Antimicrobial stewardship programmes (AMSP) seldom focus on ambulatory prescribing. Our AMSP primarily supervises in-hospital prescribing, but as we aim to include the ambulatory setting, we sought opportunities for intervention on ambulatory quinolone prescription.
Materials and methodsWe selected the prescriptions made by urologists during 2018 for analysis, and manually checked them for adequacy.
ResultsWe analyzed 237 prescriptions. Of 136 therapeutic prescriptions, 18.4% had no reported diagnosis and 31.6% had no reported symptoms. Most patients (60.3%) did not have any urinalysis or urine culture; among those who had, 27.7% had a urinalysis not suggestive of urinary tract infection and 67.4% had a positive culture, 83.9% of which had a suitable oral alternative to quinolones. Antimicrobial therapy was not indicated in 13.9% of cases; when it was, quinolones were considered inadequate in 67.8% of cases. Incorrect duration was found in 51.1% of cases. Forty-six prescriptions were made for prophylaxis; all of these were considered inadequate.
ConclusionWe found a high prevalence of inadequate ambulatory quinolone prescriptions in Urology. Many followed incomplete recordings, lack of laboratory use, or inattention to alternatives. Treatment duration and quinolone choice were frequently inadequate. Quinolone prescribing for prophylaxis was always considered inadequate. These prescribing errors could serve as a starting point for future interventions.
En la actualidad, uno de los objetivos de los programas de Antimicrobial stewardship es la optimización de antimicrobianos en el ámbito ambulatorio. En nuestro centro, el grupo responsable de Antimicrobial stewardship se centra, sobre todo, en el ámbito de hospitalización, y por ello quisimos ampliar nuestro radio de acción y centrarnos en las oportunidades de intervención sobre las quinolonas en las consultas ambulatorias.
Material y métodosFueron seleccionadas y analizadas todas las prescripciones de quinolonas hechas por urólogos en consulta durante el año 2018, clasificándolas según su adecuación.
ResultadosDoscientas treinta y siete prescripciones fueron analizadas. De ellas, 136 tuvieron intención terapéutica, no encontrándose descrito diagnostico en la historia clínica en el 18,4% de los casos. En el 31,6% de los casos no se registraron síntomas. En la mayoría de los pacientes (60,3%) no se hizo urocultivo. Entre aquellos en los que se realizaron, un 27,7% tuvo un resultado no sugestivo de infección del tracto urinario y un 67,4% tuvo resultado positivo. El 83,9% de la muestra cumplía criterios para realizar una alternativa oral. La antibioterapia no estaba indicada en el 13,9% de los casos; y cuando había indicación la elección de quinolonas fue considerada inadecuada en el 67,8% de los casos. En el 51,1% la duración no fue correcta. En 46 casos fueron prescritas quinolonas como profilaxis, considerándose inadecuadas en todos estos casos.
ConclusionesEncontramos una elevada prevalencia de prescripciones inadecuadas de quinolonas en las consultas de Urología, así como registros de historia clínica incompletos, falta de pruebas analíticas, y falta de consideración de alternativas terapéuticas. La duración del tratamiento y la selección dentro del grupo fueron mayoritariamente inadecuadas. Todas las prescripciones realizadas para profilaxis empírica fueron consideradas inadecuadas. Este análisis servirá de punto de partida para implementar acciones en el futuro.
