The objective of the study was to describe the implementation of Neurally Adjusted Ventilatory Assist (NAVA) by characteristics of patients receiving NAVA and by staff-experienced opportunities and barriers.
MethodsDesign. A retrospective review of hospital records of mechanically ventilated patients over two time periods after implementation, as well as a questionnaire survey and interviews with staff.
SettingA secondary Danish ICU.
ParticipantsICU patients, nurses, and intensivists.
InterventionImplementation of NAVA, which included theoretical education, bedside training, and frequent updates.
Main outcome measureEvaluation of NAVA implementation measured by characteristics of patients receiving NAVA and staff experiences with NAVA.
ResultsA total of 311 patients were included. Hereof 43 (27%) and 68 (44%) patients, respectively, had recieved NAVA. The patients receiving NAVA had higher severity scores and more hours on ventilators. A total of 35 nurses (76%) and 16 physicians (64%) completed the questionnaire. Most clinicians found, to a high (43%) or very high (41%) degree, that NAVA was an effective therapy option. Furthermore, 77% did not experience any barriers regarding NAVA therapy. The main advantages experienced with NAVA were increased patient comfort, respiratory synchrony with the ventilator, and improved opportunities for monitoring patient respiratory performance. The main disadvantage was the need for additional theoretical and practical knowledge.
ConclusionDespite staff experience of NAVA as a beneficial treatment option, more than half of the patients did not receive NAVA treatment two years after the start of its implementation. Implementation of a therapy which is substantially different to earlier practices is complicated.
El objetivo de este estudio fue describir la implementación de la asistencia ventilatoria ajustada neuralmente (Neurally Adjusted Ventilatory Assist [NAVA]) por características de los pacientes que lo recibieron, y las oportunidades y barreras experimentadas por el personal.
MétodosDiseño. Revisión retrospectiva de los registros hospitalarios de los pacientes que recibieron ventilación mecánica durante dos periodos temporales tras su implementación, así como una encuesta mediante cuestionario y entrevistas con el personal.
EntornoLa UCI secundaria danesa.
ParticipantesPacientes de las UCI, enfermeras e intensivistas.
IntervenciónImplementación de la NAVA que incluyó formación teórica, formación a pie de cama y actualizaciones frecuentes.
Medida principal del resultadoEvaluación de la implementación de la NAVA, medida por las características de los pacientes que lo recibieron, y experiencias del personal con el dispositivo.
ResultadosSe incluyó a un total de 311 pacientes, de los cuales 43 (27%) y 68 (44%) pacientes, respectivamente, habían recibido la NAVA. Los pacientes que recibieron la NAVA reflejaron puntuaciones de gravedad más altas y más horas de ventilación. Un total de 35 enfermeras (76%) y 16 médicos (64%) completaron el cuestionario. La mayoría de los clínicos encontraron que, en mayor (43%) o muy alto (41%) grado, la NAVA era una opción terapéutica efectiva. Además, el 77% no experimentó ninguna barrera referente a la terapia de la NAVA. Las principales ventajas experimentadas con la NAVA fueron un incremento de la comodidad del paciente, sincronía respiratoria con el ventilador y mejora de las oportunidades de monitorizar el desempeño respiratorio del paciente. La principal desventaja fue la necesidad de un conocimiento teórico y práctico adicionales.
ConclusiónA pesar de la experiencia por parte del personal, en cuanto a que la NAVA es una opción terapéutica beneficiosa, más de la mitad de los pacientes no recibieron tratamiento con la NAVA transcurridos dos años del inicio de su implementación, debido a la complejidad de introducir una terapia sustancialmente diferente a las prácticas previas.
In Denmark, more than 30,000 patients are admitted to intensive care units (ICU) each year.1 Of these, the majority has different levels of respiratory insufficiency2,3 with a need for oxygen therapy such as bi-nasal oxygen, nasal high flow, non-invasive mechanical ventilation or invasive mechanical ventilation. More than 45% of the 30,000 patients are treated with invasive mechanical ventilation and 17% with non-invasive ventilation.1
Invasive mechanical ventilation has been an integrated part of intensive therapy since the polio epidemic of the 1950s. A mechanical ventilator provides the patient with a certain and well-defined amount of pressure or volume in both controlled and assisted modes. Over the years, there has been a tendency to use ventilator modes in which some degree of spontaneous activity is preserved, which is known as partial ventilatory assist.
