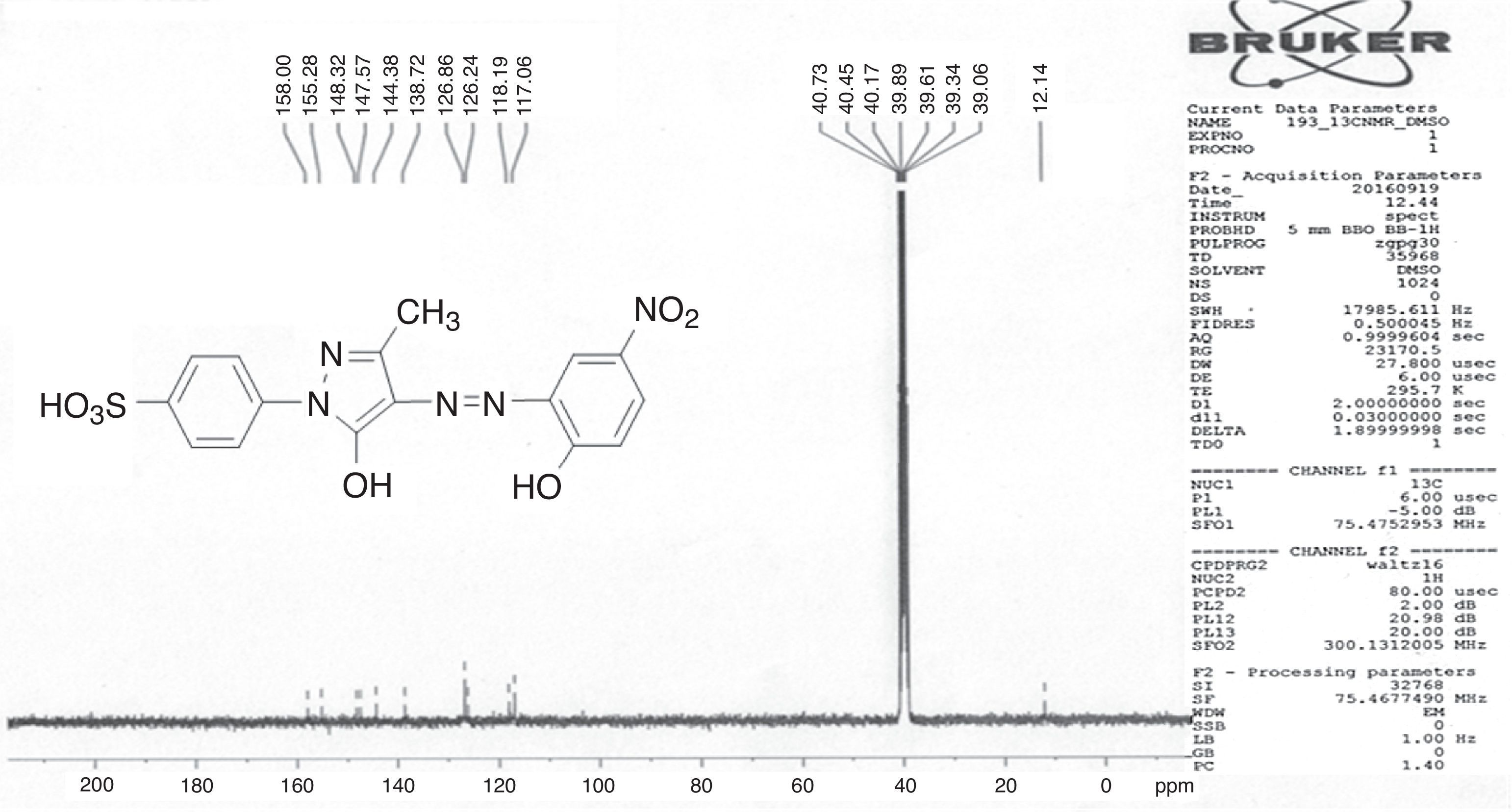
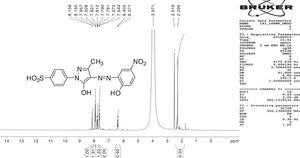
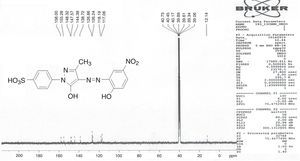
1H NMR and 13C NMR spectra were taken for ligand dyes which showed characteristic signals for different protons and carbons at different positions which evidenced the synthesis of dyes. In case of compounds 6b signal for hydroxyl group was absent due to exchangeable proton with DMSO. The doublet signal of mutually coupled set of four protons of phenyl group substituted with sulfonic group at present 7.95 and 7.68ppm having coupling constant respectively signals 8.6Hz. The multiplet signal for one proton of aromatic ring of coupling moiety is present at 7.83–7.90ppm while the same ring bearing another single proton shows signal at 8.07ppm with coupling constant 2.35Hz which evidenced the meta relationship with another proton at the same ring. The methyl group present at pyrazolone ring exhibited singlet signal at 2.20ppm (Fig. 2). In 13C NMR spectrum the signal for methyl group is present at 12.14ppm. There