The objective was to research the physical and chemical properties of collagen scaffolds (CS) obtained from bone matrix Nukbone® subject to a demineralization process using hydrochloric acid. The CS samples were characterized by optical and scanning electron microscopy, elemental chemical analysis, X-ray diffraction, spectroscopy Infrared, thermal analysis, differential scanning calorimetry and nitrogen adsorption. The microanalysis were used to set the macro- and microstructures of CS. They showed that the CS retained the morphology of Nukbone® with interconnected pores and their size between 100 and 500μm, and it is composed of 20% by weight of HA and the rest is collagen type I. By infrared spectroscopy the functional groups of collagen type I (amide A – 3285, B – 2917, I – 1633, II – 1553 and III – 1239cm−1) were identified. By thermal analysis it was determined that the phenomenon of denaturation of the collagen type I began in the range of 75–85°C and burned above 200°C.
In the mid-1980s came the so-called tissue engineering, which has continued to evolve as an exciting and multidisciplinary field, aiming to develop biological substitutes to restore, replace or regenerate defective tissues (Chan & Leong, 2008; Lanza, Langer, & Vacanti, 2013) Tissue engineering mainly uses three factors: scaffolds made of biomaterials must meet certain requirements, cells and growth-stimulating signals, which are known as the triad of tissue engineering. The scaffolds are typically made of polymeric biomaterials, must be biocompatibles and biodegradable upon implantation, with a rate matching that of the new matrix production by the developing tissue; they should be absorbable by the human organism and should be porous with interconnected pores of adequate size to allow cell adhesion and cell proliferation, giving the opportunity to subsequent tissues development, and they must also have the ability to bind to surrounding tissues and be clinically manageable (Langer & Vacanti, 1993).
There are a great variety of choices to select scaffolds for tissue engineering; in general, the biomaterials used for making porous scaffolds can be classified into two categories according to their sources, natural and synthetic (Meyer, Meyer, Handschel, & Wiesmann, 2009). The natural biomaterials generally have excellent biocompatibility so that the cells can adhere and grow with excellent viability. In this sense, the scaffolds can be obtained from different animal species: pigs, bovine and horses, using intestine, pericardium, skin, tendon, bone and demineralized bone (Carletti, Motta, & Migliaresi, 2011; Chen, Torian, Price, & McKittrick, 2011).
The main objective of this work was to determine the physicochemical properties of collagen scaffolds (CS), produced by immersion of bone matrix (BM) blocks in hydrochloric acid (HCl) at 0.5M, through different characterization techniques: the histological studies were made with optical microscopy (OM) using the following stainings: hematoxylin & eosin (H&E) (Montuenga Badía & Calvo Gonzáles, 2009), von Kossa (Penney, Powers, Frank, Willis, & Churukian, 2002), Masson's Trichrome (Sampedro, 2014) and polarized light (Narváez, 2014). Scanning electronic microscopy (SEM) and energy dispersive spectrometry (EDS) (Vázquez & Echeverría, 2000); thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) (Skoog, Holler, & Crouch, 2008); Fourier transform infrared spectrometry (FTIR) (Coman, Grecu, Baciut, Prodan & Simon, 2007; Griffiths & De Haseth, 2007) the total area was measured using N2 adsorption (BET) (Nishad, Dhathathreyan & Ramasami, 2002) was measured. For each technique at least 5 samples were used.
The collagen scaffolds characterized in this work were obtained from Nukbone®, bone matrix whose pores are interconnected in a range of size from 100 to 500μm that allow the cellular growth, and were already used without cells in patients with bone defects, successfully (Aguilar, Piña, López, Rodríguez, & Driessens, 2005; Piña, Munguía, Palma & Lima, 2006; Cueva-Del Castillo et al., 2008; León, Araiza, & Piña, 2012; Rodríguez et al., 2013).
2Material and methods2.1Sample preparationBlocks of Nukbone® (BM) of 2.5cm×2.5cm×0.5cm were employed, and each one was immersed for 10min in stirring in 50mL of chloridric acid (HCl) (TJ Baker, Germany) solution with bidistilled water at 0.5M. Then they were rinsed with bidistilled water and dried, thus collagen scaffolds were obtained, as shown in Figure 1.
2.2Optical microscopy (OM)To perform histological studies collagen scaffolds cubes of 1cm×1cm×0.5cm were used; they were dehydrated in alcohols at different concentrations: 50, 60, 70, 80, 96 and 100%, in an histoquinet during 2h in each concentration and then were included in wax cubes and 3μm thick slices were cut with a microtome.
To determine the internal structure of the CS, bright-field microscopy was used. The samples of CS were cut into slices of 3μm thickness. The samples were stained using hematoxylin & eosin (H&E), Masson's Trichrome (MT), von Kossa (vK), and they were also observed using polarized light microscopy (PLM).
2.3Scanning electron microscopy (SEM) and elemental chemical analysis (EDS)The microstructure of samples was analyzed with a JEOL electron microscope JSM7600F, using the technique of secondary electrons with a voltage of 5kV. The size of the samples used in SEM was 1cm×1cm×0.5cm, and no sample was covered with conductive material; they were placed directly in the sample holder. EDS technique was also employed for determining the main compounds of different superficial zones.