One of these modes is Neurally Adjusted Ventilatory Assist (NAVA, Servo-i and Servo-U, Maquet, Solna, Sweden) which provides pressure in proportion to and in synchrony with the patient's Electrical Activity of the diaphragm (EAdi). Spontaneous breathing starts with an impulse generated in the respiratory centre. The neural activity in this centre is predominantly a result of changes in PaCO2, pH, and PaO2: when PaCO2 rises or pH is reduced, the activity in the respiratory centre increases. The activity in the respiratory centre is transmitted via the phrenic nerve to the diaphragm,2 which induces an increase in the electric activity in the diaphragm (EAdi). The increase in diaphragmatic activity and contraction of the diaphragm will push the dome downwards, creating a negative intrathoracic pressure and causing the gas to flow into the lung. The efficacy of the diaphragm will determine the degree of output from the respiratory centre. If the muscle does not perform as much as is needed, this results in an increased output from the respiratory centre. Moreover, if the diaphragm is unable to meet the respiratory demands, the patient will develop respiratory insufficiency.
Likewise, an activity and ventilation increase will take place, albeit to a lesser extent, through a reduction in PaO2 or as a result of emotional conditions such as pain and anxiety. These regulatory mechanisms mean that blood pH, PaCO2 and PaO2 are kept within very narrow parameters in the individual.
As EAdi is a fast and precise reflection of activity in the respiratory centre, the EAdi-signals allow observation and monitoring of the synchronicity between patient and ventilator.4 A nasal-gastric tube with electrodes (EAdi-catheter, Maquet, Solna, Sweden) is placed in the oesophagus near the diaphragm, and the tube intercepts the electric diaphragmatic activity (EAdi). By connecting EAdi to the ventilator's supporting functions, the patient's respiratory activity will be supported momentarily, synchronous with and proportional to the desired strength5 and will likewise be instantly cut off when the patient expires.
Patient-triggered modes and patient-supported modes have several advantages over control modes. The modes reduce the adverse effect of prolonged sedation,6 reduce ventilator induced diaphragmatic dysfunction,7 improve gas-exchange8 and improve haemodynamic stability.9
However, even the patient-triggered modes are challenged by greater or lesser extents of asynchrony10,11 (flow asynchrony; trigger asynchrony; cycle asynchrony; and mode asynchrony), which increases respiratory muscle load9 and is associated with longer ventilator times and durations of stay at the intensive care unit.11–14 A study has shown that NAVA reduced asynchrony during assisted ventilation to a greater extent than pressure support ventilation (PSV).12 These findings also add to the data indicating that modes of ventilation which provide proportional assistance to ventilation such as NAVA markedly reduce asynchrony, and it has become more accepted that, in most circumstances, the respiratory centre of the patient is the most appropriate determinant of ventilatory pattern.
Traditionally, clinicians have been used to control the ventilatory pattern of the patient. With NAVA, clinicians are conceptually challenged in their modes of thinking. Only the proportion of help provided by the ventilator to support or supplement the patient's effort is set, while neither pressure, flow, volume nor time are. In other words, NAVA follows the lead of the patient without forcing a ventilator pattern. The major difficulty with NAVA is the inability of the clinician to control the patient's respiratory pattern. NAVA does not allow for this and the most appropriate determinant of the patient's respiratory pattern is the respiratory centre. Therefore, implementing such new ventilator strategies in an ICU may create challenges for clinicians.
The aim of this study was to describe the implementation of NAVA in a secondary Danish ICU, regarding both characteristics of patients receiving NAVA and to staff-experienced opportunities and barriers.
MethodSettingThe study was based in a Danish general ICU in a 300-bed regional hospital with eight ICU beds and one intermediary care bed. The ICU mainly receives patients from medical, surgical and oncological specialties. The sedation practice in the ICU is, as a rule, not to sedate; however, light sedation may be administered if necessary. The medical staff consists of three intensivists supplemented by senior anesthesiologists and junior anesthesiologists-in-training. The nursing staff consists of registered nurses. Hereof, 90% of the nurses have completed an additional two-year specialised education as intensive care nurses and 8% are currently in training. The nurse-patient ratio is 1:1 during the daytime and 1:1.3 after hours.
Implementation of NAVA treatmentThe implementation process included several steps:
Two hours of theoretical education for all staff; bed-side peer-to-peer courses and training for all staff; “Monthly focus” subject; frequent information and case-reviews on weekly staff meetings.
