Nano grease is found to be stable and homogeneous (wherein carbon nanotubes act as sole thickeners and oil is polyalphaolefin (PAO)). This is a good indication that three dimensional (3D) percolation network structures among carbon nanotubes (single wall and multi wall) play a crucial role in stabilization. To better understand this assumption and provide further evidence, some additional carbon nanomaterials, such as carbon nanofiber (CNF), graphene, fullerene (C60), Ni-coated single wall carbon nanotube (SWNT), are selected to make the grease. Unfortunately, CNF, graphene, fullerene (C60) and Ni-coated SWNT do not form stable greases as nanotubes do. In addition, SWNTs were mixed with CNF and Ni-coated SWNT respectively to see if stable grease could be formed. The results indicate that the CNF/SWNT did not form the stable grease, while Ni-coated SWNT/SWNT did. Inter molecular Van der Waals forces could reasonably explain these experimental results. Appropriate tube size (nanotube and nanofiber), stereo structure (nanotube, graphene and fullerene), surface energy (nanotube and Ni-coated nanotube) are critical factors that determine if Van der Waals forces could take effect or not. The scientific merit of this paper is that we understand in what manner stable greases are formed and what kinds of carbon materials are appropriate for acting as sole thickener.
Grease generally consists of a solid or semi-fluid two-phase system that is widely used in various application areas as a lubricant or sealant. Typical commercial greases are normally comprised of polyalphaolefin based oil and a metal–soap thickener such as lithium, calcium and aluminum (Hampton, 1969; Rosemary OHalloran, 1953). The thickener is used to ensure that the grease has a stable pseudo plastic property. In addition to the base oil and soap, grease contains different additives, for example, friction modifiers and extreme pressure additives. Most of the commercial greases available in markets are nonconductive greases, e.g. lithium grease and calcium grease are examples of commercially available and widely used greases (Radulescu & Radulescu, 2006). However, the rapid development in many industrial sectors has increased the demands for grease with exceptional properties. These properties include high thermal and electrical conductivity, resistance for corrosion and resistance for high temperatures. Carbon nanotubes (CNTs) attracted significant attention since they were discovered by Iijima (1991).
The remarkable structural, thermal, electrical and mechanical properties of CNTs make it a potential material in many application such as nanocomposites (Abueidda et al., 2015; Neupane et al., 2014; Younes et al., 2014), nanofluids (Younes, Christensen, Hong, & Peterson, 2013; Younes, Christensen, Li, Hong, & Ghaferi, 2015; Younes, 2014) and stable nanogrease. Hong et al. (2010) successfully fabricated nanogrease by mixing SWNTs and MWNTs with polyolefin oils respectively. The results showed that using only 11wt% of SWNTs increased the thermal conductivity by 60–70% compared to that without carbon nanotubes. Moreover, the grease was found to be electrically conductive, has a high dropping point, good temperature resistance, and does not react with copper at temperatures up to 177°C.
Hongtao, Hongmin, Haiping, and Younes (2014) studied the tribological properties of the CNT grease and compared it with the original base oil grease. CNT grease exhibited better lubricating performance and wear resistance than the base oil grease. The performance improvement of CNT grease is attributed to the unique hexagonal structure and the high thermal conductivity of CNTs. Chen et al. (2015) studied the friction, wear behavior, and wear mechanisms of in situ transformed carbon fiber toughened alumina ceramic matrix composite. Wear tests for 20vol% of carbon fiber toughened alumina ceramic matrix composite (Cf/Al2O3 composite), commercial Al2O3 ceramic and 40 Cr steel were conducted using a cylinder on blocks wear tester under PAO oil and PAO+carbon nanotube (CNT) as lubricants, applied at loads of 220N and 520N. It was found that in situ transformed carbon fibers play a toughening role in Al2O3 ceramic and the PAO+CNT lubricant has better lubricative role than PAO oil. In previous work (Hong et al., 2010) the ability of fabricating stable and homogeneous grease of SWNTs and MWNTs was demonstrated successfully. The successful fabrication is attributed to the formation of a three dimensional network structure.
In this paper, five different carbon nanomaterials; graphene/graphite, Ni-coated SWNTs, carbon nanofibers, Ni-coated SWNTs/SWNTs mixture and CNFs/SWNTs were mixed with polyolefin oil respectively to make nanogrease. The aims of the work are (i) to better understand the assumption of the three dimension network structure, (ii) answer the question; why SWNTs and MWNTs are the only successful materials to fabricate stable and homogeneous grease.
2Materials and methodsSingle-wall carbon nanotubes (SWNTs) and multiwall carbon nanotubes (MWNTs) were purchased from Carbon Nanotechnologies Incorporation (CNI, Houston, Texas) and Beijing Boda Green High Tech Co. (Beijing, China). Graphene was purchased from Graphene Supermarket (Calverton, New York). The specific surface area of the graphene is 60m2/g and the color is black; the purity is 97% with an average flake thickness of 5–30nm and an average particle (lateral) size of ∼5–25μm. Carbon nanofibers (CNFs) were purchased from Pyrograf Products, Inc. (Cedarville, Ohio). The N2 surface area (m2/g) is 35–45, dispersive surface energy (mJ/m2) is 125–145, moisture content (%) is <5, iron content (ppm) is <100 and PAH content(mgPAH/g fiber) is <1. The carbon nanotubes, graphene, carbon nanofibers were all used as received, without further purification, functionalization or surface modification. There may be some impurities in the samples, such as carbon black and metals. Ni coated SWNTs were prepared using the methodology as reported previously (Wright et al., 2007; Zhang, Franklin, Chen, & Dai, 2000). DURASYN® 166 is a commercial polyalphaolefin oil product purchased from Chemcentral (Chicago, IL). The magnetically sensitive Fe2O3 nanoparticles with an average diameter of 5–25nm were purchased from Sigma Aldrich. A three roll mill (Ross Engineering Inc., New York) was used to prepare the stable and homogeneous greases (Fig. 1).
The grease containing Fe2O3 nanoparticles was prepared by mixing the Fe2O3 particles and carbon nanotubes together in the polyalphaolefin oil under stirring while heating, and then slurry samples were poured into a three-roll mill. The milling process was repeated 5–8 times until the slurry samples became homogeneous greases. The general configuration of a three roll mill machine consists of three adjacent cylindrical rollers, each of which runs at a different velocity. The first and third rollers, called the feeding and apron rollers, rotate in the same direction while the center roller rotates in the opposite direction. The material to be mixed is fed into the hopper, where it is drawn between the feed and center rollers. When pre-dispersed, the material sticks to the bottom of the center roller, which transports it into the second gap. In this gap, the material is dispersed to the desired degree of fillers. Upon exiting, the material that remains on the center roller moves through the second nip between the center roller and apron roller, which subjects it to even higher shear force due to the higher speed of the apron roller. A knife blade then scrapes the processed material off the apron roller and transfers it to the apron This milling cycle can be repeated several times to maximize dispersion (Pötschke et al., 2013). Fe2O3 was evenly distributed through the grease structure. The oil leakage was observed visually. Digital camera images were taken by Sony-Alpha a57 Digital SLR Camera with 18–55mm Lens.
3Result and discussionHomogeneous and stable nanogrease have been made successfully by mixing SWNTs and MWNT respectively with polyalphaolefin oils (DURASYN® 166) without using chemical surfactant as reported in previous work (Hong et al., 2010).