are ten signals in the range 117.06–158.00ppm for different carbon nuclei in the compound (Fig. 3). In this way other ligand acid dyes were confirmed from their respective 1H NMR and 13C NMR spectra. When the 1H NMR spectra of metal complex dyes were conducted they showed very broad and distorted signals due to paramagnetic nature of metals used for complexation and the so the NMR study of complexes was not fruitful for structure elucidation but in other words the distorted broad signals proved the complexation when the 1H NMR of ligands acid dyes and complexes were compared.
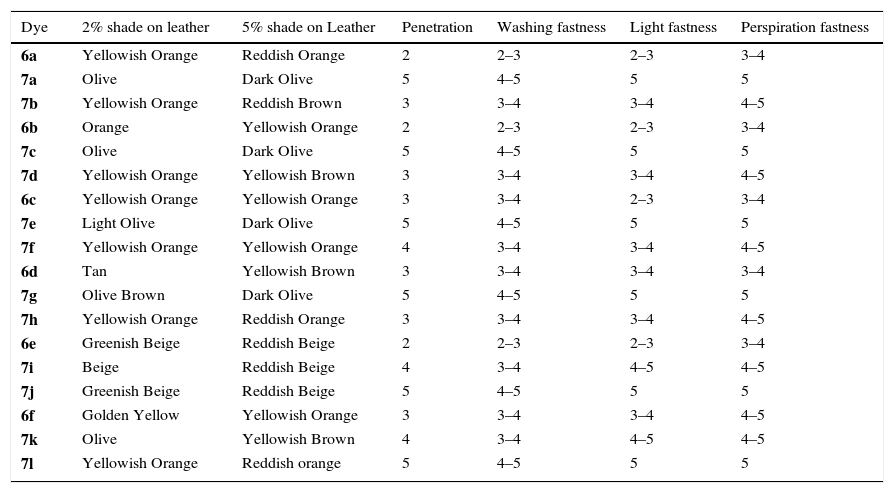
3.2Dyeing properties of substituted ligand acid dyes and their metal complexes (6a–f and 7a–l)The dyeing properties of p-substituted phenols and bis phenol dyes have been found to be very attractive. Almost all properties have been found to be of very high value (4–5). Ligand dyes had low values as per our expectation due to the presence of free hydroxyl groups. The dyeing properties of these dyes are given in Table 1.