With the advent of multidrug-resistant microorganisms and, more recently, extensively and pan-resistant microorganisms, the development of Antimicrobial Stewardship Programmes (AMSP) has been promoted around the world, supported by international recommendations that aim to reduce the rate of emergence of antimicrobial resistance. These programmes have shown efficacy in lowering inappropriate antimicrobial prescribing, consequently reducing resistance rates, adverse events (including Clostrioides difficile infection) and length of stay, without an increase in mortality.1,2
Most AMSPs focus on inpatient antimicrobial prescribing, and particularly on priority drug classes such as quinolones and carbapenems. Although comparatively less explored, outpatient antimicrobial prescribing is an area with potential for intervention as well,3,4 especially if one considers that most antimicrobial prescribing occurs in ambulatory settings and that a significant proportion of these prescriptions is potentially inappropriate.4,5 Quinolones, which can be administered orally and have a broad spectrum of activity (in some cases including Pseudomonas aeruginosa), remain a tempting drug class for outpatient prescribing, particularly in cases of upper respiratory and urinary tract infections,6,7 even though their prescription often be inappropriate.8,9 Quinolone use not only correlates with a rapid development of resistance to all drugs in this class6,7,10 but, epidemiologically more relevant, it also carries an increased risk of colonization and infection with methicillin-resistant Staphylococcusaureus10–12 and broad-spectrum beta-lactamase-producing Enterobacterales.13–15 As such, prescription of quinolones must be cautious, so as to minimize the risks associated with their use, which in the long term could severely compromise the range of available therapeutic options. In consequence, quinolones have come to be excluded as first-line drugs for most infectious syndromes generally among local and international guidelines.6,7,9,16
In our hospital, we have established an AMSP in 2017, aiming to survey antimicrobial prescription and consumption; for their association with the emergence of multidrug-resistant microorganisms, quinolone and carbapenem prescriptions have been the most targeted by this programme. As it happens in other hospitals, our AMSP focuses mainly on inpatient prescribing, although there are occasional interventions on outpatient prescriptions. The implementation of a programme for surveying outpatient antimicrobial prescribing, however, has been planned in order to generate a more systematic intervention on outpatient quinolone prescribing.
Our aim was to identify outpatient AMSP opportunities for intervention in quinolone therapy.
Material and methodsPatient selectionWe retrospectively analyzed quinolone prescriptions in outpatient episodes (consultation, day hospital and emergency department) in a hospital in Lisbon, Portugal, between January and December 2018. After counting all prescriptions by specialty, we focused on urologist prescriptions, specialty with the greatest number, for further analysis.
Collected dataThe electronic medical record was reviewed for data collection. Only the investigating team had access to the database, made in Microsoft® Excel®. The only identification for patients was their in-hospital medical record number. Each prescription was analyzed for drug, dose, dosage, and duration. When unavailable, dosage was considered as the standard and duration was considered as the maximal allowed by the prescription.
Medical records were reviewed with the objective of identifying a clinical diagnosis and associated symptoms. The symptoms were grouped according to the International Classification of Diseases 10 (ICD-10). When reviewing prescriptions of prophylactic antimicrobial therapy, the indication was obtained according to the medical record. The diagnosis that led to a therapeutic or prophylactic prescription was made available to the investigating team from a drop-down list. Clinical diagnosis was recorded only when it could be clearly inferred.
Laboratory data included inflammatory markers (white blood cell count, C-reactive protein), leukocyturia and nitrituria according to the urinalysis, and urine culture. The result of the urine culture was recorded as “negative,” “contaminated,” or, if positive, as the name of the etiologic agent. The antimicrobial susceptibility test results were grouped as sensitive or resistant; intermediate results were recorded as sensitive. The antimicrobials were grouped according to the ATC classification (2020 version) developed by the World Health Organization (WHO).17
In accordance to the clinical and laboratorial data, antimicrobial therapy was defined as empirical, directed, or prophylactic.
Other data were recorded when considered relevant by the investigation team.
Adequacy of prescriptionAntimicrobial therapy prescriptions were checked for adequacy according to our internal protocol, as well as the national and international guidelines for urinary tract infection treatment.18–22 Our internal protocol for empirical antimicrobial therapy provides recommendations for adequate antibiotic and treatment duration. It was created following our local resistance patterns and national and international guidelines. Another important tool for this assessment is the WHO AWaRe Classification,23 that divides antimicrobial drugs across three groups – Access, Watch, and Reserve – in order to emphasize the importance of their utilization in the right circumstances and their potential to generate antimicrobial resistance.
Antimicrobial prophylaxis prescriptions were checked for adequacy according to our internal protocol. This protocol provides exact recommendations about the drug, dose, timing of administration, and duration, if more than one dose is eventually indicated.