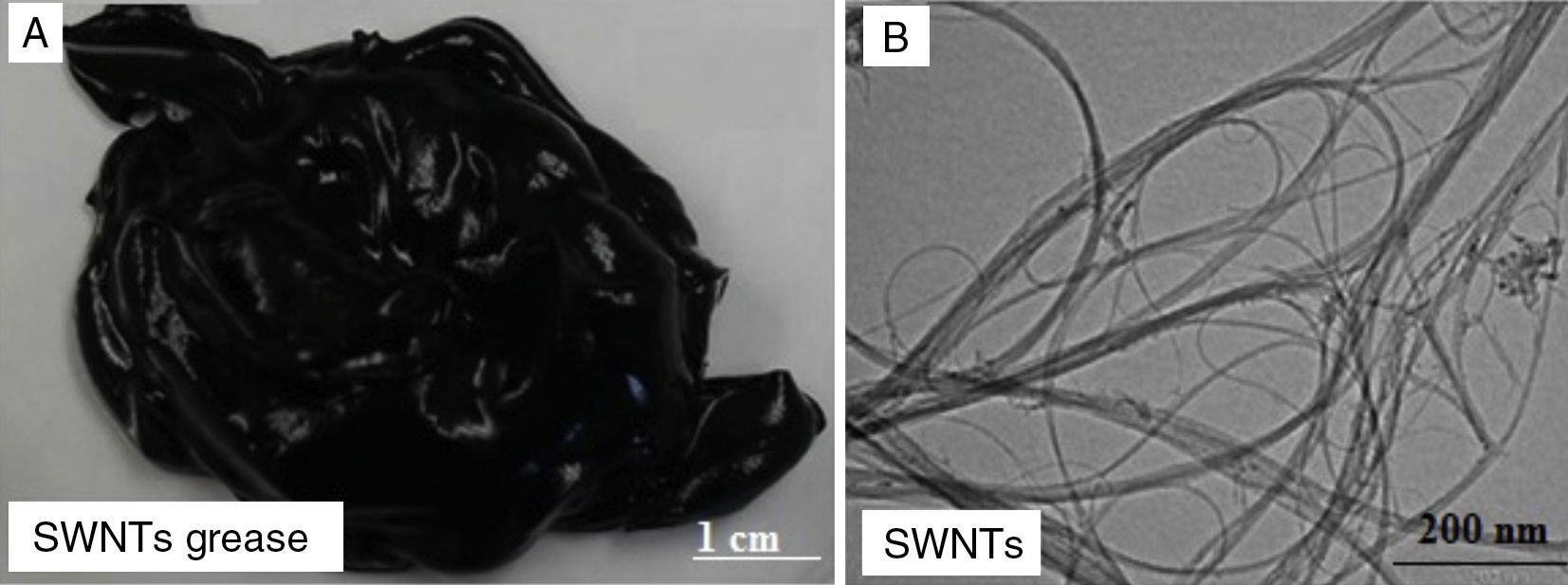
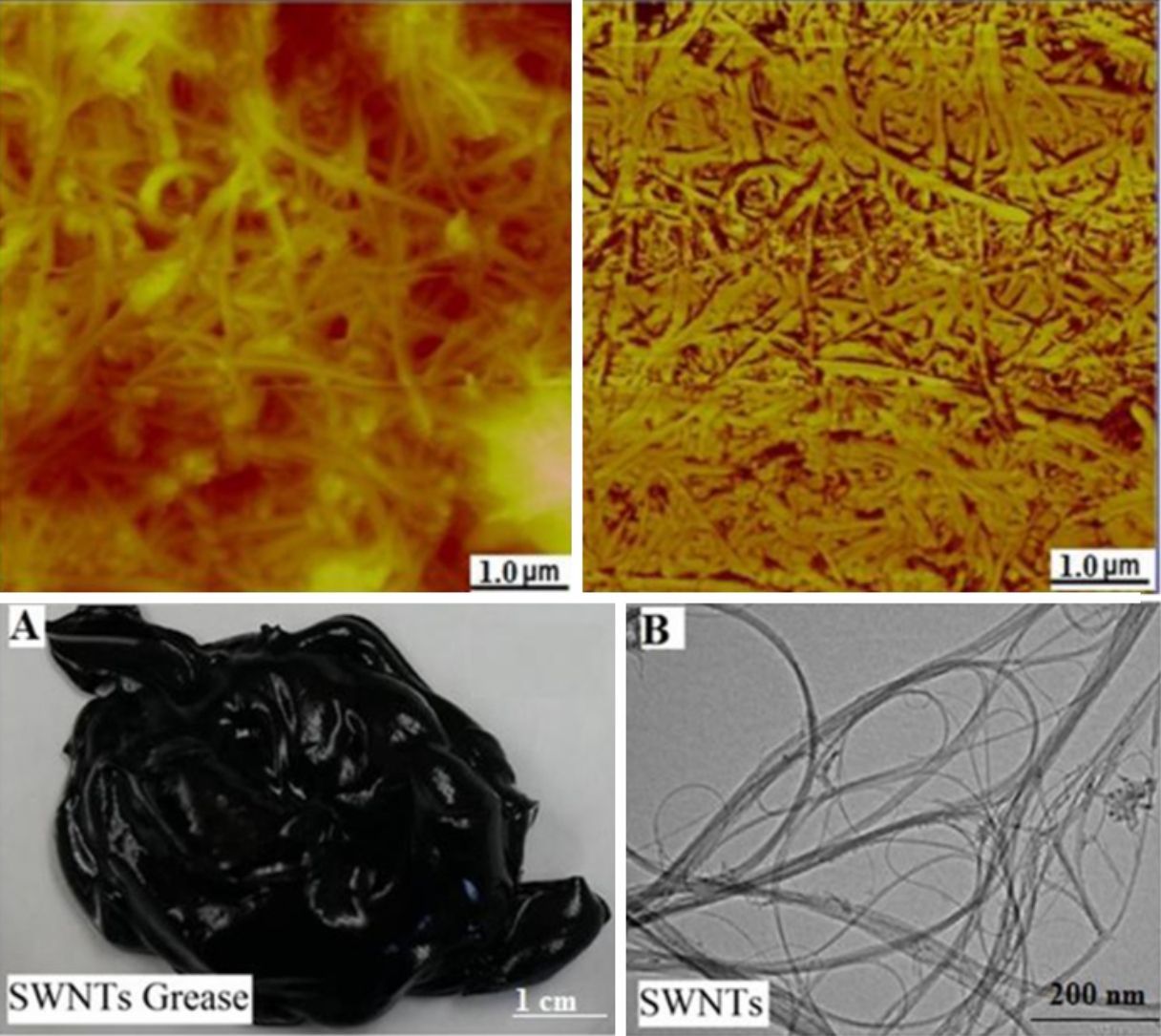
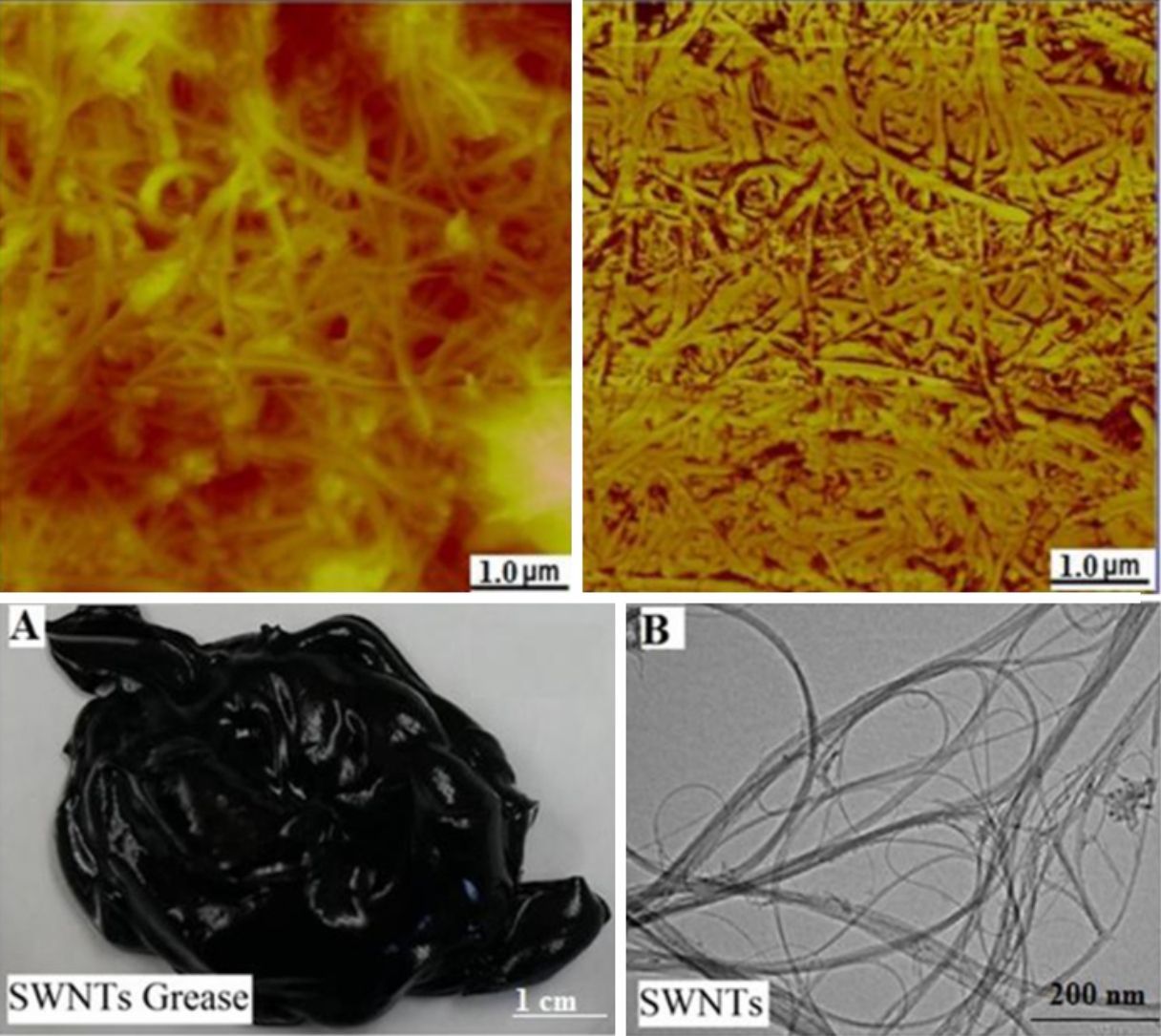
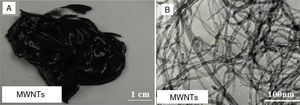
3.1Grease based on SWNTs and MWNTsFigure 2A shows a digital camera image for nanogrease that comprise 7.0wt% of SWNTs. The image shows that the grease is homogeneous and stable with no oil leaking observed from the image. Figure 2B shows a TEM image for SWNTs that were used to fabricate the stable grease, it can be seen in the image that SWNTs are grouped into ropes and they are tangled together into