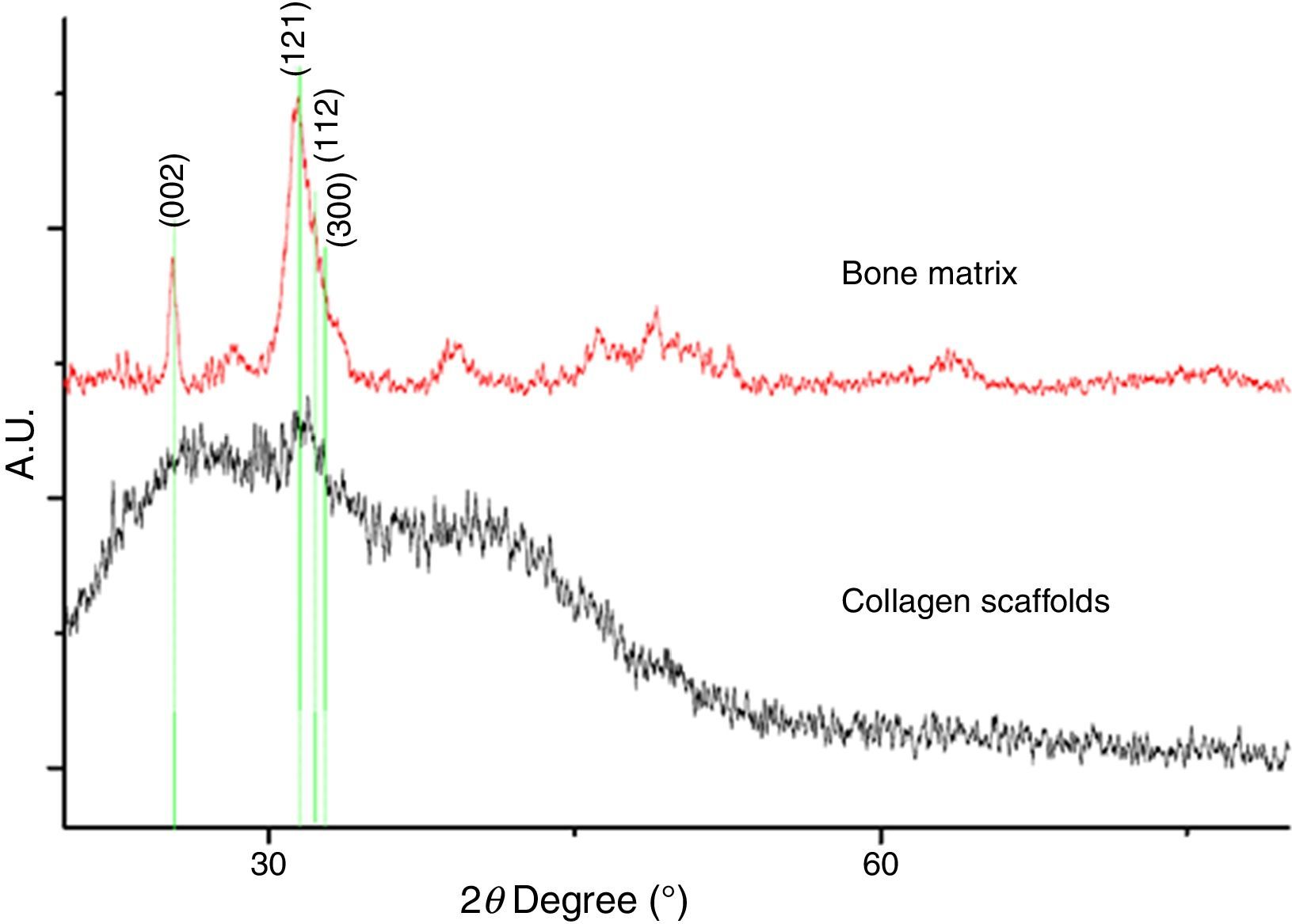
2.4X-ray diffraction (XRD)The powders of BM and CS were tested by an X-ray diffractometer D8 Advance of Bruker AXS, with Cu Kα radiation (λ=0.154nm) and 2θ from 18° to 80°, to obtain structural information on an atomic scale from both materials (BM and CS).
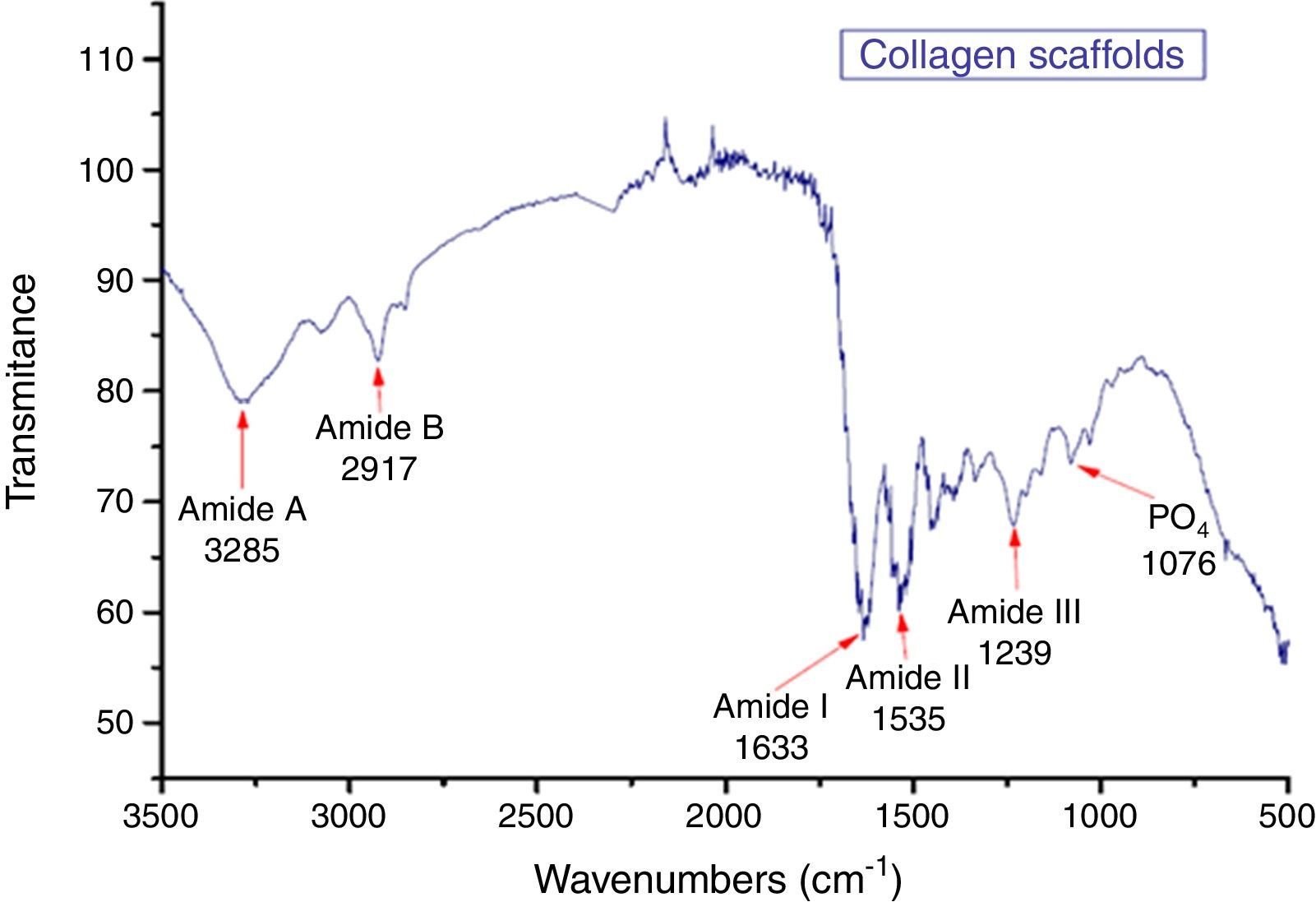
2.5Fourier transform infrared (FTIR)To identify the functional groups of collagen used the Fourier transform infrared (FTIR) analysis of CS was done with a Scientific Nicolet™ 6700 FT-IR spectrometer, in a wave number range of 500–3500cm−1; the samples of 0.2cm thickness were placed directly in the spectrometer.