Fastness properties of ligand acid dyes (6a–f) and their metal complexes (7a–l).
| Dye | 2% shade on leather | 5% shade on Leather | Penetration | Washing fastness | Light fastness | Perspiration fastness |
|---|---|---|---|---|---|---|
| 6a | Yellowish Orange | Reddish Orange | 2 | 2–3 | 2–3 | 3–4 |
| 7a | Olive | Dark Olive | 5 | 4–5 | 5 | 5 |
| 7b | Yellowish Orange | Reddish Brown | 3 | 3–4 | 3–4 | 4–5 |
| 6b | Orange | Yellowish Orange | 2 | 2–3 | 2–3 | 3–4 |
| 7c | Olive | Dark Olive | 5 | 4–5 | 5 | 5 |
| 7d | Yellowish Orange | Yellowish Brown | 3 | 3–4 | 3–4 | 4–5 |
| 6c | Yellowish Orange | Yellowish Orange | 3 | 3–4 | 2–3 | 3–4 |
| 7e | Light Olive | Dark Olive | 5 | 4–5 | 5 | 5 |
| 7f | Yellowish Orange | Yellowish Orange | 4 | 3–4 | 3–4 | 4–5 |
| 6d | Tan | Yellowish Brown | 3 | 3–4 | 3–4 | 3–4 |
| 7g | Olive Brown | Dark Olive | 5 | 4–5 | 5 | 5 |
| 7h | Yellowish Orange | Reddish Orange | 3 | 3–4 | 3–4 | 4–5 |
| 6e | Greenish Beige | Reddish Beige | 2 | 2–3 | 2–3 | 3–4 |
| 7i | Beige | Reddish Beige | 4 | 3–4 | 4–5 | 4–5 |
| 7j | Greenish Beige | Reddish Beige | 5 | 4–5 | 5 | 5 |
| 6f | Golden Yellow | Yellowish Orange | 3 | 3–4 | 3–4 | 4–5 |
| 7k | Olive | Yellowish Brown | 4 | 3–4 | 4–5 | 4–5 |
| 7l | Yellowish Orange | Reddish orange | 5 | 4–5 | 5 | 5 |
Almost all un-metalized p-substituted phenol dyes have been found to be different; both in 2% and 5% dyed leathers, along with a variation of the depth of shades. This can be attributed to the difference of chromophoric systems in all dyes. However, it is clear from shades that this difference is due to the difference of p-substituents in different phenols. All of the phenol homologues had dark and redder shades except p-sulphophenol substituted dyes. The variation of the depth can be attributed to the participation of peripheral group's variation in different phenols.
Almost all iron-metalized phenol dyes had been found to be similar (olive); both in 2% and 5% dyed leathers, along with a variation of depth of shades. However, it is clear from shades that the depth difference is due to the difference of p-substituents in different phenols. All the phenol homologues had dark and redder shades except p-sulphophenol. Both nitro phenols had similar shades with yellowish tone. Almost all copper-metalized phenol had been found to be different; both in 2% and 5% dyed leathers, along with a variation of depth of shades. This can be attributed to the difference of chromophoric systems in all dyes. However, it is clear from shades that this difference is due to the difference of p-substituents in different phenols. All of the phenol homologues had dark and redder shades except p-sulphophenol. Both nitro phenols had similar shades with yellowish red tone.
The dyeing properties of bis-phenols dyes have been found to be very good and attractive. Almost all properties have been found to be of very high value (4–5). The dyes with bisphenol-A have been found to be much darker as compared to the dyes of bisphenol-S. This can be attributed to the n→п* electronic transitions occurring in sulphone group of bisphenol-S.
As it is clear from dye shades among the un-metalized bisphenol dyes, the dye with bisphenol-A had a high color value while bisphenol-S dye gave a low color yield on leather. This difference can be ascribed to the participation of sulphone group present in bisphenol-S.
It is clear from the depth of shades that iron metalized bisphenol dyes had greater color value than their parent un-metalized bisphenol dyes. These dyes are much greener as they were expected. The iron complex with ligand acid dye based on bisphenol-S had a low color value while bisphenol-A dye provided a darker shade and high color yield on leather along with a much redder effect. This difference can be attributed to the participation of sulphone group in bisphenol-S.