bunches. The nanotubes have good structure and length. The diameter of a single tube is around 1–2nm.
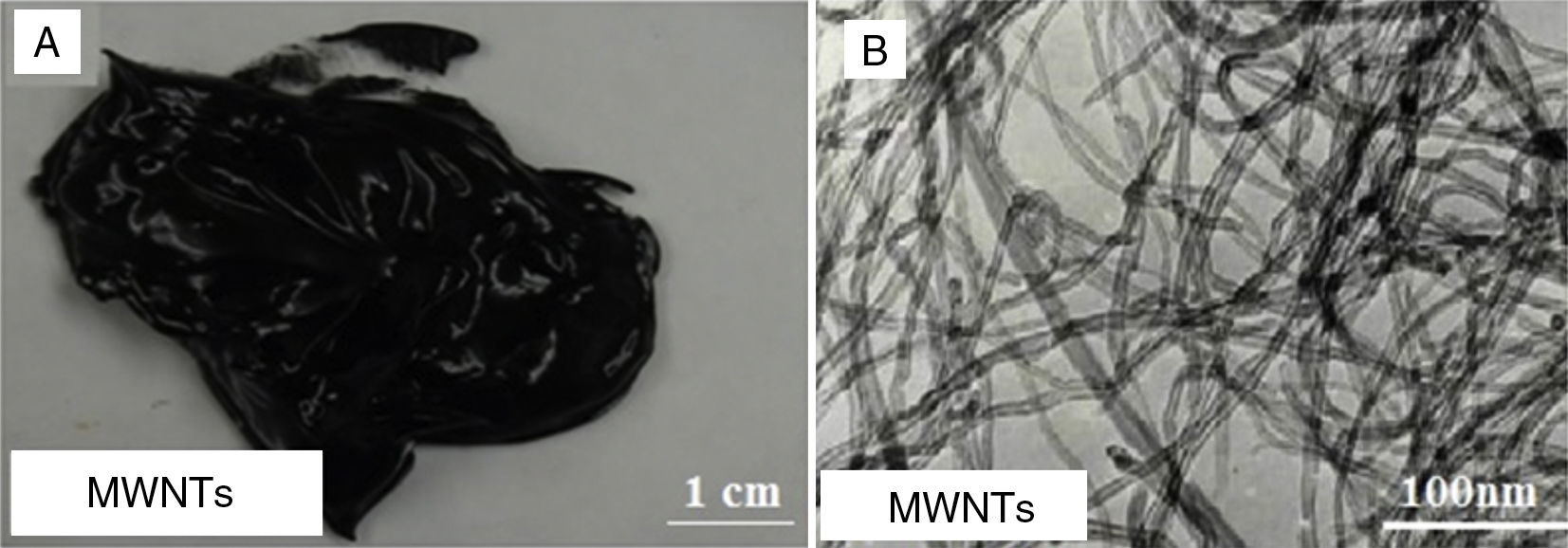
Figure 3A shows a digital camera image for nanogrease made of 20wt% of MWNTs. The MWNTs nanogrease was found to be very homogeneous and smooth without large chunks that are visible by naked eye. In addition, the grease was found to be stable and no oil leaking even after 48h. Figure 3B shows a TEM image for MWNTs, the image shows that the tubes are highly tangled into bunches of tubes with diameter ranges from 5 to 10nm.