2.6Thermo gravimetric analysis (TGA)–differential scanning calorimetry (DSC)The behavior of the samples with temperature was studied using TA Instruments SDTQ600 in nitrogen atmosphere. TGA and DSC curves were taken in the range 25–700°C at a heating rate of 10°C/min.
2.7Nitrogen adsorption (BET)The total area of the samples was determined using a Quantachrome Instruments Autosorb equipment, employing cubes of 20mm×20mm×20mm of BM and CS were used. The analysis was carried out using liquid nitrogen, and the samples were cleaned prior to the analysis at 77K. The area was determined before and after the demineralization process (Chen & McKittrick, 2011)
3Results and discussion3.1Preparation of samplesActually, HCl is used for demineralization of hard tissue with allograft purposes, as reported by Gruskin, Doll, Futrell, Schmitz, and Hollinger (2012), or for obtaining matrices from demineralized human and bovine bone, for use in tissue engineering; Murugan et al. reported 3 days of treatment to demineralize the samples of 10mm3 at environment temperature (Murugan, Ramakrishna, & Rao, 2008); meanwhile our treatment requires only 10min.
The results showed that the demineralization of bone matrix using HCl did not alter its structure and hold the organic compound that was identified as type I collagen.
3.2Optical microscopy (OM)The samples of CS cut into 3μm thick slices were used in bright-field optical microscopy, with several staining techniques mentioned above.
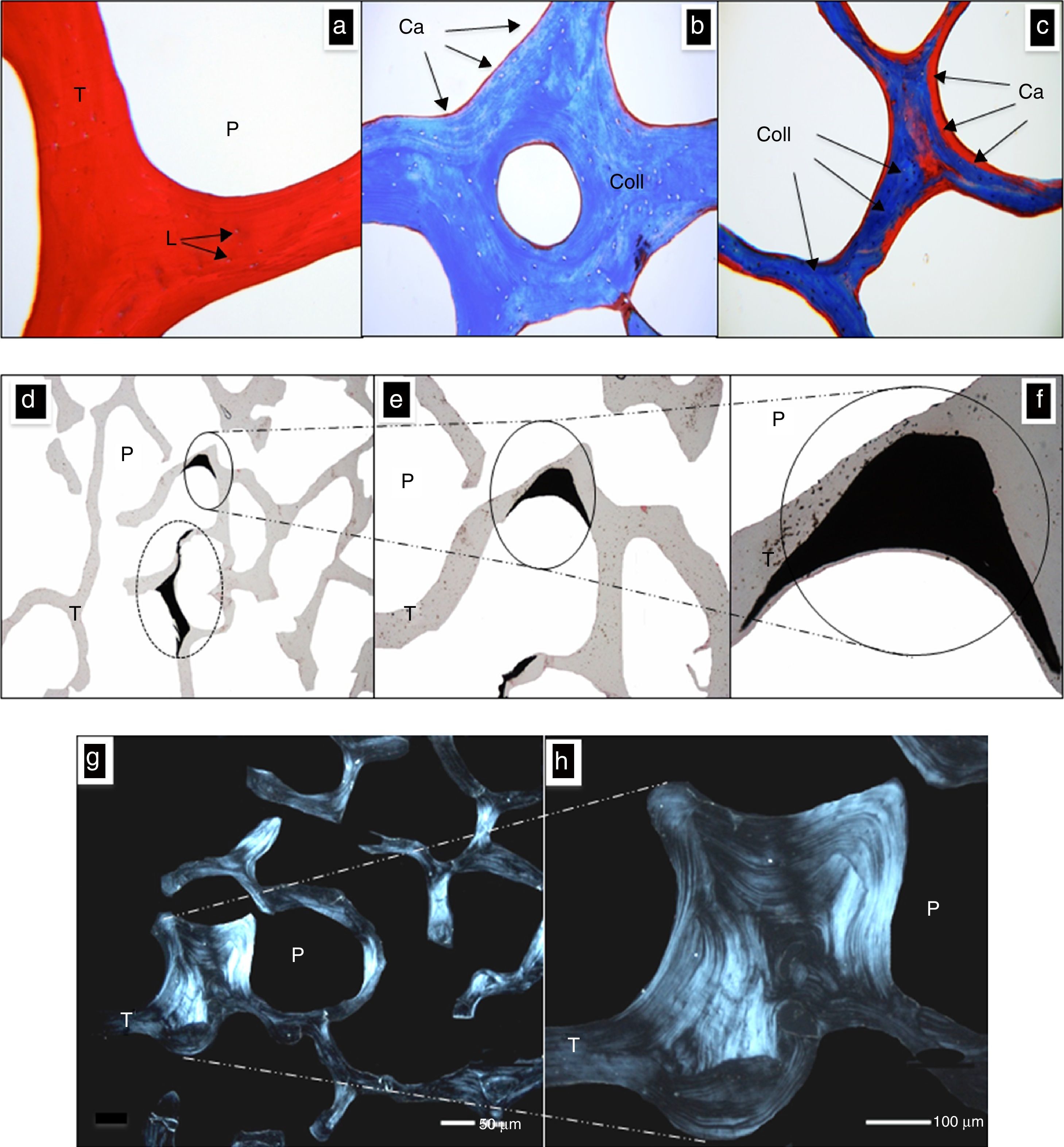
Figure 2a shows a cut of CS stained with H&E; P denotes the space of a pore while T denotes the space occupied by a trabecula, now without the ceramic component. The morphology of this tissue is observed with eosinophilic pigment (red), thus expressing its affinity by the organic structure, which in this case is the collagen. The bone gaps were spaces (L) occupied by osteocytes, preserved their architecture (size and form), and they were basophilic pigmented (blue), which matches with description reported by Domínguez and Torres (2006).