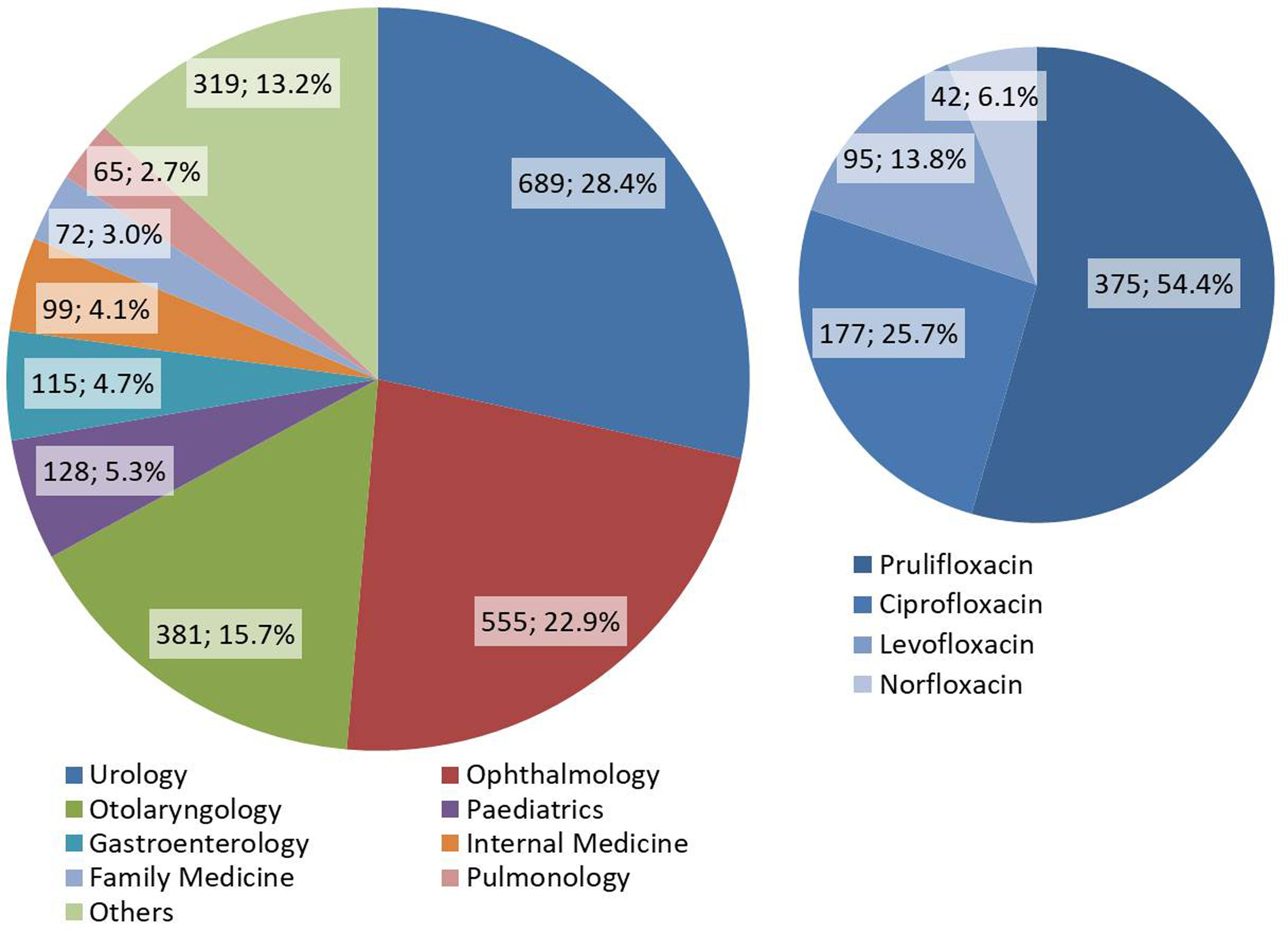
ResultsCounting of quinolone prescriptionsWe identified a total 2423 quinolone prescriptions made during the year 2018. Of these, 689 were made by urologists. The distribution of all prescriptions made by specialty, as well as the distribution of all quinolone antibiotics prescribed in Urology, is shown in Fig. 1. Of note, prulifloxacin was prescribed in more than half of cases (54.4%). We analyzed in detail a total 237 quinolone prescriptions in Urology.
Symptoms and diagnoses reported for antimicrobial therapyOf the 237 analyzed prescriptions, 136 were made for therapy (Table 1), most of which empirical therapy (79.4%). The most frequently presumed diagnosis was acute prostatitis (20.6%). In 38 cases (27.9%) a diagnosis of unspecified urinary tract infection was recorded; we could not presume a definite diagnosis in these cases. In 25 cases (18.4%) the diagnosis was not reported, although the patient was symptomatic.