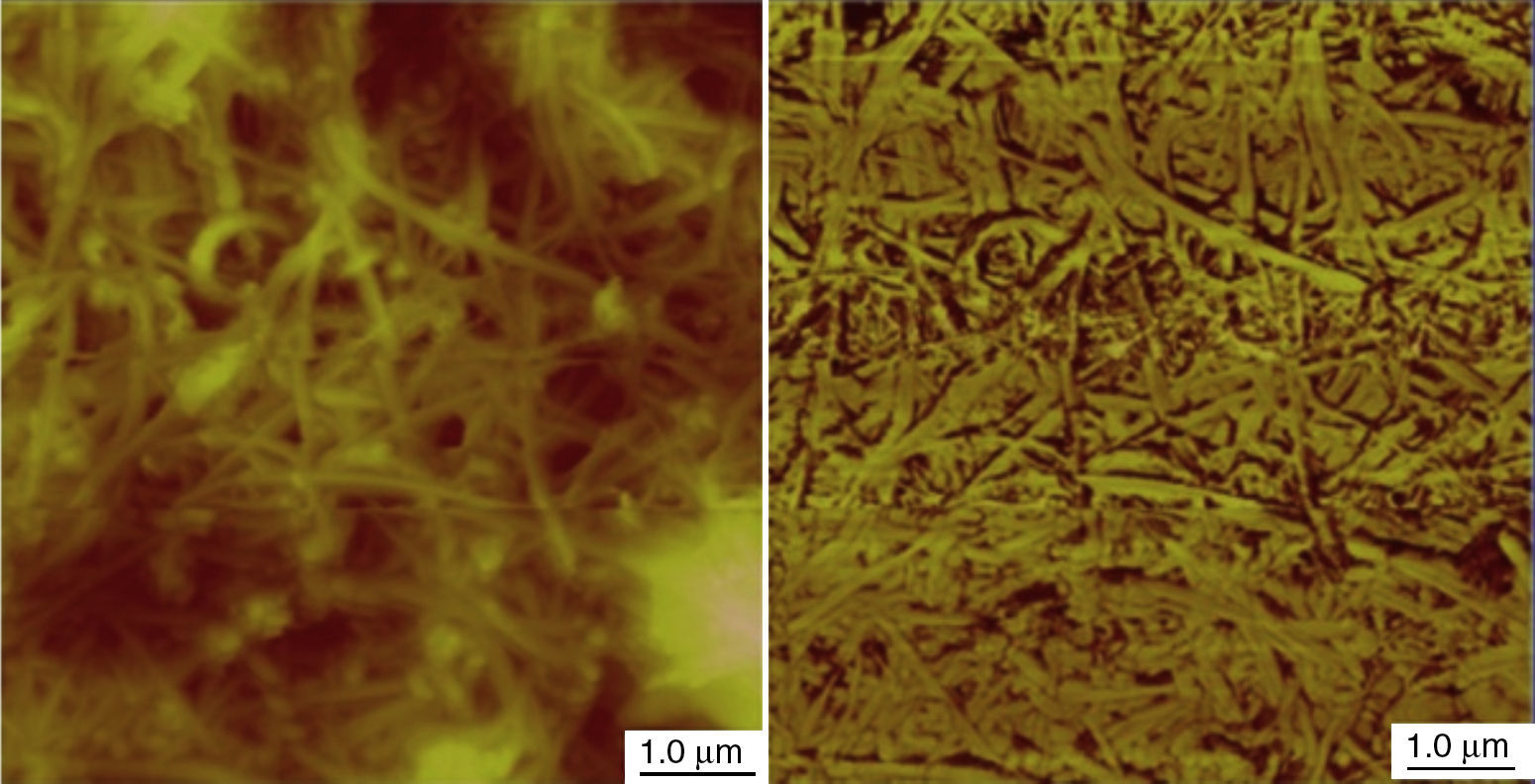
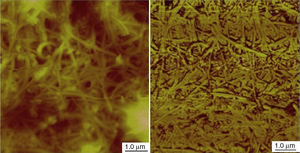
Using 7.0wt% of SWNTs was enough to make the stable and homogeneous nanogrease, whereas 20wt% of MWNTs were needed. This can be attributed to the Van der Waals forces which are very strong in nanostructures (Pötschke et al., 2013; Yang et al., 2012). Van der Waals forces in SWNTs are stronger than that in MWNTs, because of the very small diameter of 1–2nm in case of SWNTs as the TEM image (Fig. 2B) tells us. In contrast, MWNTs have larger diameters of 5–10nm as can be seen in the TEM image of Figure 3B, which makes Van der Waals forces in the case of MWNTs weaker than the Van der Waals forces in the case of SWNTs, because Van der Waals forces tend to increase with decrease of the tube diameter. Figure 4 shows an AFM images for SWNTs grease. It can be seen in the figure that SWNTs are randomly dispersed in the grease.
The AFM images show that the CTNs are interconnected with each others through a 3D network structures and this 3D network structure occurs either (1) tube–tube within the structure or (2) between neighbor bundles through their contacts. Therefore, the 3D network depends on the Van der Waals forces of the nanotubes themselves and the ability of creating strong attractive forces between adjacent nanotubes or adjacent bundles.
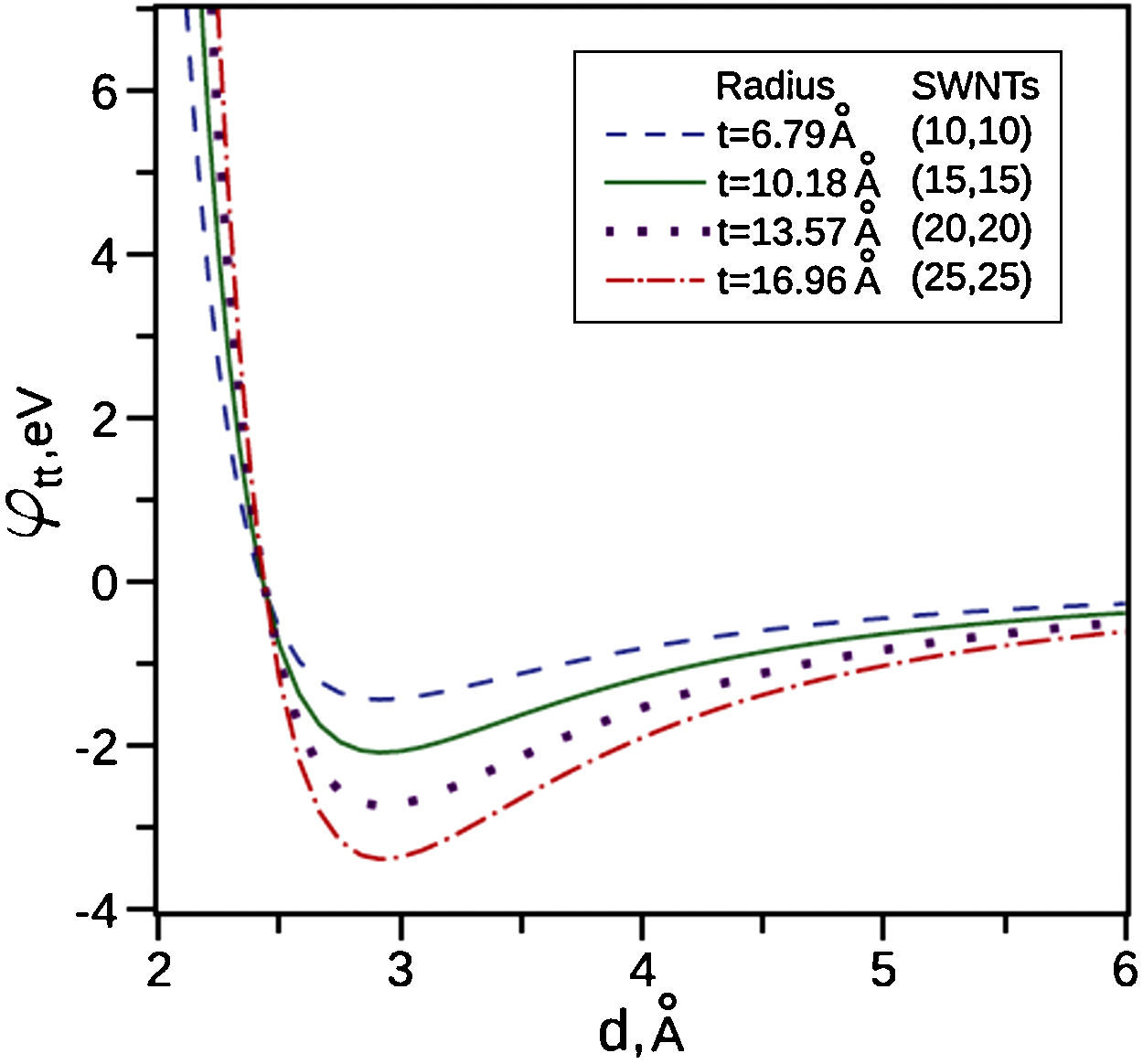
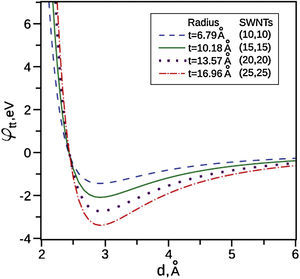
Zhbanov, Pogorelov, and Chang (2010) did an analytical integrations for the potential energy for the interaction between two identical SWNTs as can be seen in Figure 5. It is found that the real gap between surfaces of interacting SWNTs of different radii in an equilibrium state is changed only slightly in the range d0=2.92–2.93Å. After this range the potential energy decreases significantly. This means that Van der Waals forces are very strong for very small radii and Figure 5 depicts that when the tubes radii increases Van der Waals forces decreases significantly. The SWNTs that used in this study have a very small diameter as shown in the TEM images (Figs. 2B and 3B) and the AFM images (Fig. 5). It is clearly seen that SWNTs are grouped into ropes and they are tangled together into bunches. Therefore, the distance between the tubes is very small and less than 3nm. At this very small distance, Van der Waals forces are very strong, so carbon nanotubes become more associated with each other and also with the oil by Van der Waals forces. We hypothesize that this leads to formation of a 3D network structure, and as it stated above the 3D network structure could occur between tubes or bundles and reserve the oil inside the 3D network structure.
