Positron emission tomography–computed tomography (PET–CT) and bone marrow biopsy are currently the common clinical examination of lymphoma infiltration. The aim of this research is to evaluate the value of PET–CT in diagnosis of bone marrow infiltration, clinical staging and pathological typing of lymphoma.
Methods153 cases were analyzed retrospectively to compare the consistency of PET–CT and bone marrow biopsy. We analyzed the sensitivity, accuracy and specificity of PET–CT in different clinical pathology of lymphoma.
ResultsThe PET–CT sensitivity in detecting bone marrow infiltration is 54.3% with a specificity of 80.5% and accuracy of 74.5%. In aggressive B-cell lymphoma (DLBCL, HG-BL) and MZL, PET–CT results of bone marrow infiltration showed high accuracy of 88.1% and 83.3% respectively. The median value of SUVmax in the patients detected to have bone marrow infiltration by BMB was significantly higher than patients with BMB negative results among subgroups of aggressive B-cell lymphoma, MZL and T-NHL (p<.05).
ConclusionPET–CT is significant in detecting bone marrow infiltration in certain pathological types of lymphoma. However pathological inconsistencies still exist between bone marrow biopsy and PET–CT, thus PET–CT cannot completely replace biopsy.
La tomografía computarizada por emisión de positrones (PET/TC) y la biopsia de médula ósea (BMB) son actualmente los exámenes clínicos comunes para valorar la infiltración linfomatosa. El objetivo de este estudio es evaluar el valor de la PET/TC en el diagnóstico de la infiltración de la médula ósea, la estadificación clínica y la tipificación patológica del linfoma.
MétodosSe analizaron retrospectivamente 153 casos para comparar la consistencia de la PET/TC y la BMB. Analizamos la sensibilidad, la fiabilidad y la especificidad de la PET/TC en las diferentes enfermedades clínicas del linfoma.
ResultadosLa sensibilidad de la PET/TC para detectar infiltración de la médula ósea es del 54,3%, con una especificidad del 80,5% y una precisión del 74,5%. En los casos de linfoma agresivo de células B (DLBCL, HG-BL) y MZL, los resultados de la PET/TC para la infiltración de la médula ósea reflejaron una alta precisión del 88,1 y 83,3%, respectivamente. El valor medio SUVmáx en los pacientes en los que se detectó infiltración de la médula ósea mediante BMB fue significativamente superior al de los pacientes con resultados negativos de BMB entre los subgrupos de linfoma agresivo de células B, MZL y T-NHL (p<0,05).
ConclusiónLa PET/TC es significativa a la hora de detectar infiltración de la médula ósea en ciertos tipos patológicos de linfoma. Sin embargo, siguen existiendo inconsistencias patológicas entre la biopsia de médula ósea y la PET/TC, aunque esta técnica no puede sustituir por completo a la biopsia.
Lymphoma is a common malignant tumor origin from the lymphatic hematopoietic system. It is usually accompanied by bone marrow infiltration thus affecting the Ann Arbor staging. Therefore, the evaluation of lymphoma bone marrow infiltration is particularly important. Bone marrow biopsy is the most direct method of detecting lymphoma infiltration, but it is an invasive procedure which is accompanied by several complications such as pain and hemorrhage.1 On the other hand, PET–CT is a non-invasive examination that can comprehensively evaluate the state of bone marrow with extremely high sensitivity in detecting bone marrow infiltration of lymphoma.2–4 Therefore, many clinical institutions put forward the idea of replacing bone marrow biopsy with PET–CT as the first choice in the diagnosing of lymphoma bone marrow infiltration.