Presumed diagnoses for antimicrobial therapy and proportion of cases with and without symptoms.
| Number | Percentage | |
|---|---|---|
| Proportion of cases with and without symptoms | ||
| Cases with symptoms | 88 | 64.7 |
| Asymptomatic | 5 | 3.7 |
| Not reported | 43 | 31.6 |
| Diagnosis | ||
| Renal abscess | 2 | 1.5 |
| Asymptomatic bacteriuria | 3 | 2.2 |
| Balanitis | 3 | 2.2 |
| Cystitis | 5 | 3.7 |
| Epididymo-orchitis | 14 | 10.3 |
| Unspecified urinary tract infection | 38 | 27.9 |
| Repeat urinary tract infections | 8 | 5.9 |
| Pyelonephritis | 1 | 0.7 |
| Acute prostatitis | 28 | 20.6 |
| Chronic prostatitis | 4 | 2.9 |
| Not reported | 25 | 18.4 |
| Other | 5 | 3.7 |
| Type of treatment | ||
| Empirical antimicrobial therapy | 108 | 79.4 |
| Directed antimicrobial therapy | 23 | 16.9 |
| Empirical and directed antimicrobial therapy | 5 | 3.7 |
| Total | 136 | 100 |
Among these patients, 88 (64.7%) had symptoms described in the medical record. Antimicrobial therapy was prescribed to 5 patients described as asymptomatic, and, in 43 patients (31.6%), symptoms (or their absence) were not recorded at all.
Of the remaining prescriptions, 55 were made without any clear motive.
Indications for antimicrobial prophylaxisOf the 237 analyzed prescriptions, 46 were made for prophylaxis. Symptoms were not considered in this case. The most common indication for antimicrobial prophylaxis with quinolones was prostate biopsy (32 cases), followed by cystoscopy (8 cases).
Laboratory resultsThe laboratory results are shown in Table 2. In all groups of patients (antimicrobial therapy, antimicrobial prophylaxis, no clear indication) only in a minority (27.8%) laboratory tests were run. This was more frequent when antimicrobial therapy was prescribed (39.7%). A significant proportion of patients had a urinalysis not suggestive of urinary tract infection (27.7% of tested samples), as well as a negative or contaminated urine culture (32.6% of tested samples).
Results of laboratory testing.
| Antimicrobial therapy (n=136) | Antimicrobial prophylaxis (n=46) | Antimicrobials with unspecified motive (n=55) | |
|---|---|---|---|
| Inflammatory markers | 31 (22.8%) | 5 (10.9%) | 5 (9.1%) |
| High inflammatory markers | 15 (48.4%) | 0 (0.0%) | 0 (0.0%) |
| Urinalysis | 47 (34.6%) | 4 (8.7%) | 5 (9.1%) |
| Leukocyturia | 34 (72.3%) | 2 (50.0%) | 1 (20.0%) |
| Nitrituria | 9 (19.1%) | 1 (25.0%) | 0 (0.0%) |
| Urine culture | 46 (33.8%) | 3 (6.5%) | 3 (5.5%) |
| Positive | 31 (67.4%) | 1 (33.3%) | 0 (0.0%) |
Among patients with positive urine cultures that were given antimicrobial therapy (Table 3), only 77.4% had reported sensitivity results for quinolones (in none sample resistance was reported), and 71.0% had sensitivity to oral antimicrobials in the Access group of the WHO AWaRe classification.23 Sensitivity to oral antimicrobials in the Access group was similar among samples with reported sensitivity to quinolones and the whole of samples with positive urine cultures. When also considering the oral antimicrobials in the Watch group of the same classification, the proportion of sensitive microorganisms was 83.9% in the whole of samples with positive urine culture and 83.3% among the samples with reported sensitivity to quinolones.
Analysis of the susceptibility profiles in samples with positive urine culture.
| Antimicrobial therapy (n=31) | Antimicrobial prophylaxis (n=1) | |
|---|---|---|
| Sensitive to quinolones | 24 (77.4%) | 1 (100.0%) |
| Sensitive to oral agents of the Access group (AWaRe) | 17 (70.8%) | 1 (100.0%) |
| Sensitive to oral agents of the Access/Watch groupa (AWaRe) | 20 (83.3%) | 1 (100.0%) |
| Resistant to quinolones | 0 (0.0%) | 0 (0.0%) |
| Sensitivity to quinolones not reported | 7 (22.6%) | 0 (0.0%) |
| Sensitive to oral agents of the Access group (AWaRe) | 6 (85.7%)* | NA |
| Sensitive to oral agents of the Access/Watch groupb (AWaRe) | 6 (85.7%)* | NA |
| Total | ||
| Sensitive to oral agents of the Access group (AWaRe) | 22 (71.0%) | 1 (100.0%) |
| Sensitive to oral agents of the Access/Watch groupa (AWaRe) | 26 (83.9%) | 1 (100.0%) |
NA, not applicable.