Potential energies for interaction between pairs of identical SWNTs, γ=μ/2.
In addition to SWNTs and MWNTs, graphene was mixed with the polyalphaolefin oil in an attempt to fabricate stable and homogeneous smooth grease similar to the one which was obtained by SWNTs and MWNTs.
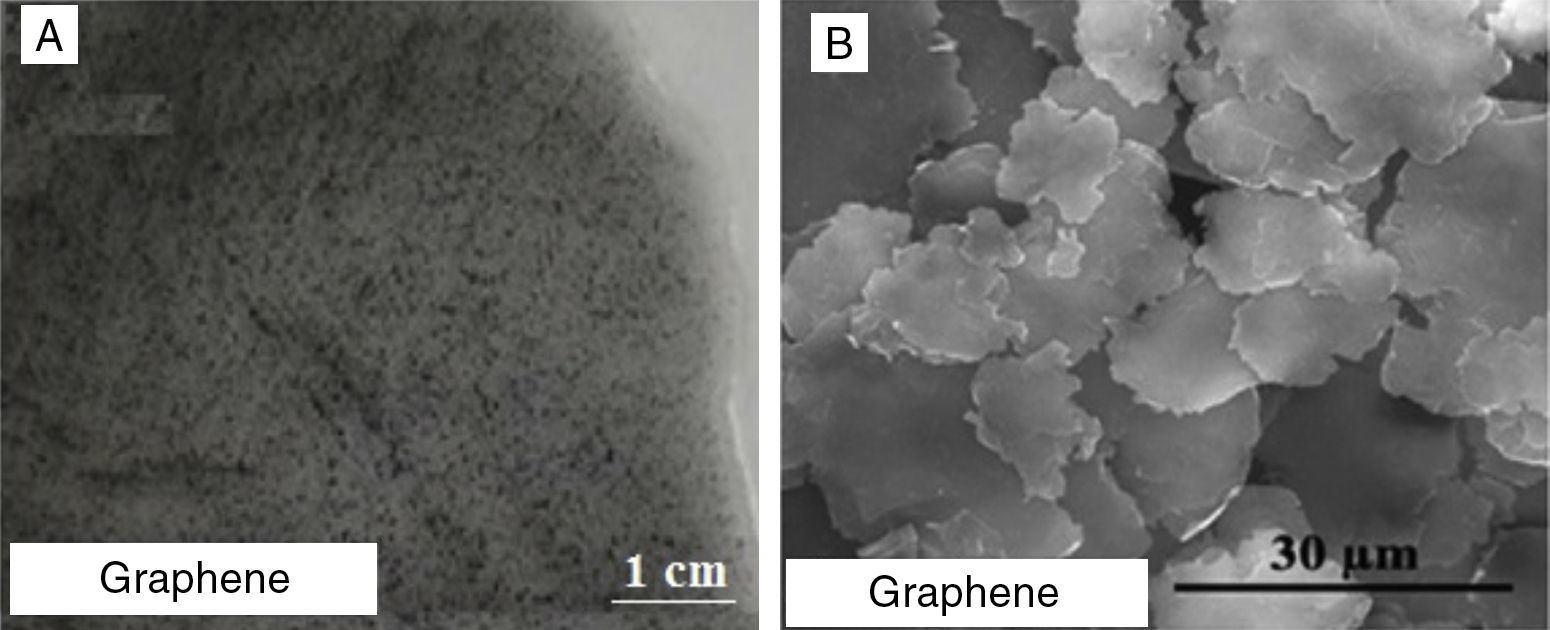
Figure 6A shows a digital camera image for grease made from 8wt% of graphene, but as it can be seen in the digital camera image the grease is not stable or homogeneous and lacked the same thickening ability as SWNTs grease. Oil leakage is clearly can be seen by the image. Figure 6B shows a SEM image for graphene that used to make the grease. The graphene that was used here is multilayer graphene that fabricated, according to the vendor, using mechanical exfoliation method. The attempt to fabricate stable, homogeneous, and smooth grease from 8wt% of graphene was unsuccessful, which can be attributed to the weak Van der Waals forces between graphene's flakes. Graphene is two dimensional multi sheets with an average flake thickness of 5–30nm and an average particle (lateral) size ∼5–25μm. Van der Waals forces are defined as the sum of the molecular interaction for all pairs of molecules composed of one molecule in each particle, as well as to all pairs of molecules with one molecule in a particle and one in the surrounding medium such as solvent (Cao, 2004).