Meanwhile, many other researchers share different opinions. Relevant research indicates that PET–CT cannot replace bone biopsy in the diagnosing of bone marrow infiltration in DLBCL and FL,5 and the accuracy of PET–CT in detecting bone marrow infiltration is dissatisfactory.6,7 Some researchers came to the conclusion that the clinical significance of diffuse infiltration of bone marrow in PET–CT remains uncertain because the high uptake focus of FDG in PET–CT cannot represent the bone marrow infiltration very well.8,9 It is not objective to do a comparison between whole body PET–CT and one sample point bone marrow biopsy.10
At this moment, there is no clear international guideline between the indications of PET–CT and bone marrow biopsy. The aim of this research is to discuss the significance of PET–CT in detecting lymphoma bone marrow infiltration and clinical staging.
Data and methodsInclusion criteria and exclusion criteria of patientsInclusion criteria includes: specific histological type; accepted PET–CT and bone marrow biopsy before treatment; the interval between PET–CT and bone marrow biopsy is less than 2 weeks; detailed and believable clinical information.
Exclusion criteria includes: undefined histological type; accepted PET–CT and bone marrow biopsy after treatment; the interval between PET–CT and bone marrow biopsy is more than 2 weeks; missing or suspicious clinical information.
Data collectionCollected clinical information includes name, age, sex, pathological type, specific PET–CT description, bone marrow biopsy report, serum LDH level, etc. Patients were categorized based on the Ann Arbor Staging Criteria. Patients are then scored based on the IPI index.
MethodsPET–CT image collection and analysisPatients were imaged with equipment MCT31067. After a 4-h fasting period, patients were intravenous injected with imaging agent and rested for 1h before CT was performed. After attenuation correction and iterative reconstruction, multifaceted and multi-piece PET imaging was performed and fused with CT images. Grayscale was used to compare the imaging agent uptake level of liver and bone marrow. If a high-uptake focus in bone marrow was observed and the uptake level of the focus was higher than liver (or the mean SUV of bone marrow was higher than 2.7), the patient was defined abnormal bone marrow glucose metabolism. If no high-uptake focus in bone marrow was observed and the mean uptake level of bone marrow was lower than liver (or lower than 1.7), the patient was defined normal bone marrow glucose metabolism. We acquired the SUVmax of the bone marrow focuses in patients with abnormal bone glucose metabolism.
Bone marrow biopsy and pathological analysisUnilateral iliac crest biopsies were performed before treatment. BMB specimens were evaluated morphologically by hematopathologists. Immunohistochemistry of the bone marrow were carried out to determine the immunophenotype of the lymphoma and to quantify marrow involvement.
Statistical analysisWe used SPSS 19.0 to analyze continuous variables with non-parametric tests and grouping variables with Chi-square test or Fisher's exact test.
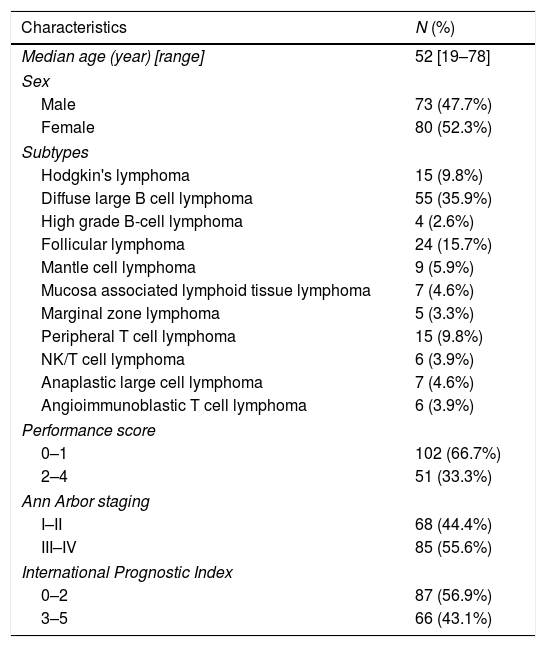
ResultsGeneral dataOur research included 153 patients who were diagnosed with lymphoma in the First Affiliated Hospital of China Medical University between September 2016 and October 2017. The basic information of the patients can be found in Table 1.