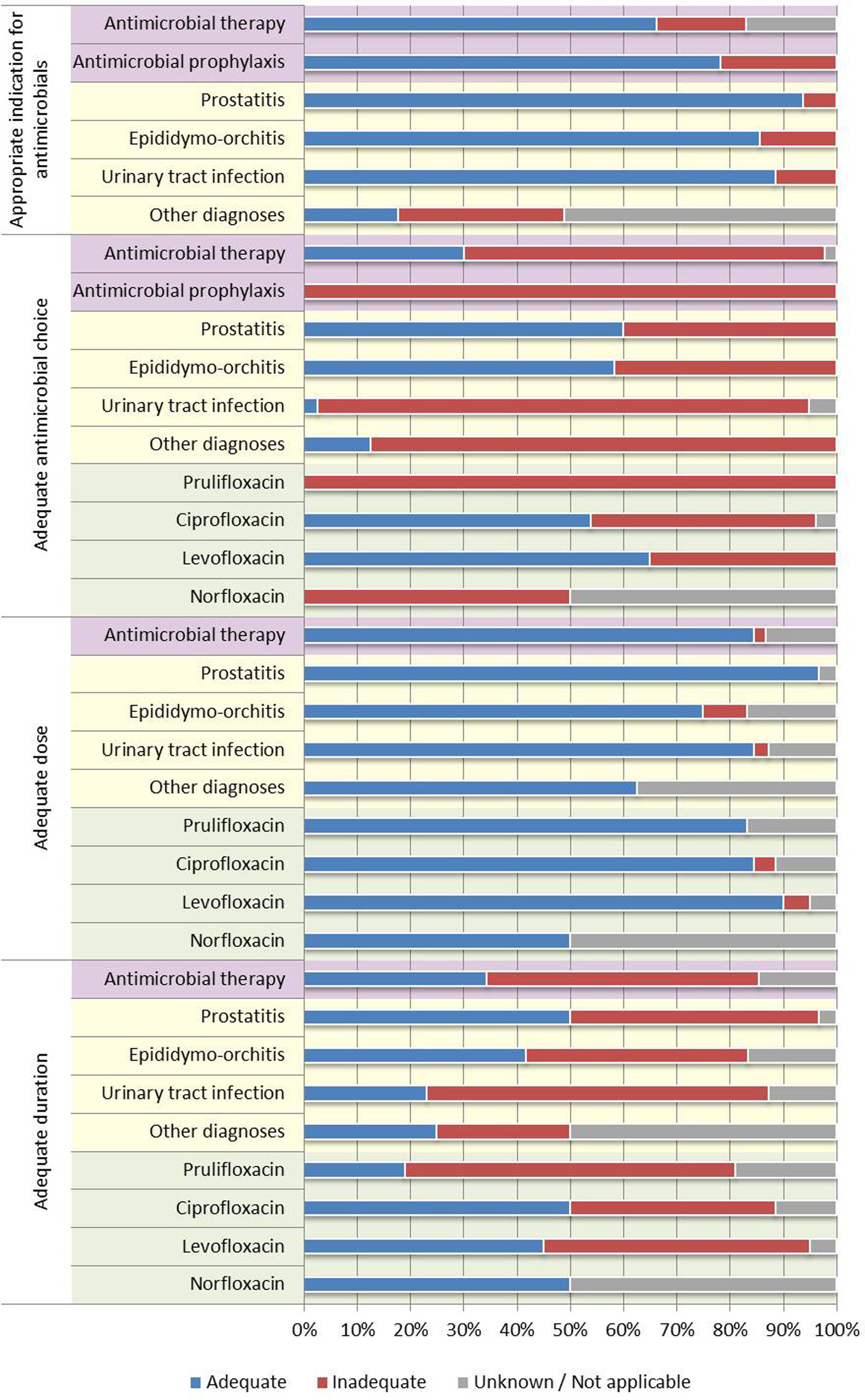
In most analyzed prescriptions (53.2%), we found a clear and correct motive for antimicrobial prescribing. It is remarkable, however, that in 13.9% of cases antimicrobials were not indicated. In the remaining cases (32.9%), we did not find a clear motive for antimicrobial prescribing, so we could not assess with certainty whether it was indicated or not.