NAVA as a ventilator mode was introduced in the ICU in 2012. From 2014, the local ICU guidelines state that, by default, NAVA should be used for all patients with respiratory failure who require mechanical ventilation. However, if this is not possible, other ventilation modes such as pressure regulated volume control (PRVC) or pressure control/support (PC/PS) can be used.
The ICU Clinical Information System (CIS) was used to collect patient data for all patients staying in the ICU for more than 24h in two time periods: 01.04.2012 to 30.09.2013 and 01.04.2014 to 30.09.2015. Patient data included age, gender, body mass index (BMI), medical specialty, reason for ICU admittance, initial blood gas, hours receiving mechanical ventilation (hereof hours of NAVA when applicable), reintubation, hours receiving sedation and vasopressors and ICU length of stay, as well as APACHE II (Acute Physiology and Chronic Health Evaluation), SAPS II (Simplified Acute Physiology Score) and SOFA (Sepsis-Related Organ Failure Score) scores. Additionally, ICU –, 30 days – and 90 days mortality were registered.
Staff experiencesStaff experiences were examined using a questionnaire survey and semi-structured interviews.
QuestionnaireThe questionnaire contained 11 questions: four personal questions about profession, number of years as a professional, years in the department and how often the participant worked with NAVA; five questions about experienced advantages, disadvantages and possible barriers (with options for adding comments), and two open questions about ideas for further implementation/improvements and other comments about NAVA.
The first questionnaire draft was developed based on literature and NAVA experience in the ICU.
The draft was tested by heads of departments (both physicians and nurses) and adjusted accordingly.
The second draft was then tested by three physicians and five nurses from the ICU for face and content validity.
When a consensus was obtained for the final version, all ICU nurses and all physicians working full- or part-time in the ICU received the questionnaire electronically by e-mail. If the questionnaire was not completed within three weeks, a reminder was sent by e-mail.
InterviewsA strategic sample of physicians and nurses was individually interviewed using a semi-structured interview guide with questions about perceived advantages, disadvantages, barriers and possibilities regarding NAVA treatment. The sample consisted of the four physicians: the head of Department, the head of the ICU, a senior anaesthesiologist rarely working in the ICU and a junior anaesthesiologist with five month's experience in the department. Furthermore, the sample included three nurses: the head of the ICU, a senior nurse with a special interest in NAVA and a junior nurse with three years’ experience in the ICU. All interviews were audio recorded.
Data analysesAs the quantitative part of the study was descriptive, no sample size calculation was conducted. Data was analysed with descriptive and comparative analyses, using the Chi-square test or Fishers exact test for categorical data, and the Mann–Whitney U-test or Kruskall–Wallis test for ordinal and non-normally distributed continuous data. Ordinal and non-normally distributed continuous data are presented with median and interquartile range (IQR). P-value <0.05 was considered significant.
Interview data was analysed using content analyses with a focus on manifest content.15
EthicsPermission to register patient data without consent from the patients was obtained from the Danish Health and Medicine Authority (3-3013-1345/1), and the study was registered with the Danish Data Protection Agency (15/5384). All staff participants were informed about the study and assured confidentiality. Completion of the questionnaire was considered acceptance of study participation.
Interview participants received written and verbal information about the study before acceptance of participation.