Morphological characterization with optical microscopy. (a) CS stained with H&E, pores (P) and trabecules (T) with multiples lacunae (L) can be seen. (b) CS stained with Masson's Trichrome, identifying the fiber of collagen (Coll) with a blue color and residue of calcium (Ca) identified by red color. (c) Elsewhere in the CS in which a higher concentration of Ca is observed. a–c: 100×. (d–f) CS stained with von Kossa technique. The dark areas involving a higher concentration of calcium remaining after demineralization. P denotes a pore, T denotes a trabecula and Ca denotes calcium. d: 10×, e: 40×, f: 100×. (g and h) Photographs of the CS taken with polarized light; the collagen fibers orientation gives the architecture of bone trabeculae (T) and the pores (P). g: 10×, h: 40×.
Meanwhile Figure 2b and c shows a 3μm thickness histological section of CS stained with Masson's technique where the structure was occupied by a trabecula meshwork, now without ceramic compound. Here, the red color denotes the calcium presence (Ca) and the blue color denotes collagen (Coll); it is possible to see that the calcium is found mostly on the edges, and it is not distributed uniformly in the volume as could be expected.
Von Kossa staining, shown in Figure 2d–f, is employed to determine Ca concentration in new formation tissue (Zong Ming, Jian Yu, Rui Xin, Hao, & Yong, 2013). This stain allows to observe the concentration Ca places; these places are related with the sites where the HA is associated with the collagen in Masson's stain, observed in Figure 2c (in red color). In Figure 2e only two dark zones correspond to high concentration of Ca, and in the rest of the body of CS, small sites of Ca were observed. The architecture of trabeculae is conserved; in Figure 2f the details of the deposition of Ca including a circle way of the trabeculae are shown.
Figure 2g and h are cuts of 3μm thickness; these images were obtained with a polarized light microscope, and it corresponds to the collagen of a trabeculae. This technique showed how the collagen fibers are arranged in bundles that determine the final morphology of the bone matrix and the scaffolds; the pores are preserved and their sizes varied in the range of 100–500μm.
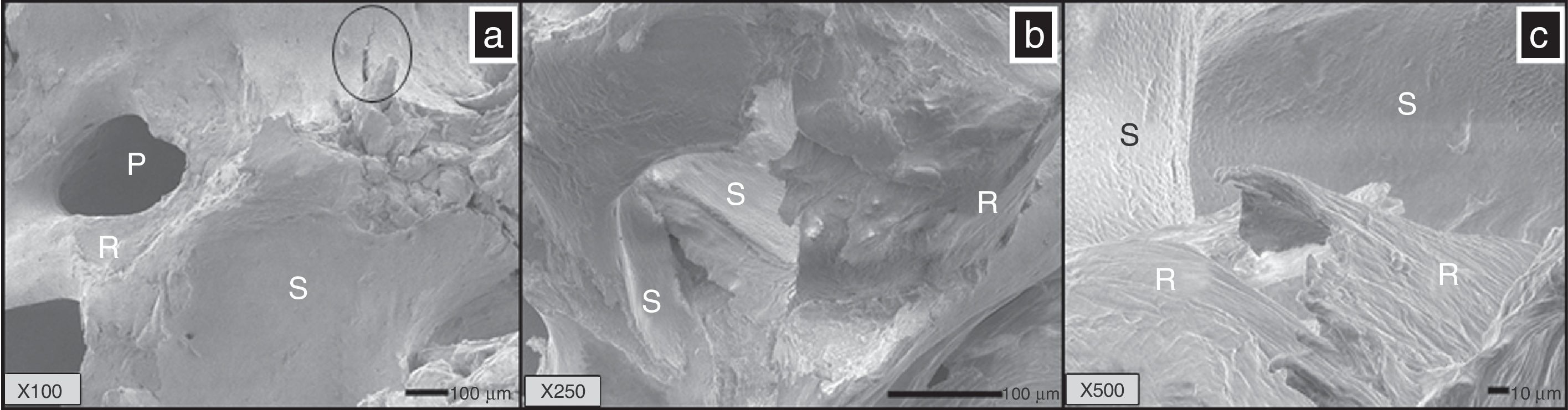
3.3Scanning electron microscopy (SEM) and elemental chemical analysis (EDS)Figure 3a (X100) shows the microstructure of the BM; three zones may be seen; one of them is rough (R) and corresponds to a cut from trabecula; another zone is smooth (S) which corresponds to the extern surface of the trabeculae constituted by hydroxyapatite (ceramic component of the bones), and the last zone is a porous zone (P), that corresponds to opened and interconnected pores, which have a range size of 100–200μm, and a fracture (circle) due to excessive charge of compression is also observed.