Among the cases with indication for antimicrobials, quinolones were considered inadequate in 77.0% of cases. None of the prescriptions for antimicrobial prophylaxis were considered adequate. The duration of therapy was considered adequate in only 34.4% of cases with indication for treatment (we found a trend towards higher treatment duration than recommended in urinary tract infections and epididymo-orchitis and lower than recommended in prostatitis). The dosing, in most cases, was considered adequate.
Therapy with quinolones was considered adequate in most cases of prostatitis (60.0%) and epididimo-orchitis (58.3%) that had indication for antimicrobial therapy. On the other hand, among urinary tract infections (cystitis, pyelonephritis, others not specified), 92.3% quinolone prescriptions in patients with indication for treatment were considered inadequate.
In the case of prescriptions by drug, prulifloxacin was disproportionally prescribed when compared to other quinolones, and was considered inadequate in all. It is of importance that the prescription of prulifloxacin happened several times in cases where another quinolone would have been an adequate option. The remaining quinolones (ciprofloxacin, levofloxacin, norfloxacin) were considered adequate in no more than 50% of cases.
Fig. 2 and Table 4 summarize the adequacy of prescribed antimicrobials regarding these variables.
Adequacy of quinolone prescribing.
| Antimicrobial therapy (n=136) | Antimicrobial prophylaxis (n=46) | Antimicrobials with unspecified motive (n=55) | Prostatitis (n=32) | Epididymo-orchitis (n=14) | Urinary tract infection (n=44) | Other diagnoses (n=45) | |
|---|---|---|---|---|---|---|---|
| Appropriate indication for antimicrobials | |||||||
| Yes | 90 (66.2%) | 36 (78.3%) | 0 (0.0%) | 30 (93.8%) | 12 (85.7%) | 39 (88.6%) | 8 (17.8%) |
| No | 23 (16.9%) | 10 (21.7%) | 0 (0.0%) | 2 (6.2%) | 2 (14.3%) | 5 (11.4%) | 14 (31.1%) |
| Unknown | 23 (16.9%) | 0 (0.0%) | 55 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 23 (51.1%) |
| Adequate antibiotic choice | |||||||
| Yes | 27 (30.0%) | 0 (0.0%) | NA | 18 (60.0%) | 7 (58.3%) | 1 (2.6%) | 1 (12.5%) |
| No | 61 (67.8%) | 36 (100.0%) | NA | 12 (40.0%) | 5 (41.7%) | 36 (92.3%) | 7 (87.5%) |
| Unknown | 2 (2.2%) | 0 (0.0%) | NA | 0 (0.0%) | 0 (0.0%) | 2 (5.1%) | 0 (0.0%) |
| Adequate dose | |||||||
| Yes | 76 (84.5%) | NA | NA | 29 (96.7%) | 9 (75.0%) | 33 (84.6%) | 5 (62.5%) |
| No | 2 (2.2%) | NA | NA | 0 (0.0%) | 1 (8.3%) | 1 (2.6%) | 0 (0.0%) |
| Unknown/not applicable | 12 (13.3%) | NA | NA | 1 (3.3%) | 2 (16.7%) | 5 (12.8%) | 3 (37.5%) |
| Adequate duration | |||||||
| Yes | 31 (34.4%) | NA | NA | 15 (50.0%) | 5 (41.7%) | 9 (23.1%) | 2 (25.0%) |
| No | 46 (51.1%) | NA | NA | 14 (46.7%) | 5 (41.7%) | 25 (64.1%) | 2 (25.0%) |
| Unknown/not applicable | 13 (14.5%) | NA | NA | 1 (3.3%) | 2 (16.6%) | 5 (12.8%) | 4 (50.0%) |
NA, not applicable.
In this study we found a high rate of inadequate prescribing of quinolones. Many of these prescriptions were accompanied by incomplete recordings, lack of laboratory testing, and existence of alternative antimicrobials with lesser potential for selective pressure. Quinolone prescribing in patients with a diagnosis of prostatitis or epididymo-orchitis was considered adequate in most cases, but the duration of treatment was inadequate in more than half of cases.