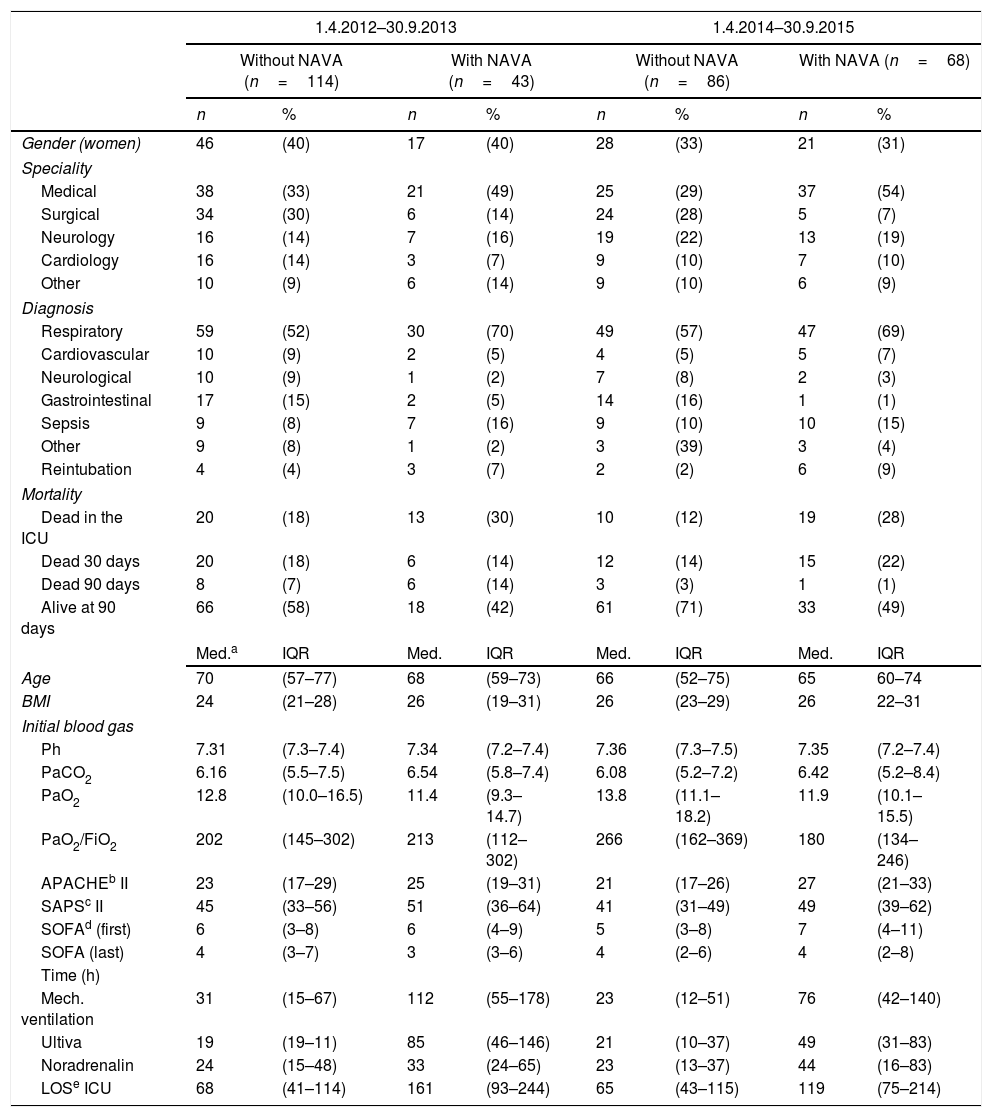
ResultsA total of 311 patients were included and hereof 43 patients in the first time period and 68 in the second period had received NAVA. Table 1 presents an overview of patient characteristics for patients with and without NAVA treatment in the two time periods.
Patient characteristics.
| 1.4.2012–30.9.2013 | 1.4.2014–30.9.2015 | |||||||
|---|---|---|---|---|---|---|---|---|
| Without NAVA (n=114) | With NAVA (n=43) | Without NAVA (n=86) | With NAVA (n=68) | |||||
| n | % | n | % | n | % | n | % | |
| Gender (women) | 46 | (40) | 17 | (40) | 28 | (33) | 21 | (31) |
| Speciality | ||||||||
| Medical | 38 | (33) | 21 | (49) | 25 | (29) | 37 | (54) |
| Surgical | 34 | (30) | 6 | (14) | 24 | (28) | 5 | (7) |
| Neurology | 16 | (14) | 7 | (16) | 19 | (22) | 13 | (19) |
| Cardiology | 16 | (14) | 3 | (7) | 9 | (10) | 7 | (10) |
| Other | 10 | (9) | 6 | (14) | 9 | (10) | 6 | (9) |
| Diagnosis | ||||||||
| Respiratory | 59 | (52) | 30 | (70) | 49 | (57) | 47 | (69) |
| Cardiovascular | 10 | (9) | 2 | (5) | 4 | (5) | 5 | (7) |
| Neurological | 10 | (9) | 1 | (2) | 7 | (8) | 2 | (3) |
| Gastrointestinal | 17 | (15) | 2 | (5) | 14 | (16) | 1 | (1) |
| Sepsis | 9 | (8) | 7 | (16) | 9 | (10) | 10 | (15) |
| Other | 9 | (8) | 1 | (2) | 3 | (39) | 3 | (4) |
| Reintubation | 4 | (4) | 3 | (7) | 2 | (2) | 6 | (9) |
| Mortality | ||||||||
| Dead in the ICU | 20 | (18) | 13 | (30) | 10 | (12) | 19 | (28) |
| Dead 30 days | 20 | (18) | 6 | (14) | 12 | (14) | 15 | (22) |
| Dead 90 days | 8 | (7) | 6 | (14) | 3 | (3) | 1 | (1) |
| Alive at 90 days | 66 | (58) | 18 | (42) | 61 | (71) | 33 | (49) |
| Med.a | IQR | Med. | IQR | Med. | IQR | Med. | IQR | |
| Age | 70 | (57–77) | 68 | (59–73) | 66 | (52–75) | 65 | 60–74 |
| BMI | 24 | (21–28) | 26 | (19–31) | 26 | (23–29) | 26 | 22–31 |
| Initial blood gas | ||||||||