(a) Bone matrix (BM) observed by SEM, pores are observed (P) and a smooth zone corresponding to the surface of HA (S), and a rough area (R) lacking of HA, corresponding to collagen fibers. SEM images of CS (b and c) showed two areas well identified; the smooth (S) surface corresponded with the area of HA which was removed by the demineralization process. The other zone was observed as rough (R) corresponding to collagen fibers. a: 100×, b: 250×, c: 500×.
Figure 3b shows a photomicrograph of CS (X250); it is interesting to note two different surfaces; one of them is a smooth zone feature to hydroxyapatite (HA, main compound of bone) and which maintains its morphology (S) and is covering to rough volumes (R) that correspond to the trabecula conformed by collagen fibrous; this fact speaks of the kindness of the demineralization method.
Figure 3c (X500) also shows a microstructure of CS, and it can be seen that the exterior surface (S) retains its smooth morphology while the volume is made of long fibers as expected. This was also reported by Chen & McKittrick, (2011).
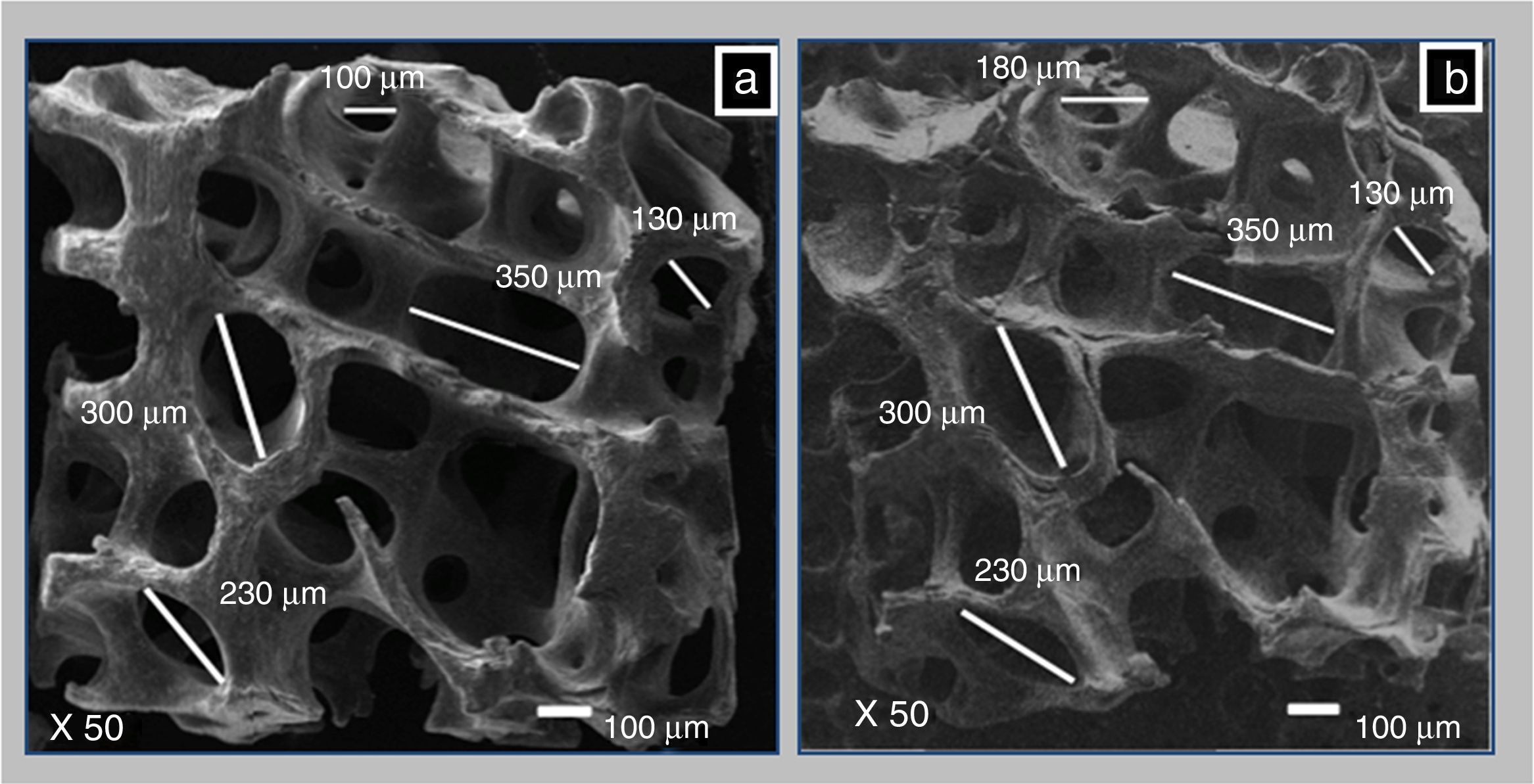
3.4Macropores measured by SEMThrough SEM the pore size before and after demineralization was compared. Figure 4a and b are SEM images (X50), and they allowed measurements to the bone matrix, first mineralized (BM) and after demineralized (CS). The pores were measured and there was no difference in the measurements before and after demineralization. The pore sizes remained in a range of 180–350μm. Murphy reported that the optimum pore size to promote cell proliferation and migration in bone tissue engineering is 325 microns (Murphy, Haugh, & O’Brien, 2010). The pores generally have an oval shape that the largest diameter was in a range between 100 and 500μm, and was ideal for use as a cell scaffold.
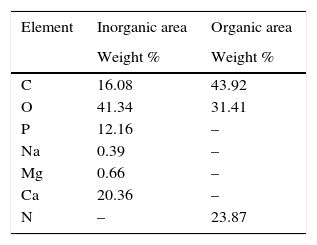
3.5Energy dispersive X-ray analysis (EDS)Elemental analysis was made using EDS of the smooth and rought areas identified by SEM in Figure 4a and b, to be punctual is a semiquantitative analysis. On the surface, the identified elements of higher percentage in weight were: O (41.34%); Ca (29.36%); C (16.08%); P (12.16%); Mg (0.66%) and Na (0.39%) that can be associated to calcium phosphates like HA, as observed in Figure 3a. In the fibrous zone under the surface, the identified elements were C (43.92%), N (23.87%) and O (23.87%); these elements are part of the amide functional groups of the collagen (NH, CO) between others, as observed in Figure 3c. In Table 1, the elements found on CS and their proportion are reported.