After our AMSP was implemented, in 2017, we have been observing a gradual decline in in-hospital quinolone prescribing.24 In-hospital quinolone prescribing is relatively easy to tackle using simple notification systems, as there is a greater control over the medication administered to inpatients and, consequently, a larger window of opportunity for suggesting effective alternatives to quinolones to the assistant physician.
The control of outpatient quinolone prescribing is hampered by a greater complexity in notification of prescriptions and in changing antimicrobial regimens in patients that have left the hospital. On the other hand, because of a lesser viability of parenteral therapy, alternative drugs may be shorter, especially in the absence of cultural exams.
The surveillance of outpatient antimicrobial prescribing has thus been left behind in most AMSPs. In our hospital, we are planning a start to this activity, so we made an initial attempt at identifying priority problems for intervention.
About two thirds of outpatient quinolone prescribing in our hospital are made by the specialties of Urology, Ophthalmology, and Otolaryngology. Urology, with 28.4% of all prescriptions, is thus our largest outpatient quinolone prescriber. Although Ophthalmology and Otolaryngology have made, jointly, a larger number of prescriptions, these are specialties that have topical formulations available. Such formulations carry a theoretically lower risk of development of antimicrobial resistance due to a smaller systemic exposure.25,26
The choice of quinolone is, in itself, a potential target for discussion. All analyzed prescriptions involved one of four quinolones: norfloxacin, levofloxacin, ciprofloxacin, and prulifloxacin. Although levofloxacin and ciprofloxacin are considered practically equivalent in the treatment of urinary tract infections, including prostatitis,16,19,20,22 it is important to note that ciprofloxacin is active against the majority of the most common aetiological agents, including Pseudomonas aeruginosa and staphylococci.27 The spectrum of activity of levofloxacin is similar, but extends to other Gram-positive microorganisms such as the streptococci,27 which is unnecessary in the empirical treatment of urinary tract infections. Norfloxacin has a similar spectrum of activity to ciprofloxacin, except for its inactivity against atypical agents.27 Also, as norfloxacin fails to attain therapeutic concentrations in serum and tissues, it can only be used in uncomplicated urinary tract infections.19,27 Peculiarly, for reasons we ignore, prulifloxacin was the most commonly prescribed quinolone. Prulifloxacin is the most active quinolone against Enterobacterales and its activity against Pseudomonas aeruginosa is equivalent to that of ciprofloxacin. Its broad spectrum of activity still includes the atypical agents, streptococci, enterococci, and staphylococci.28 Because of its broader spectrum of activity when compared to second-generation quinolones such as ciprofloxacin, and taking into consideration that its efficacy is similar, prulifloxacin should not be preferred in the treatment of urinary tract infections.
A significant problem we found was the relative scarcity of clinical records. Description of patient symptoms was not made in a large proportion of cases: in 31.6% of patients with a diagnosis for antimicrobial therapy there was no mention of symptoms and in 55 patients (23.2% of all prescriptions) there was no mention of symptoms or diagnosis. Even among cases with a recorded diagnosis, in 27.9% of cases there was mention of an unspecified urinary tract infection; this hampers the assessment of antimicrobial therapy adequacy, as this should be different whether it be for cystitis, pyelonephritis, or other infections. Such incomplete records hinder the action of AMSPs, as they prevent the auditing clinician to know the motives behind antimicrobial prescribing.29
Focusing on antimicrobial prophylaxis, although quinolones have been recommended in several procedures, and continue to be in specific cases16; owing to the potential development of antimicrobial resistance there has been an effort towards a gradual removal. In our hospital, according to our internal protocol, quinolones are not recommended in any regimen of antimicrobial prophylaxis for urologic procedures. As such, all prescriptions of quinolones for antimicrobial prophylaxis have been considered inadequate.
Laboratory tests are, as well, of paramount importance to guide antimicrobial therapy. Urine culture is recommended in the generality of complicated urinary tract infections,16,18–22 as in the identification of asymptomatic bacteriuria before urologic procedures.16,30 The urinalysis provides another opportunity of an adequate use of antimicrobials: a normal test (without leukocyturia or nitrituria) allows for the exclusion of urinary tract infection.30 Among the studied population, only in a minority such laboratory testing was performed: urinalysis was made in 34.6% of patients, of which 27.7% had samples not suggestive of urinary tract infection; 33.8% of patients had a urine culture. These numbers were lower if one considers antimicrobial prophylaxis only, where only 6.5% of patients had a urine culture.