| Ph | 7.31 | (7.3–7.4) | 7.34 | (7.2–7.4) | 7.36 | (7.3–7.5) | 7.35 | (7.2–7.4) |
| PaCO2 | 6.16 | (5.5–7.5) | 6.54 | (5.8–7.4) | 6.08 | (5.2–7.2) | 6.42 | (5.2–8.4) |
| PaO2 | 12.8 | (10.0–16.5) | 11.4 | (9.3–14.7) | 13.8 | (11.1–18.2) | 11.9 | (10.1–15.5) |
| PaO2/FiO2 | 202 | (145–302) | 213 | (112–302) | 266 | (162–369) | 180 | (134–246) |
| APACHEb II | 23 | (17–29) | 25 | (19–31) | 21 | (17–26) | 27 | (21–33) |
| SAPSc II | 45 | (33–56) | 51 | (36–64) | 41 | (31–49) | 49 | (39–62) |
| SOFAd (first) | 6 | (3–8) | 6 | (4–9) | 5 | (3–8) | 7 | (4–11) |
| SOFA (last) | 4 | (3–7) | 3 | (3–6) | 4 | (2–6) | 4 | (2–8) |
| Time (h) | ||||||||
| Mech. ventilation | 31 | (15–67) | 112 | (55–178) | 23 | (12–51) | 76 | (42–140) |
| Ultiva | 19 | (19–11) | 85 | (46–146) | 21 | (10–37) | 49 | (31–83) |
| Noradrenalin | 24 | (15–48) | 33 | (24–65) | 23 | (13–37) | 44 | (16–83) |
| LOSe ICU | 68 | (41–114) | 161 | (93–244) | 65 | (43–115) | 119 | (75–214) |
Table 1 shows that the percentage of patients receiving NAVA increased from 27% to 44% from the first period to the second period. The patients receiving NAVA in both the first and second time periods were characterised by being mainly medical patients with respiratory problems as the main reason for admittance to the ICU. Compared to patients not receiving NAVA, the NAVA patients tended to have higher APACHE scores, more hours on mechanical ventilation, longer lengths of stay in the ICU, and higher mortality rates (Table 1).
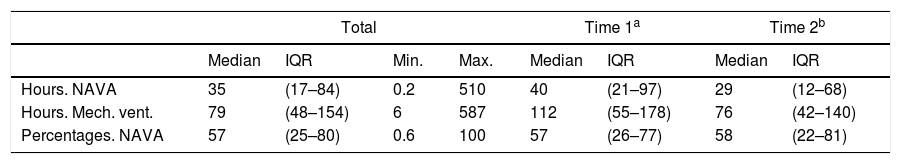
When looking only at patients receiving NAVA treatment, the patients had received NAVA between 0.2 and 150h. When compared to total time on mechanical ventilation, the patients had received NAVA treatment for a median of 57% of the mechanical ventilation time (IQR 25–80) (Table 2).
Treatment with NAVA compared to total time on mechanical ventilation.