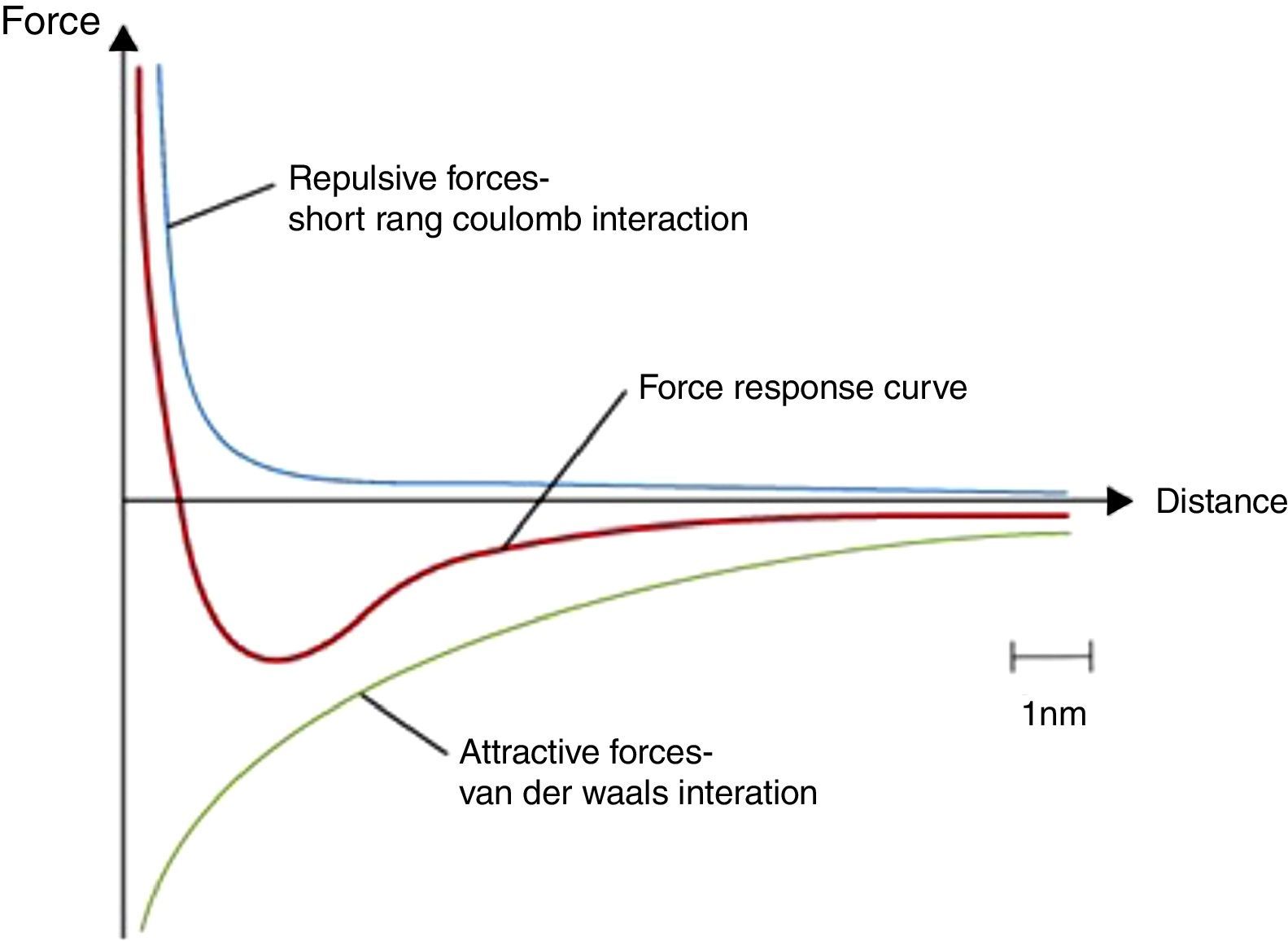
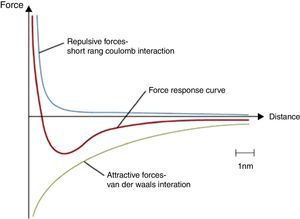
Figure 7 shows the curve for the total potential energy of a particle. The total potential energy of a particle is the sum of the repulsion energy and attractive (Van der Waals) forces. As it can be seen in Figure 7, at very small tube diameter and very short distance between tubes, Van der Waals forces become very strong and so many surfaces involved. This is the reason why it is very hard to disperse carbon nanotubes in solvents without using surfactants or ultra-high sonication power to break the attraction forces among the tubes. So using high loading of CNTs (>7wt%), carbon nanotubes become more associated with each other and with the oil by Van der Waals forces in the grease. We hypothesize that this leads to formation of a three dimensional network structure (Sun, Wang, & Kang, 2013). Currently available graphene consists of mulitple layers and also has micron x and y dimensions so Van der Waals forces decreases dramatically and it becomes very hard to form the three dimensional network structures necessary to prevent oil leakage, which explains why graphene is not a beneficial materials to formulate grease.
A general total potential energy curve (repulsion forces+attractive forces). © DoITPoMS, University of Cambridge.
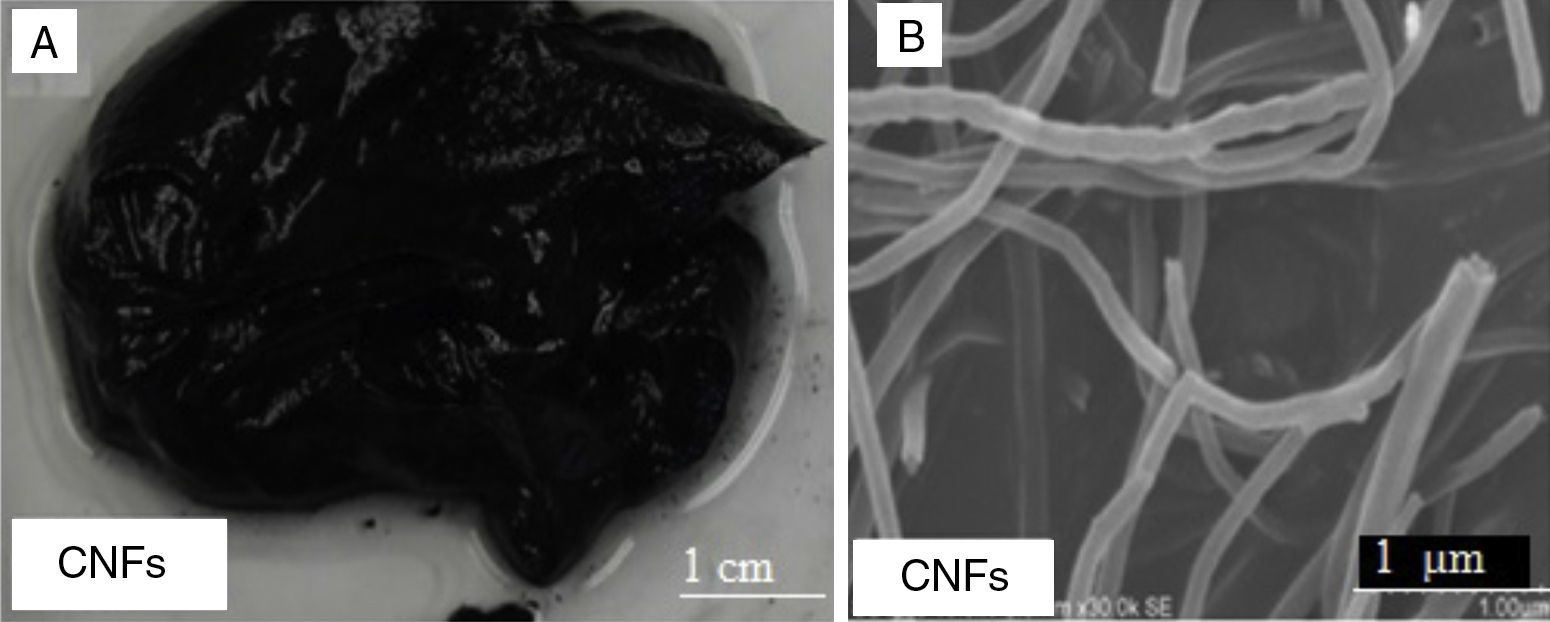
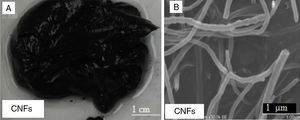
Figure 8A shows a digital camera image for nanogrease made of 7wt% of CNFs. The formed grease was found to be unstable. The oil leaked from the grease and as it can be seen clearly in the digital camera image. CNFs grease did not have the same thickening ability as the SWNTs grease or MWNTs grease. Figure 8B is a SEM image for CNFs that shows the fibers have good structure and length. The diameter of the carbon nanofibers is much greater than the diameter of SWNTs.