Among patients with positive cultures, most had viable alternatives to quinolones as per the antimicrobial susceptibility testing. Of all the patients that underwent antimicrobial therapy, 77.4% had reported sensitivity to quinolones. In no samples resistance was reported; the remaining samples did not report on sensitivity to quinolones. In most patients (71.0%) there was sensitivity to oral agents of the WHO AWaRe classification (this group includes amoxicillin, amoxicillin/clavulanate, nitrofurantoin, and trimethoprim/sulfamethoxazole) and 83.9% patients had sensitivity to oral agents in either the Access or Watch groups (the latter includes cefuroxime and fosfomycin). Many patients were thus given quinolones when sensitivity to quinolones was not reported (which is not synonymous of existing resistance) and/or when the results of susceptibility testing allowed for the use of antimicrobials with lesser selective pressure.
As a consequence of all these factors, as might be expected, the rate of adequate quinolone prescribing was globally low. The low proportion of cases with a clear indication for antimicrobial prescribing (53.2%) is problematic, as is the proportion of cases in which, there not being an indication for antimicrobial prescribing, this was made nonetheless (13.9%). Among the cases with indication for antimicrobial prescribing, quinolones were considered inadequate drugs in 77.0% of cases. The rate of concordance was higher in patients with a diagnosis of prostatitis (60.0%) or epididymo-orchitis (58.3%); this reflects the renowned greater efficacy of quinolones in such cases, which makes quinolones an adequate choice even in the presence of alternatives.16,20,21 In the remaining urinary tract infections, where first-line drugs belong to other classes16,18,19,22 and almost always have alternatives that are at least as effective, in only 2.6% of cases was the prescription considered adequate. These numbers, however, are likely deflated by the disproportional prescribing of prulifloxacin, which made the choice inadequate when another quinolone (such as ciprofloxacin or levofloxacin) could have been an adequate choice. The duration of treatment is another problem, as, in more than half of cases (51.1%), this was inadequate. There was a trend towards higher than recommended treatment duration in urinary tract infections and epididymo-orchitis and lower than recommended in prostatitis.
Our study has limitations: the number of analyzed prescriptions was relatively low and prevents some subgroup analyses, even if, following the initial objective of identifying opportunities for intervention, the most priority prescribing faults have been identified. Another limitation is the analysis of prescribing in a single specialty; analysis of prescribing in other specialties is also important and may be a target for auditing in the future. This auditing was itself hampered by the scarcity of medical records and by frequent laboratory testing outside the hospital (to which we had no access except when recorded electronically). Finally, the assessment of the adequacy of prescriptions, although guided by national and international guidelines, carries a share of subjectivity; by having several investigators tasked with assessing prescriptions for adequacy it is likely that some have been considered either adequate or inadequate when another investigator could have had a different opinion. We believe, however, that these prescriptions would be exceptional and that, in all but a few such cases, the opinions would be concordant.
In conclusion, this analysis makes clear several opportunities for intervention during the act of prescription:
- •
Incomplete recordings must be avoided in order to facilitate the input from AMSPs. The obligatory insertion of a diagnosis is a simple measure, but an accurate description of symptoms must also be encouraged.
- •
The urinalysis and urine culture are essential tools in the process of decision on the antimicrobial to administer and must be done in all patients with suspected complicated urinary tract infection. Making sure that the result of the urine culture will be observed is important for later adjustment of therapy.
- •
Prescription of quinolones for prophylaxis in Urology is inappropriate and reflects the need to implement strategies for better adhesion to an internal protocol for antimicrobial prophylaxis.
- •
Prescription of incorrect treatment durations is frequent and guidance that makes this clearer can easily be created.
- •
Different quinolones have different spectra of activity and the chosen drug must reflect it. In particular, although prulifloxacin is equivalent in efficacy when compared to other quinolones in urinary tract infections, its prescription in this context is potentially inappropriate. We found a disproportionately high number of prescriptions of prulifloxacin, the cause of which shall be explored in the future.
- •
If possible, alternative antimicrobials with lesser selective pressure must always be preferred. The WHO AWaRe classification is a potential aid for this choice.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare no conflicts of interest.