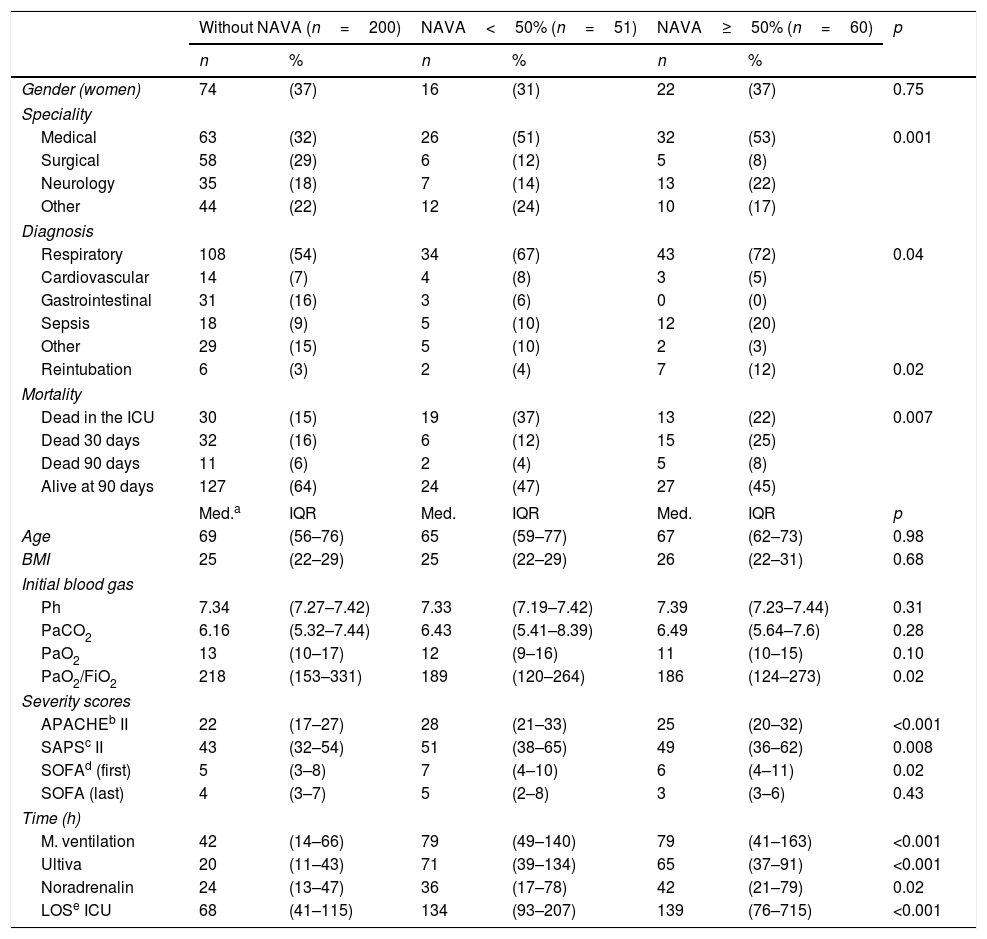
In Table 3, patient characteristics are compared for three groups (for the two time periods combined): patients without any NAVA treatment, patients receiving NAVA for less than 50% of the time on mechanical ventilation, and patients receiving NAVA treatment for 50% or more of the time on mechanical ventilation. The main statistically significant differences were found between the three groups regarding specialty, severity scores, time on mechanical ventilation, lengths of stay in the ICU and mortality rates.
Patient characteristics. Non-NAVA, NAVA<50% and NAVA 50% and more.