The instability of the 7wt% of CNFs can be attributed again for the weakness in the Van der Waals forces between the tubes themselves and not between the tubes and the oil, which leads to the inability to form the 3D network structures. CNFs have diameters over a hundred nanometers as seen in SEM images (Fig. 8B), which decreases Van der Waals forces among them; we hypothesize that this in turn prevents the formation of the 3D structures necessary to prevent oil leakage.
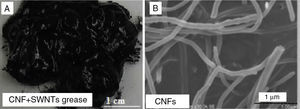
Figure 9A shows a digital camera image for nanogrease made of a mixture of SWNTs and CNFs. SWNTs were added to the CNFs grease in order to help in making stable and homogeneous grease. Nonetheless, as it can be seen in the digital camera image the formed grease is inhomogeneous and unstable. Oil leaking can be seen clearly in the digital camera image. The grease took virtually two days before the leakage occurred. Figure 9B is a SEM image for CNFs. SWNTs were mixed with CNFs grease in order to help in making stable and homogeneous grease. The formed grease was stable and homogeneous for two days, but after that, the formed grease seemed to be inhomogeneous, unstable and started to leak. Oil leaking can be seen clearly in the digital camera image (Fig. 9A). Obtaining a homogeneous mixture from SWNTs and CNFs is a very difficult process and we hypothesize this is possibly due to the strong Van der Waals forces between SWNTs, which make it hard to mix SWNTs with CNFs. The weak Van der Waals forces between CNFs due to the large diameter of CNFs shown in Figure 9B could make it also very hard to form the proposed three dimensional network structures consisting of the two materials.
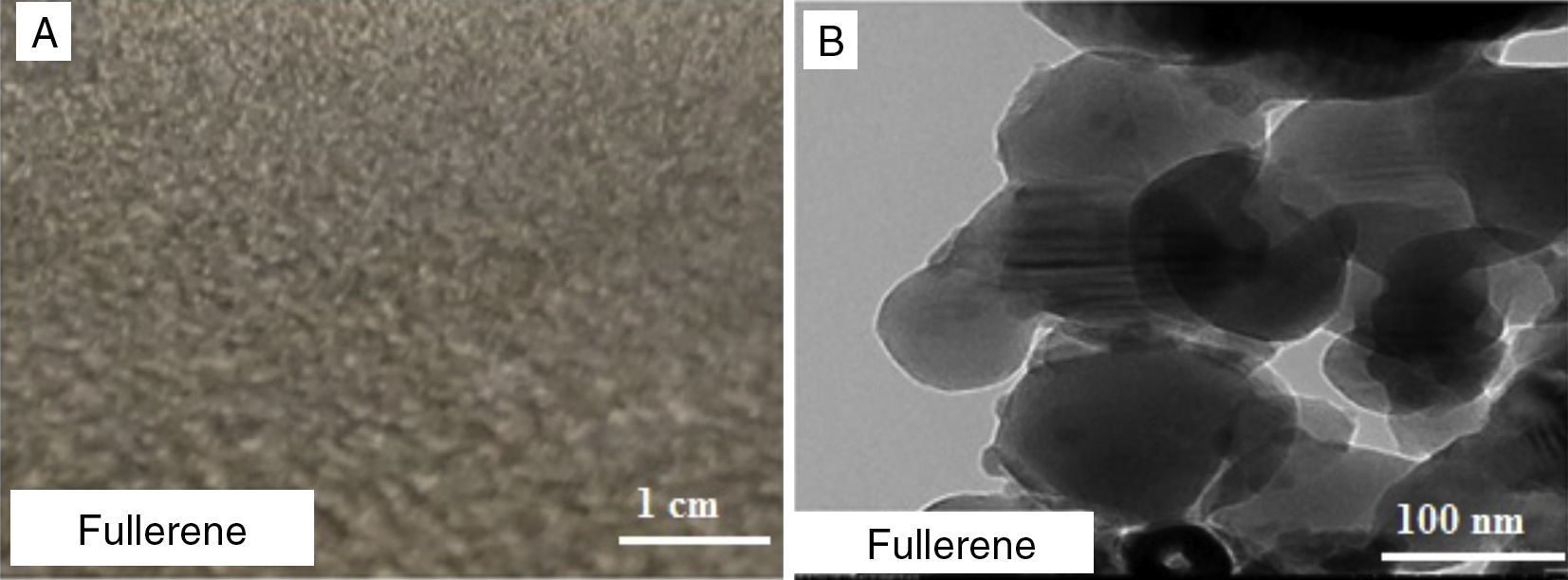
3.4Grease based on fullereneFigure 10A shows a digital camera image for grease made from 8wt% of fullerene, but as can be seen in the digital camera image the grease is not stable or homogeneous and did not have the same thickening ability as the SWNTs grease. The oil leakage can be seen clearly in the image. Figure 10B shows a TEM image for fullerenes; it can be seen that fullerenes have spherical structure with large diameter so the voids between the spherical particles will be large compared to SWNTs. In addition, although the edges of the spheres may still touch each other, but there are still larger voids and less surface interaction. As can be seen in Figure 5 with large distance between particles, Van der Waals forces are weak and as a result the 3D network could not be formed.
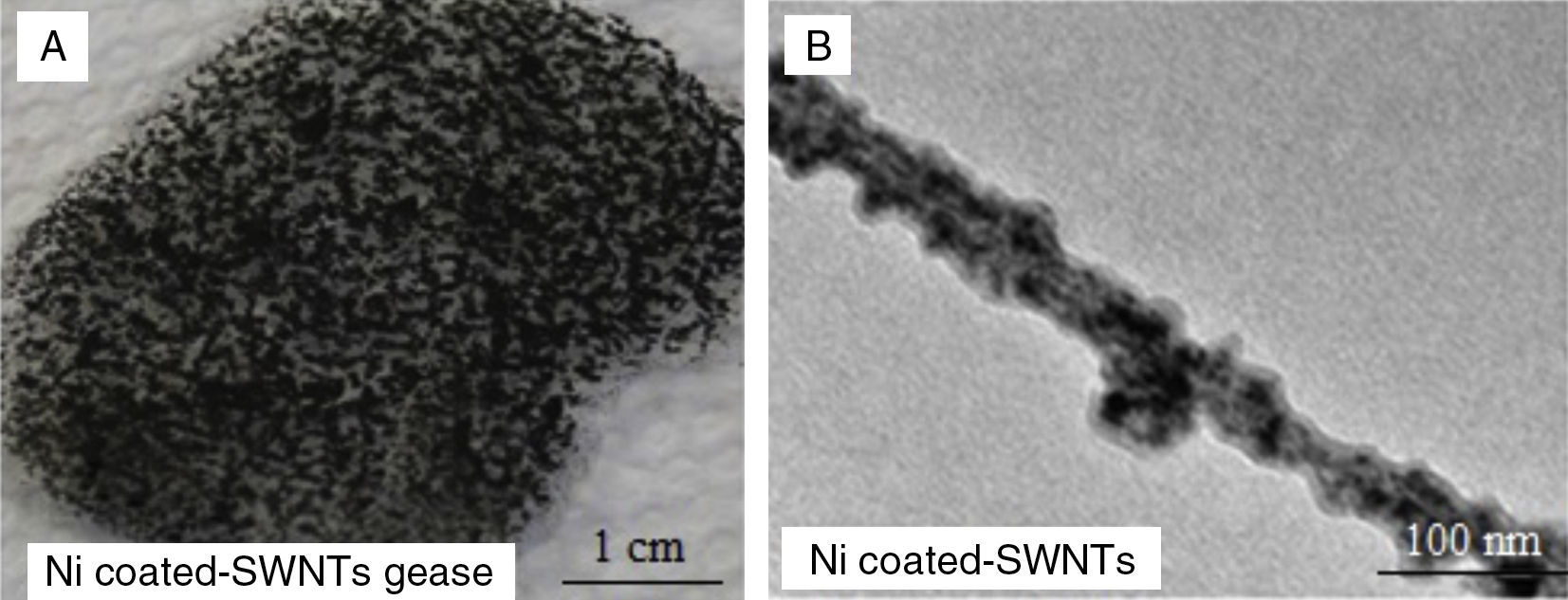
3.5Grease based on Ni-coated SWNTsFigure 11A shows a digital camera image for grease made from 8wt% of Ni-coated SWNTs, but as can be seen in the digital camera image the grease is not stable or homogeneous and did not have the same thickening ability as the SWNTs grease. The oil leakage can be seen clearly in the image. Figure 11B is a TEM image for one tube of Ni-coated SWNTs, and the image shows that the tube has a uniform coating of Ni.