The XRD of BM and CS is shown in Figure 5 and was compared with the data sheet 9-432 from Joint Committee Powder Diffraction (JCDP), of PDF-2 (Powder Diffraction Field) of International Center for Diffraction Data (ICDD, 2006), that corresponds to hydroxyapatite, mineral compound of bone, which is a crystalline ceramic. The diffractogram of the CS appears as an amorphous material; however, the main peaks of HA: 2θ=25.8 (002), 2θ=30.4 (121), 2θ=30.6 (112), 2θ=32.9 (300) are distinguished, which indicates that a certain amount of it is present in the sample.
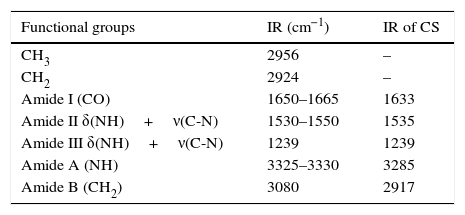
3.6Infrared spectroscopy (FTIR)The results obtained through this technique allow us to identify the functional groups of the organic part of the CS, see Figure 6. However, there is a phosphate group PO4 (1076cm−1), corresponding to remainder hydroxyapatite (Xiao, Cai, & Liu, 2007). Functional groups identified in the IR spectra were amide A, B, I, II and III corresponding to collagen (Xiao et al., 2007).
The sample of CS shows the following bands: 1650–1665cm−1 (CO) corresponding to Amide I; 1530–1550cm−1 corresponding to Amide II δ(NH)+ν(CN); 3325–3330 for Amide A (NH); the peaks at 2956cm−1 corresponding to functional group CH3; 2924cm−1 to CH2; 1239cm−1 to Amide III δ(NH)+ν(CN); and 3080cm−1 to Amide B (CH2) can be seen. Those functional groups are corresponding to collagen (Sionkowska & Kozłowska, 2010). Table 2 summarizes the data obtained for the CS IR spectra.
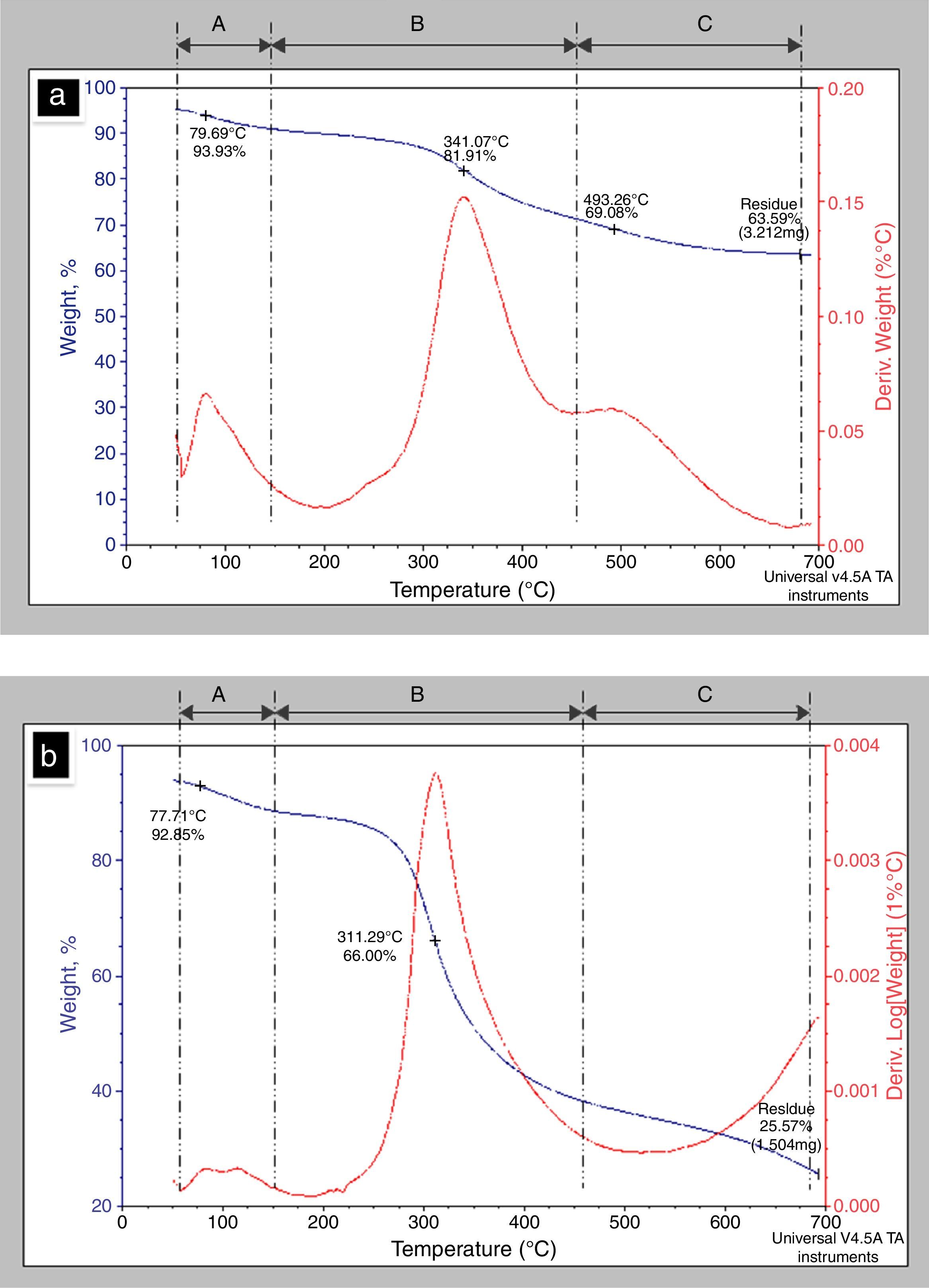
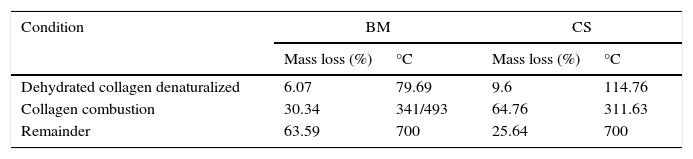
3.7Thermo gravimetric analysis (TGA)The TGA provided information about the behavior with the temperature presented by the BM (Fig. 7a) as compared with CS (Fig. 7b). The main loss is presented in three different temperature ranges given by A: (50–200°C); B: (200–450°C) and C: (450–700°C), as shown in Figure 7a and b. The curve in A corresponded to the loss of the physisorbed and chemical water in the material, which represented the 6.07%wt of the bone matrix, which occurred at 79°C. Meanwhile for CS the loss of water corresponded to 9.6%wt and occurred at 114.74°C. These findings are similar to those reported by Lozano, Peña & Heredia, (2003).