Similarly, copper metalized bisphenol dyes had also greater color value than their parent un-metalized bisphenol dyes. These dyes are much redder as per our expectation. The copper complex dye with bisphenol-A had a high color value while bisphenol-S dye gave a lighter and low color yield on leather along with a much redder effect. This difference can be attributed to the participation of sulphone group in bisphenol-S (Figs. 4 and 5).
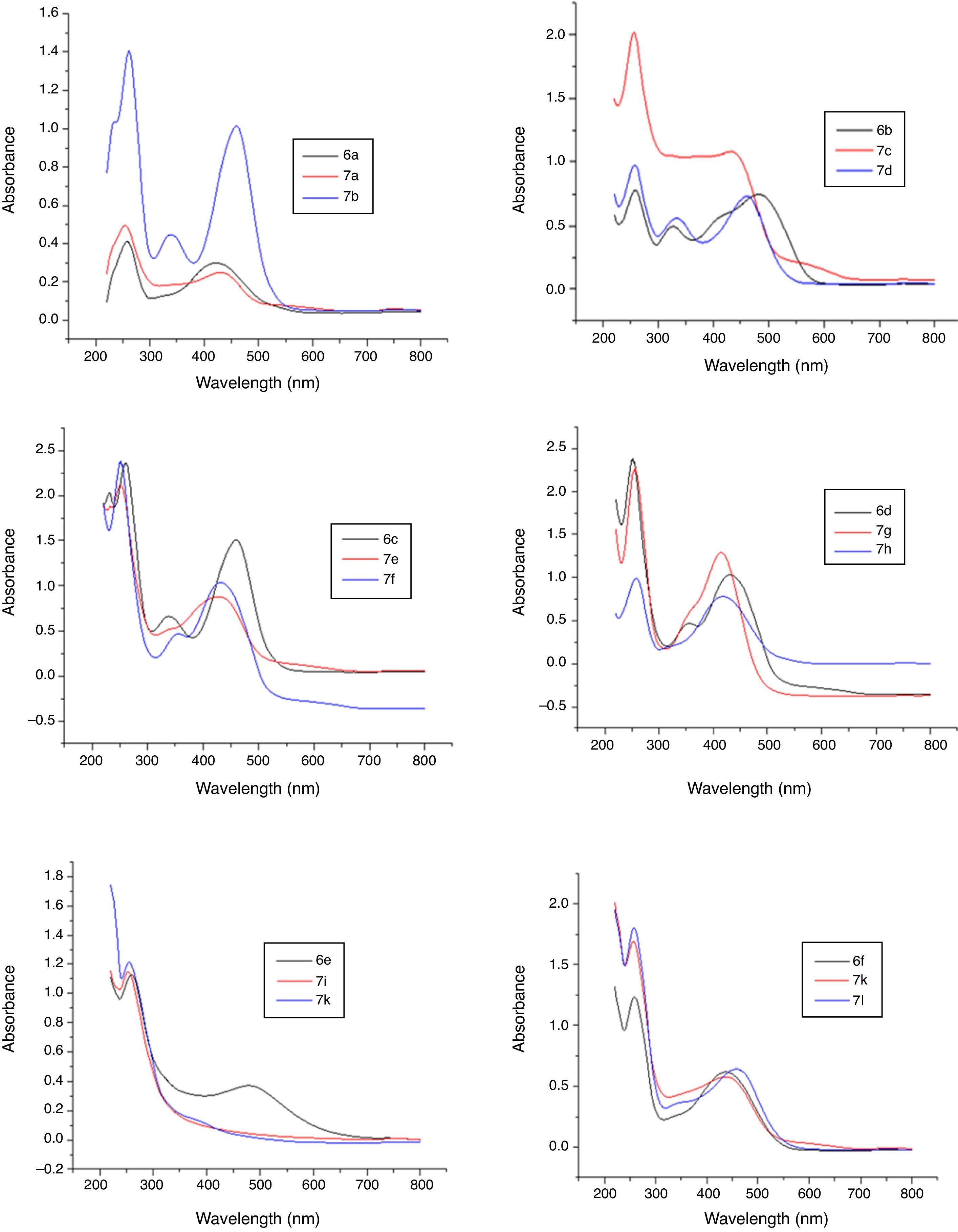
3.3Optical studies of dyesIn case of optical studies, dyes were conducted their UV–vis studies to observe the changes rendered by metal complex formation. The UV–vis data presented in Table 2 is supported by combined UV–vis spectra of ligand acid dyes 6a–f. The un-metalized dye-6a was an orange dye with λmax 460nm while on metallization of this with iron, Olive Brown dye (Dye-7a) was produced with λmax 490nm undergoing a bathochromic shift of 30nm along with hypochromic effect. Metallization of dye 6a with copper resulted in the formation of a Reddish Violet dye (Dye-7b) with λmax 500nm and showed a bathochromic shift of 40nm with hypochromic shift in intensity.