Patient characteristics.
| Characteristics | N (%) |
|---|---|
| Median age (year) [range] | 52 [19–78] |
| Sex | |
| Male | 73 (47.7%) |
| Female | 80 (52.3%) |
| Subtypes | |
| Hodgkin's lymphoma | 15 (9.8%) |
| Diffuse large B cell lymphoma | 55 (35.9%) |
| High grade B-cell lymphoma | 4 (2.6%) |
| Follicular lymphoma | 24 (15.7%) |
| Mantle cell lymphoma | 9 (5.9%) |
| Mucosa associated lymphoid tissue lymphoma | 7 (4.6%) |
| Marginal zone lymphoma | 5 (3.3%) |
| Peripheral T cell lymphoma | 15 (9.8%) |
| NK/T cell lymphoma | 6 (3.9%) |
| Anaplastic large cell lymphoma | 7 (4.6%) |
| Angioimmunoblastic T cell lymphoma | 6 (3.9%) |
| Performance score | |
| 0–1 | 102 (66.7%) |
| 2–4 | 51 (33.3%) |
| Ann Arbor staging | |
| I–II | 68 (44.4%) |
| III–IV | 85 (55.6%) |
| International Prognostic Index | |
| 0–2 | 87 (56.9%) |
| 3–5 | 66 (43.1%) |
Among the 153 patients, 42 patients were reported bone marrow infiltration by PET–CT (27.5%), 35 patients were reported bone marrow infiltration by bone marrow biopsy (22.9%). Setting bone marrow biopsy as golden standard, the sensitivity, specificity and accuracy of PET–CT in detecting bone marrow infiltration are 54.3%, 80.5% and 74.5% respectively (Table 2).
Correlation analysis of bone marrow biopsy and PET–CT in various pathological types of lymphomaSetting bone marrow biopsy as golden standard, we analyzed the sensitivity, specificity and accuracy of PET–CT in detecting bone marrow infiltration of different pathological types of lymphoma. The sensitivity of PET–CT is 0%, specificity and accuracy are both 46.7% in HL patients due to no bone marrow infiltration detection by bone marrow biopsy. In aggressive B-cell lymphoma (DLBCL, HG-BL) and MZL, PET–CT results of bone marrow infiltration showed high accuracy of 88.1% and 83.3% respectively. In other pathological types, PET–CT showed lower consistency with bone marrow biopsy. The median value of SUVmax in the patients detected bone marrow infiltration by BMB was significant higher than patients with BMB negative results among subgroups of aggressive B-cell lymphoma, MZL and T-NHL (Table 3). Detailed information for each histological subgroups can be found in supplementary Table 1.
Correlation analysis of bone marrow biopsy and PET–CT in various pathological types of lymphoma.
| Pathological subtype | PET–CT positive | PET–CT negative | Sensitivity | Specificity | Accuracy | Median and range of SUVmax | p |
|---|---|---|---|---|---|---|---|
| HL (N=15) | |||||||
| BMB positive | 0 | 0 | 0% | 46.7% | 46.7% | – | – |
| BMB negative | 8 | 7 | 2.9 (0–4.7) | ||||
| Aggressive B-cell lymphoma (N=59) | |||||||
| BMB positive | 8 | 2 | 80% | 90.0% | 88.1% | 10.4 (1.2–18.9) | <0.05 |
| BMB negative | 5 | 44 | 1.9 (0–5.3) | ||||
| FL (N=24) | |||||||
| BMB positive | 3 | 5 | 37.5% | 87.5% | 70.8% | 1.52 (0–3.7) | 0.62 |
| BMB negative | 2 | 14 | 1.44 (0–2.9) | ||||
| MCL (N=9) | |||||||
| BMB positive | 2 | 3 | 40% | 50% | 44.4% | 1.49 (0–9.3) | 0.47 |
| BMB negative | 2 | 2 | 1.98 (0–5.5) | ||||
| MZL (N=12) | |||||||
| BMB positive | 3 | 2 | 60% | 100% | 83.3% | 6.5 (0–7.4) | <0.05 |
| BMB negative | 0 | 7 | 1.3 (0.1.6) | ||||
| T-NHL (N=34) | |||||||
| BMB positive | 4 | 3 | 57.1% | 81.5% | 76.5% | 7.8 (1.3–17.9) | <0.05 |
| BMB negative | 5 | 22 | 1.7 (0–4.8) | ||||
BMB=bone marrow biopsy, HL=Hodgkin's lymphoma, FL=follicular lymphoma, MCL=mantle cell lymphoma, MZL=marginal zone lymphoma, T-NHL=T cell non-Hodgkin's lymphoma.