| Without NAVA (n=200) | NAVA<50% (n=51) | NAVA≥50% (n=60) | p | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Gender (women) | 74 | (37) | 16 | (31) | 22 | (37) | 0.75 |
| Speciality | |||||||
| Medical | 63 | (32) | 26 | (51) | 32 | (53) | 0.001 |
| Surgical | 58 | (29) | 6 | (12) | 5 | (8) | |
| Neurology | 35 | (18) | 7 | (14) | 13 | (22) | |
| Other | 44 | (22) | 12 | (24) | 10 | (17) | |
| Diagnosis | |||||||
| Respiratory | 108 | (54) | 34 | (67) | 43 | (72) | 0.04 |
| Cardiovascular | 14 | (7) | 4 | (8) | 3 | (5) | |
| Gastrointestinal | 31 | (16) | 3 | (6) | 0 | (0) | |
| Sepsis | 18 | (9) | 5 | (10) | 12 | (20) | |
| Other | 29 | (15) | 5 | (10) | 2 | (3) | |
| Reintubation | 6 | (3) | 2 | (4) | 7 | (12) | 0.02 |
| Mortality | |||||||
| Dead in the ICU | 30 | (15) | 19 | (37) | 13 | (22) | 0.007 |
| Dead 30 days | 32 | (16) | 6 | (12) | 15 | (25) | |
| Dead 90 days | 11 | (6) | 2 | (4) | 5 | (8) | |
| Alive at 90 days | 127 | (64) | 24 | (47) | 27 | (45) | |
| Med.a | IQR | Med. | IQR | Med. | IQR | p | |
| Age | 69 | (56–76) | 65 | (59–77) | 67 | (62–73) | 0.98 |
| BMI | 25 | (22–29) | 25 | (22–29) | 26 | (22–31) | 0.68 |
| Initial blood gas | |||||||
| Ph | 7.34 | (7.27–7.42) | 7.33 | (7.19–7.42) | 7.39 | (7.23–7.44) | 0.31 |
| PaCO2 | 6.16 | (5.32–7.44) | 6.43 | (5.41–8.39) | 6.49 | (5.64–7.6) | 0.28 |
| PaO2 | 13 | (10–17) | 12 | (9–16) | 11 | (10–15) | 0.10 |
| PaO2/FiO2 | 218 | (153–331) | 189 | (120–264) | 186 | (124–273) | 0.02 |
| Severity scores | |||||||
| APACHEb II | 22 | (17–27) | 28 | (21–33) | 25 | (20–32) | <0.001 |
| SAPSc II | 43 | (32–54) | 51 | (38–65) | 49 | (36–62) | 0.008 |
| SOFAd (first) | 5 | (3–8) | 7 | (4–10) | 6 | (4–11) | 0.02 |
| SOFA (last) | 4 | (3–7) | 5 | (2–8) | 3 | (3–6) | 0.43 |
| Time (h) | |||||||
| M. ventilation | 42 | (14–66) | 79 | (49–140) | 79 | (41–163) | <0.001 |
| Ultiva | 20 | (11–43) | 71 | (39–134) | 65 | (37–91) | <0.001 |
| Noradrenalin | 24 | (13–47) | 36 | (17–78) | 42 | (21–79) | 0.02 |
| LOSe ICU | 68 | (41–115) | 134 | (93–207) | 139 | (76–715) | <0.001 |
A total of 35 nurses (76%) and 16 physicians (64%) completed the survey. The majority had more than 10 years’ experience as professionals, whereas the number of years working in the ICU were more equally divided between younger and older staff.
Advantages with using NAVA were “Faster correction of respiratory insufficiency” (61%), “Monitoring of respiratory capacity” (47%), “Decreased ventilator time” (37%), “Increased patient comfort” (84%), “Increased patient involvement” (39%) and “Other” (4%), which included a reduction in the need for sedatives and higher levels of alertness in patients.
Disadvantages were found to be “Applying a particular gastric tube” (37%), “Need for training in the NAVA modus” (42%), “Troublesome to adjust correctly” (23%), “Demands more in regard to collaboration with the patient” (26%) and “Other” (21%), which included the tube bothering the patients due to heaviness, a short cable which can complicate mobilisation and not all patients being able to cooperate with NAVA.
Most participants found, either to a high (43%) or very high (41%) degree, that NAVA was an effective therapy option to be able to offer patients with respiratory distress, and 77% did not experience any barriers regarding NAVA therapy. Those experiencing barriers found that the main ones were lack of experience (both nurses and physicians), difficulties interpreting the alarms, the lack of control of most respiratory parameters, and the time needed to apply the EAdi catheter for respiratory challenged patients.
InterviewAll invited nurses and physicians agreed to participate, and interviews lasted between 9 and 17min.
The main experienced advantages of NAVA were increased patient comfort, respiratory synchrony with the ventilator and improved opportunities for monitoring patient respiratory performance. The main disadvantages were the need for extra knowledge both theoretically and in practice, and the consequent need to unlearn traditional ways of ventilator treatment for experienced staff. Economic costs were also mentioned as a disadvantage.
The interviewees found that the implementation had been successful overall. As many new practices had been introduced in the ICU around the time of the introduction of NAVA, progress was slow to begin with because knowledge was often insufficient and the known respiratory modi were then resorted to. However, when a united commitment was made for both physicians and nurses to move forward with NAVA, the implementation process accelerated. For most nurses and physicians, the potential need for NAVA was now a natural part of the assessment when a new patient was admitted to the ICU.
The main perceived barriers were lack of knowledge (partly due to the organisational structure), uncertainty, lack of courage to relinquish control over the patient's respiration, and the time and effort needed to find the appropriate setting for the individual patient, which is described as a triangle between patient, ventilator and physician/nurse.