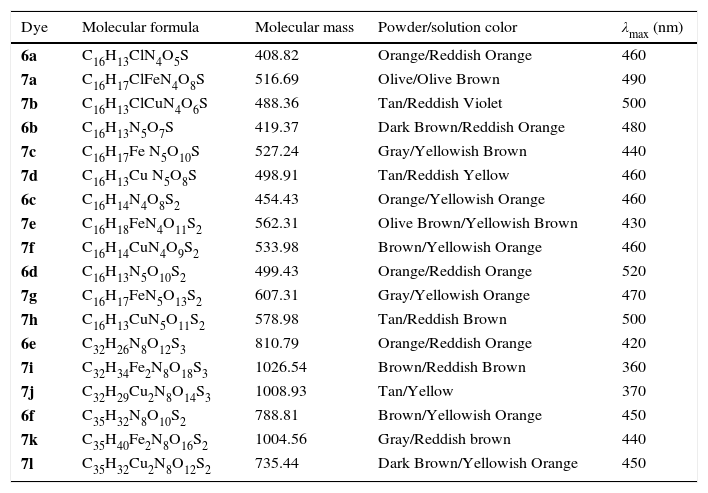
UV–vis data of ligand acid dyes (6a–f) and their Fe (II) and Cu(II) complexes (7a–l).
| Dye | Molecular formula | Molecular mass | Powder/solution color | λmax (nm) |
|---|---|---|---|---|
| 6a | C16H13ClN4O5S | 408.82 | Orange/Reddish Orange | 460 |
| 7a | C16H17ClFeN4O8S | 516.69 | Olive/Olive Brown | 490 |
| 7b | C16H13ClCuN4O6S | 488.36 | Tan/Reddish Violet | 500 |
| 6b | C16H13N5O7S | 419.37 | Dark Brown/Reddish Orange | 480 |
| 7c | C16H17Fe N5O10S | 527.24 | Gray/Yellowish Brown | 440 |
| 7d | C16H13Cu N5O8S | 498.91 | Tan/Reddish Yellow | 460 |
| 6c | C16H14N4O8S2 | 454.43 | Orange/Yellowish Orange | 460 |
| 7e | C16H18FeN4O11S2 | 562.31 | Olive Brown/Yellowish Brown | 430 |
| 7f | C16H14CuN4O9S2 | 533.98 | Brown/Yellowish Orange | 460 |
| 6d | C16H13N5O10S2 | 499.43 | Orange/Reddish Orange | 520 |
| 7g | C16H17FeN5O13S2 | 607.31 | Gray/Yellowish Orange | 470 |
| 7h | C16H13CuN5O11S2 | 578.98 | Tan/Reddish Brown | 500 |
| 6e | C32H26N8O12S3 | 810.79 | Orange/Reddish Orange | 420 |
| 7i | C32H34Fe2N8O18S3 | 1026.54 | Brown/Reddish Brown | 360 |
| 7j | C32H29Cu2N8O14S3 | 1008.93 | Tan/Yellow | 370 |
| 6f | C35H32N8O10S2 | 788.81 | Brown/Yellowish Orange | 450 |
| 7k | C35H40Fe2N8O16S2 | 1004.56 | Gray/Reddish brown | 440 |
| 7l | C35H32Cu2N8O12S2 | 735.44 | Dark Brown/Yellowish Orange | 450 |
Three dyes were prepared with p-nitrophenol. The first one being an un-metalized dye and the other two were iron and copper complexes, respectively. The detailed properties of p-nitrophenol dyes are given in Table 1. The unmetallized dye 6b was an orange dye with λmax 480nm which developed yellowish brown dye on complexation with iron (Dye 7c) with λmax 440nm. It showed hypsochromic shift of 40nm along with hyperchromic effect. Similar behavior was observed for copper complexes of this dye. The dye 6c was prepared from phenol-4-sulphonic acid which was Yellowish Orange colored dye having λmax 460nm. Metallization of the dye 6c with iron and copper produced yellowish brown (Dye 7e) and Reddish Orange dye (Dye 7f) dyes with λmax 430 and 460nm respectively which exhibited pattern of hypso and hypochromic effects. Dyes 7g and 7h were iron and Cu complexes of 1:1 type of dye 6d. The un-metalized dye 6d was a Reddish Orange dye with λmax 520nm. Its metallization with Iron produced a yellowish brown dye (Dye 7g) with λmax 470nm and enhanced the absorbance intensity than parent dye and showed a hypsochromic shift of 50nm. While metallization of the same with copper resulted in the formation of a Reddish Brown dye (Dye 7h) with λmax 500nm and a hypsochromic shift of 20nm was observed.