Bone marrow biopsy and PET–CT are two kinds of common examinations in detecting bone marrow infiltration of lymphoma. The former is an invasive examination which has high true positive rate (sensitivity) and false negative rate (rate of missed diagnosis). The latter is a noninvasive examination which has high true negative rate (specificity) and false positive rate (misdiagnosis rate). Consequently, the clinical value of bone marrow biopsy and PET–CT needed to be reevaluated.
Lymphoma is a common malignant proliferative disease in which bone marrow infiltration may alters Ann Arbor staging of patients, and thus affects the prognosis of the disease. Therefore, the evaluation of lymphoma bone marrow infiltration is particularly important.3,4,11 In our research, twenty patients from the Ann Arbor stage directed by PET–CT differ from the Ann Arbor stage directed by bone marrow biopsy. Among them, 13 patients were upgraded by PET–CT and 7 patients were downgraded. Except for the 8 patients with HL, the change of stage did not affect the clinical treatment of other types of lymphoma which is similar to related literature reports. Also, as expected, The SUVmax value of bone marrow in PET–CT showed significant correlation with bone marrow infiltration among patients with aggressive B-cell lymphoma and T-NHL (p<0.05), which may be associated with a highly aggressive nature of the disease.12,13
There are several recent research that have discussed the clinical status of PET–CT in detecting bone marrow infiltration of lymphoma with different pathological types, however, no unified conclusion has been drawn to guide the clinic so far.
For patients with HL, most investigators put forward that PET–CT is very precise in detecting bone marrow infiltration thus may replace bone marrow biopsy as the preferred examination.14,15 As for HL, Ujjani et al. reported that PET–CT detected over 90% bone marrow infiltration including the ones escaped from bone marrow biopsy.16–18 Subocz et al. believed that PET–CT is the most valuable imagological examination in lymphoma, and may even replace bone marrow biopsy now and then.19 Chen-Liang et al. recommended PET–CT as the first choice examination in detecting bone marrow infiltration of HL as well.11 However, our research does not include HL which has positive bone marrow biopsy result due to rare bone marrow infiltration in early HL. Among all the 15 patients with HL, 8 had positive PET–CT results about bone marrow infiltration, thus the sensitivity of PET–CT bone marrow infiltration detection of HL is 0% and the specificity and accuracy are both 46.7%. Based on our data, it is not appropriate to take PET positivity as an unequivocal sign of BM involvement in HL, since treatment options may be changed in some of the patients in this event. Sometimes, PET–CT may be too sensitive and detect small degrees of BMI that are clinically irrelevant. It is questionable to pursue high sensitivity blindly.
Some guidelines indicated that PET–CT is now recommended as the gold standard for staging DLBCL patients. Thus, biopsy is no longer required when a PET/CT scan demonstrates bone or marrow involvement indicating advanced-stage disease but is appropriate in case of negative PET–CT results.20 Teagle and El Karak et al. revealed that as for DLBCL, the sensitivity and specificity of PET–CT are higher than bone marrow biopsy.16,21,22 However, low-volume involvement (<10–20%) and discordant lymphoma may be missed by PET/CT imaging.23 Our study showed that the sensitivity, specificity and accuracy of PET–CT in detecting bone marrow infiltration of DLBCL were 80%, 90.0% and 88.1% respectively. As for HG-BL, Chen-Liang et al. recommended to check PET–CT first. Bone marrow biopsy is necessary only in cases where PET–CT showed no bone marrow infiltration.11 The consistency of bone marrow biopsy and PET–CT is relatively convincing thus it was concluded that PET–CT can be used as a first line examination in detecting bone marrow infiltration of DLBCL and HG-BL.