The following loss, occurring in the range of temperatures B in the thermogram, for BM was observed between 341 and 493°C corresponding to 30.34wt% loss compared to 67.76% corresponding to 311.63°C for CS. These losses are related to the combustion of collagen in the two samples. The differences in mass loss in the BM could be due to the structure of the trabeculae devoid from mineral phase (HA), so that the combustion occurs at a lower temperature. Finally, the weight loss in C, corresponding to 450–700°C, presenting a minimum change in mass loss, leaving a residue of 63.59% for the BM and 25.64% for CS surely corresponds to calcium compounds. The use of derivative of thermal analysis (DTG) allowed determining that the degradation process of collagen had maximum rate at 311.95°C. The data obtained from the thermograms for BM and CS are summarized in Table 3.
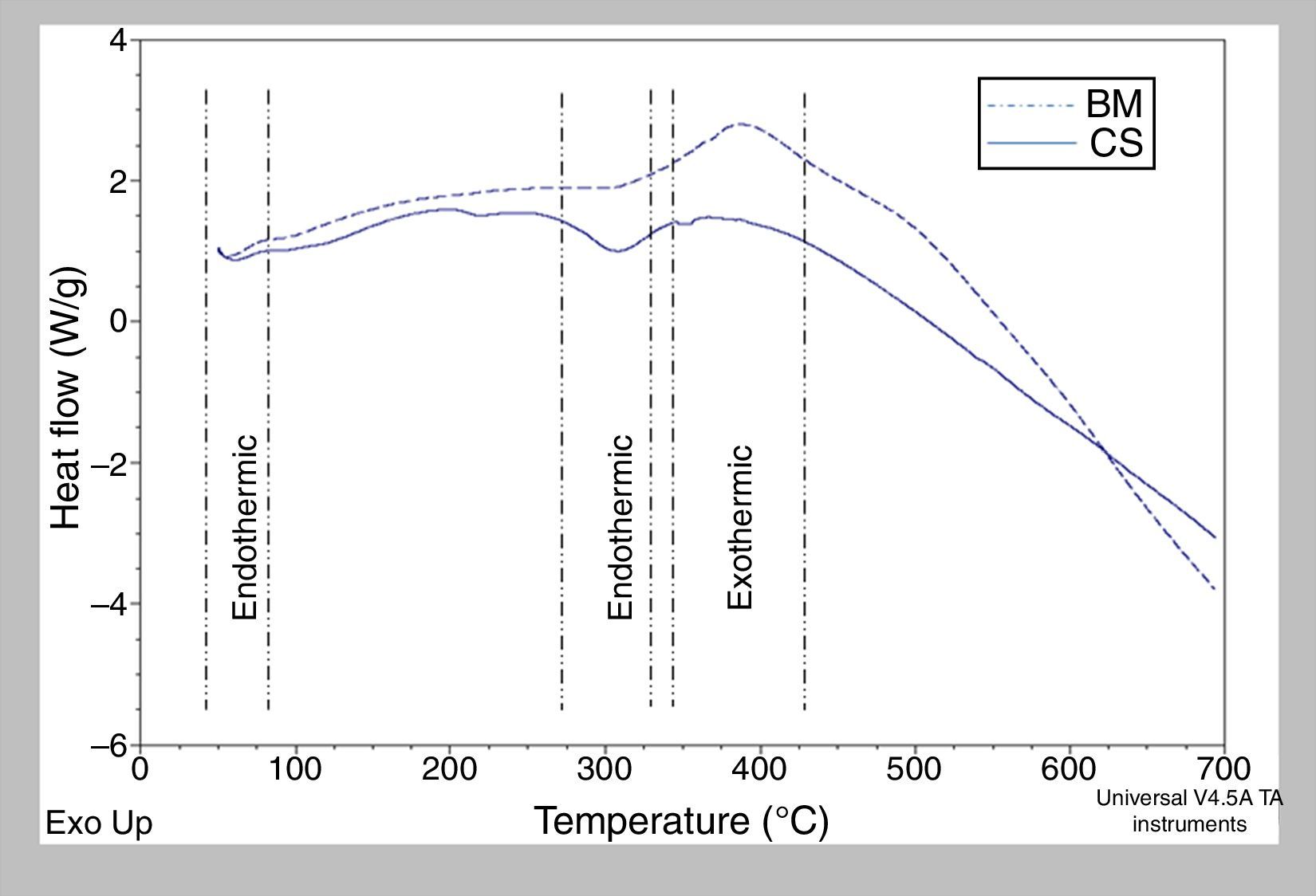
3.8Differential scanning calorimetry (DSC)This technique allowed to identify changes in the thermodynamic variables that occurred during the physic-chemical transformations induced by heating or cooling the BM and CS. The first endothermic peak observed in BM and CS was between 85 and 90°C, corresponding to dehydration process of surface water, see Figure 8. This result is also presented in the study by Lozano et al. (2003). The second endothermic phenomenon observed in the BM and CS was in the temperature range between 275°C and 325°C. This is related to the loss of hydrogen bonds, so that the phenomenon of protein denaturation, could be initiated in this interval in which the tertiary structure is lost. This phenomenon is reversible if the protein is newly hydrated.