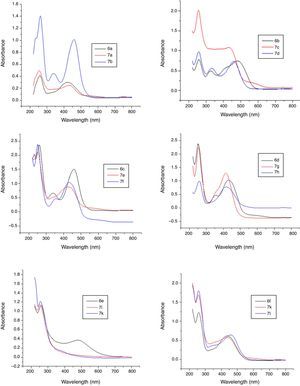
In the bisphenol series six dyes were prepared. For this purpose two different bisphenols, namely 4,4′-dihydroxy biphenyl sulphone (BPS), 4, 4′-dihydroxy biphenyl propane (BPA), were used as couplers. Three disazo dyes were accomplished with bisphenol-S. The first dye being an un-metalized one and another two were iron and copper bis-metal complexes, respectively. The un-metalized dye 6e was a Reddish Orange dye with λmax 420nm. Its metallization with iron produced a Reddish Brown dye (Dye 7i) with λmax 410nm and a hypsochromic shift of 10nm was seen. While the metallization of same dye 6e with copper resulted in the formation of a Yellow dye (Dye 7j). It had λmax 370nm and absorbance 1.6, showed a hypsochromic shift of 50nm. The metal complexes of dye 6f with iron and copper exhibited the similar behavior like bisphenol S dye metal complexes in absorption intensity while no conceivable change was found in λmax position. From the detailed UV–vis study of dyes it was gathered that metal complexes of ligand dyes bearing electron with drawing groups showed hypsochromic shit and those having electron donating groups showed bathochromic shift in absorbance which is in accordance with well established UV–vis absorption pattern of compounds (Figs. 6 and 7).
4ConclusionsA series of new acid dyes 6a–f and their metal complexes (7a–l) have been synthesized in high yields from 1-(p-sulphophenyl)-3-methyl-4-aminopyrazolone with p-substituted phenols and bisphenols. Metal complexation has changed the absorption maxima of acid dyes which resulted in hypsochromic shift in iron and copper complexes of those acid dyes which carried electron withdrawing groups like nitro and sulfonic while iron and copper complexes of dyes bearing electron donating groups caused bathochromic shifts. Moreover, metal complex dyes had better application properties on leather as compared to ligand dyes due to elevated substantivity owing to electron deficient metals and polar substituents.
Conflict of interestThe authors have no conflicts of interest to declare.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.