As for FL, PET–CT seems to be less precise in detecting bone marrow infiltration thus cannot replace bone marrow biopsy as the golden standard in clinic. According to study conducted by Gallamini et al., PET–CT can stage and evaluate the prognosis of FL precisely.24 However, Ujjani and Teagle reported that the sensitivity and specificity of PET–CT in detecting bone marrow infiltration of FL are unsatisfactory.16,21 The sensitivity, specificity and accuracy of PET–CT in detecting bone marrow infiltration of FL in our research were 37.5%, 87.5% and 70.8%. The consistency of bone marrow biopsy and PET–CT is not ideal, so we believe PET–CT have less value in detecting bone marrow infiltration of FL.
A recent study conducted by Koh et al.25 analyzed 109 (63 PTCL and 46 NKTCL) patients. Biopsy revealed BM involvement in 35.8% of cases. Sensitivity and specificity of PET for diagnosing positive BM biopsy were 61.5% and 75.7% respectively. Despite fair correlation with BM biopsy result, PET may not replace BM biopsy in PTCL and NKTCL. However, the BM finding on PET is an independent prognostic factor, suggesting additional biological implication of PET findings. Abe et al.26 revealed that PET/CT exhibited a higher sensitivity for BM involvement than BMB. Furthermore, BM assessment using PET/CT identified patients at high risk of disease progression and mortality. Shao puts forward that PET–CT is of vital importance in AITL.27 The sensitivity, specificity and accuracy of PET–CT in detecting bone marrow infiltration of AITL and ALCL in our research were relatively high. Due to the fact that there was only 1 patient with bone marrow infiltration for each pathological type, we cannot draw any convincing conclusion for ALCL and AITL from our research.
There is no report discussing the correlation between bone marrow biopsy and PET–CT in MCL, MALT, and MZL for the time being.
Still, our research had certain limitations. First of all, our sample was not large enough so that our conclusion may not represent the total population. Secondly, the pathological types included in our research were not comprehensive enough; the distribution among each type was not well-proportioned. As a consequence, we cannot draw conclusions for each type of lymphoma. Thirdly, our sample was collected only from our own hospital, thus our conclusion has regional limitation. Lastly, this retrospective study omitted the blind method in clinical information such as PET–CT and B symptom. As a result, the information bias may affect the results of our research.
PET–CT has many other functions besides diagnosing and staging of lymphoma. PET–CT can distinguish invasive lymphoma from noninvasive lymphoma.12,13,28 PET–CT is also an important evidence for differential diagnosis in many diseases.29–33 Furthermore, PET–CT can detect lymphoma infiltration in different locations.34–41
In addition, a delayed PET–CT may have high clinical value.42,43 Recent studies on PET-MRI stated that the combination of PET–CT with MRI resulted in a reduction of radiation from CT, thus giving it a promising future development.44 More future researches with larger sample size are needed to determine if delayed PET–CT is better than the standard PET–CT and whether PET-MRI can replace PET–CT in the evaluation of bone marrow condition without radiation.
ConclusionPET–CT may be sometime useful in the diagnosis of bone marrow infiltration in lymphoma. At some point it may show influence on Ann Arbor staging of lymphoma even lead to changes of clinical treatment. However, the clinical significance of PET in the diagnosis of bone marrow infiltration remains uncertain in certain pathological types. So, PET–CT cannot replace bone marrow biopsy completely.
Conflict of interestThe authors declare no conflicts of interest.
This work was supported by Demonstration of Regional Application of innovative diagnosis and treatment equipment in Liaoning Province (2017YFC0114200) and the National Natural Science Foundation of China (NSFC, 81900153).