9wt% Ni-coated SWNTs were added to Durasyn 166 in an attempt to produce stable and homogeneous nanogrease. It was found that Ni-coated SWNTs did not have the same thickening ability as the pristine SWNTs. Thus, the weight ratio of the Ni-coated nanotubes was increased in order to have grease with thickening ability such as the pristine SWNTs. The mixture was reheated and the total Ni-coated SWNT concentration was taken up to roughly 16.5wt%. No significant change in the thickness of the mixture was found. The total potential energy of a particle is defined as the sum of the repulsion and attractive (van der Waals) energies as can be seen in Figure 6. In Ni-coated SWNTs case, the presence of the Ni increases the repulsion forces because of increasing the surface energy; as a result, formation of the proposed 3D network structure is less likely. Higher surface energy does not allow the oil to effectively wet the surface. The oil could not wet the surface of Ni-coated nanotubes and the three dimensional network could not be formed.
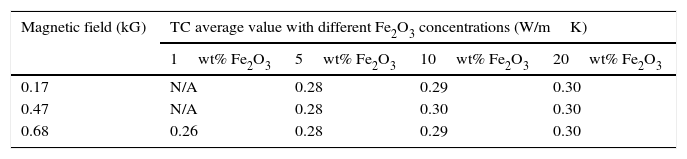
3.6The effect of using Fe2O3 nanoparticles and magnetic field on the thermal conductivity of the SWNTs greaseThe thermal conductivity of the SWNTs grease was measured and the effect of using different concentration of Fe2O3 nanoparticles on the thermal conductivity of SWNTs grease at different magnetic field strength was studied. Table 1 shows the thermal conductivity for 10wt% SWNTs versus time under different magnetic field strengths and different Fe2O3 loading concentrations of 1wt%, 5wt%, 10wt%, and 20wt%. It can be seen that there is no significant change on the thermal conductivity of the 10wt% SWNTs with using different loading concentrations of Fe2O3 nanoparticles and different magnetic field strengths. The formation of a three dimensional network due to van der Waals forces could explain the TC results shown in Table 1. The strong entanglement between CNTs with each other due to van der Waals forces hinders the movement of CNTs under an applied magnetic field and prevents alignment, which is observed in the case of nanofluids (Inoue, Gunjishima, & Okamoto, 2007). Even under the strong external magnetic field, there is no significant TC enhancement observed. Also the slight increased for the TC at 20wt% Fe2O3 is due to the higher concentration of Fe2O3 nanoparticles. It is known that Fe2O3 nanoparticles have a high TC value around hundreds of W/mK higher concentration of Fe2O3 increases the chances for particles to contact each other, hence the enhanced in TC is observed. The high viscosity of the SWNT grease is another possible reason for not observing a change of the thermal conductivity, since the high viscosity of the grease could impedes the movement of SWNTs.
Thermal conductivity values in different magnetic fields with 10wt% single wall nanotube and different weight percentages of Fe2O3 nanoparticles.
| Magnetic field (kG) | TC average value with different Fe2O3 concentrations (W/mK) | |||
|---|---|---|---|---|
| 1wt% Fe2O3 | 5wt% Fe2O3 | 10wt% Fe2O3 | 20wt% Fe2O3 | |
| 0.17 | N/A | 0.28 | 0.29 | 0.30 |
| 0.47 | N/A | 0.28 | 0.30 | 0.30 |
| 0.68 | 0.26 | 0.28 | 0.29 | 0.30 |
In recent times, only 1.9wt% nanotube was required to make the stable grease, compared to previous usage of 7–11wt%. The dropping point is >650 (F). They are normal grease with penetration 288 (265–295 is regarded as normal grease by NLGI classification) (Waynick, 2013). It will be very interesting to investigate why different carbon nanotubes would make the significant differences in grease performance!
4ConclusionGreases were made by mixing DuraSyn 166 oil with SWNT, MWNT, CNF, graphene, Ni-coated SWNT, Ni-coated SWNT/SWNT mixture and CNF/SWNT. Only SWNT, MWNT and Ni-coated SWNT/SWNT mixtures could form stable and homogeneous greases. The 3D network structure is believed to be the main reason for the stable and homogeneous grease. Some oil leakage was observed in CNF grease, probably because of the weak Van der Waals forces between CNF that has big diameter (um level) compared to nano level diameter of SWNT and MWNT. In addition, it has less surface area as well and the surface area per gram of carbon is less, therefore less oil is required to coat the surface. The structure is disrupted and the voids are larger, but the internal forces cannot hold sufficient oil to fill these large voids. The two-dimensional and multiple sheet structure of graphene make Van Der Waals force too weak to form 3D network structures, and thus making stable grease difficult as well. The grease made of CNFs/SWNTs mixture was also unstable, possibly due to voids in the grease between CNFs that could not be filled with SWNTs. In addition, SWNTs were not mixed thoroughly with CNFs due to the strong Van Der Waals forces between SWNTs. The Ni-coated SWNT based greases were not stable at any concentration because of the repulsion forces between Ni on the surface of SWNTs, whereas mixing Ni-coated SWNTs with SWNTs at a ratio of 3 to 1 obtained stable grease. The TC of SWNTs based greases with different load of Fe2O3 nanoparticles did not change under an applied magnetic field as it is observed before with nanofluids. It is assumed that no change was noticed due to the pull of the magnetic field being weaker than the strength of the 3-D network structures. The experimental results demonstrate that three dimensional (3D) percolation network structures is the key to form stable carbon nanogrease. Since carbon nanogrease has many promising properties such as enhanced thermal and electrical conductivities and high temperature resistance, the present study opens the way to better understand the nature of this novel grease based on the nano scale.
Conflict of interestThe authors have no conflicts of interest to declare.
The financial support of Army Research Lab (Cooperative agreement W911NF-08-2-0022) and NASA EPSCoR (award No. NNX09AU83A) are acknowledged. Thanks to Dr. Edward Duke for assistance with the optical microscope and thanks for Dustin Thomas for the thermal conductivity measurements.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.