An exothermic process was found in both samples (BM and CS) observed in a temperature range between 350°C and 425°C, corresponding to the combustion of the collagen fibers. The results obtained with the DSC technique are complemented by TGA, which suggests that BM and CS are basically composed of three components, which are water, collagen and hydroxyapatite.
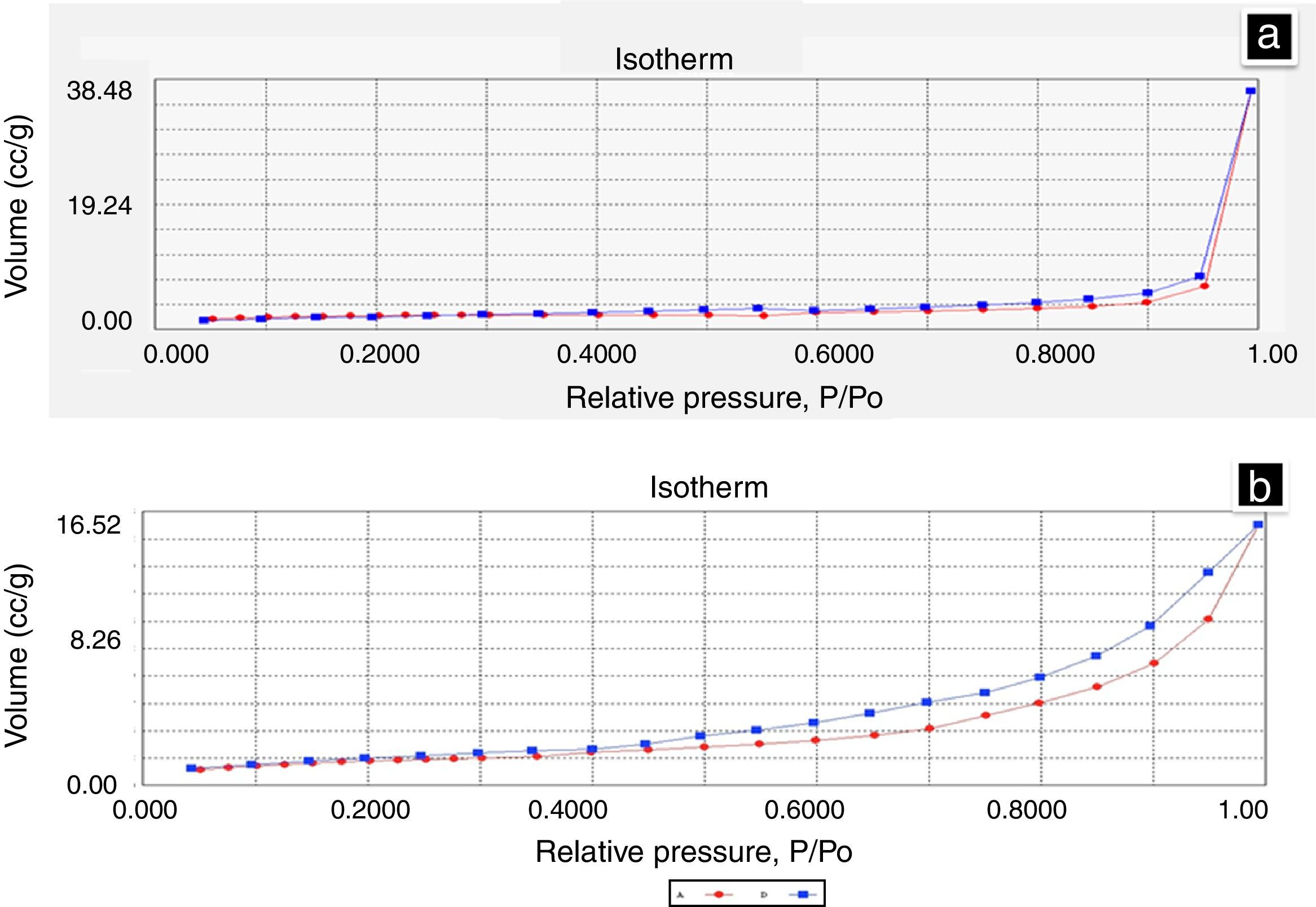
3.9Nitrogen adsorption (BET)The total area of the BM was determined by BET and was compared with the area of CS. The results obtained were 5.39m2/g for BM and 7.34m2/g for CS. The difference of the total surface area between the BM and CS was 36.18%, the result can be used for in vitro studies, because it is the area that will be in contact with the cells. Figure 9a shows the isotherm presented by the BM, and it shows that there were no changes (hysteresis) between adsorption (red line) and desorption (blue line). Figure 9b is the isotherm from CS, and there is a minimum difference between the adsorption and desorption of gas. The isotherms observed are Type 2 and correspond to an open macropores type, according to the IUPAC. Table 4 summarizes all data obtained.
These scaffolds have the advantage that they can be molded to give them different shapes. The scaffolds preserved the morphology from trabecular bone, the pores are open and interconnected, and the pore sizes are adequate for use as cell scaffolds (tissue regeneration). This biomaterial is proposed for use as a scaffold in tissue regeneration. The collagen scaffolds obtained are completely biocompatible, biodegradable and easy to use.
Thanks to E. Fregoso, V. Rodríguez, A. Pérez, O. Novelo, A. Tejeda, M.A. Canseco, M. Herrera, A. Zepeda, F. Pasos and V. Maturano for their technical support. Also thanks to DGAPA-UNAM for financial support through projects: IG100114, IT114911, IT119111 and IN113108.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.