Ideas for improving the original implementation included more efficient timing of the implementation start, ensuring time is planned for all staff to participate in the NAVA theoretical course, education of 10–15 “Super-NAVA nurses” to ensure expert knowledge in the ICU 24/7, and more structured peer-to-peer training in practice. To maintain the foundation of NAVA as the first choice for respiratory therapy, the interviewees suggested both theoretical and peer-to-peer education for all new staff, especially for senior physicians used to traditional respiratory therapy, and regularly refresher courses with NAVA theory and “Tips and tricks”.
DiscussionNAVA was meant to be the first choice for patients requiring mechanical ventilation, but less than half of the patients received NAVA treatment in the follow-up period. The data analyses showed that the patients receiving NAVA were mainly the most severely ill. “Unlearning” knowledge and practice is always difficult and may especially be a barrier for experienced staff.16 The need for individual adjustment is opposed to traditional respiratory treatment where a setting is chosen and then checked with a blood gas test sometime later.
Therefore, NAVA therapy requires greater physician presence. As the ICU was staffed by only three intensivists, a number of shifts were covered by anesthesiologists who did not have the same opportunities to become familiar with NAVA therapy due to infrequent ICU shifts. This prolonged the implementation process, and the probability of the patient receiving NAVA was influenced depending on which physician was on duty.
However, both nurses and physicians experienced few barriers regarding NAVA treatment and, to a high or very high degree, the majority found that NAVA was an advantage for respiratory therapy. The main experienced advantages were better patient comfort, which is in accordance with studies showing that NAVA reduces patient-ventilator asynchrony,12,17 and an experienced shorter period of ventilation time. Patient-ventilator asynchrony is associated with prolonged duration of mechanical ventilation,18,19 even though this was not confirmed by the quantitative data from the current study. The participants also found that the use of NAVA influenced ordinary treatment with mechanical ventilation regarding focus on patient involvement in respiration and an significant decrease in the need for sedation. Sedation is among other things used to decrease patient-ventilator asynchrony, but studies have shown that it may instead increase asynchrony.20,21 As sedation is associated with lengths of stay and mortality,22–25 practices that help to minimise the perceived need for sedation are important.
The implementation process included a bundle of evidence-based implementation elements such as theoretical education, one-to-one bed-side instructions, and frequent reminders in weekly staff-meetings and as a “monthly focus”.26 However, one of the staff-experienced barriers was still lack of knowledge, and ideas for an improved implementation process included more structured one-to-one tuition at the bedside and the education of “super-NAVA nurses” to help ensure expert knowledge would always be available. One of the most important aspects of implementation success is that the health care professionals involved perceive the change as an improvement.27 This was the case for most of the participants in this study as they found that NAVA increased patient comfort, respiration synchrony with the ventilator, and improved opportunities for monitoring patient respiratory performance and, therefore, experienced NAVA as an improvement. A danger in all implementation processes is the risk of going back to old practices if the “critical mass” (a sufficient number of adopters) is not reached.27 To maintain this, the participants suggested both theoretical and peer-to-peer education for all new staff, especially for senior physicians used to traditional respiratory therapy, and regular refresher courses with NAVA theory and “Tips and tricks”.
Strengths of the study include the multi method design, a large patient cohort, inclusion of both physicians and nurses, and a high response rate. Limitations of the study include the focus on only a single centre and that experiences expressed by nurses and physicians were general and not connected with individual patients. As the ICU's electronic registration database was introduced at around the same time as the implementation of NAVA, it was not possible to compare the NAVA patients with a baseline group. Therefore, and due to the retrospective design and confounding by indication possibilities, it is not possible via this study to examine whether NAVA decreases time in mechanical ventilation, weaning, ICU length of stay or mortality. Randomised studies are needed to provide evidence for the effect of NAVA on these parameters.
ConclusionDespite an evidence-based implementation process and staff experiences of NAVA as a beneficial treatment option, more than half of the patients did not receive NAVA treatment one to two years after the start of implementation. Implementation of a therapy which is substantially different to earlier practices is complicated and is among other factors influenced by organisational issues.
FundingThis work was supported by a grant of 50,000 DKK from Maquet to cover some of the time used for hospital record review in connection with the study. Maquet had no influence on planning of the study, hospital record review, data analyses, conclusions or content of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.