Aminobisphosphonates are widely used in the treatment of osteoporosis. They have a high affinity for hydroxyapatite, binding primarily to resorbing surfaces, but also to forming surfaces and to some extent to resting surfaces. They inhibit osteoclasts, thereby decreasing remodelling units. Consequently, they increase bone mass and reduce stress risers. This decreases the risk of fractures. If this decrease is sufficient, they can be temporarily withdrawn (drug holidays), which prevents serious complications (atypical femoral fracture). They probably reduce mortality. Virtually all patients with osteoporosis can benefit from them at some point in the course of their disease (at the beginning of treatment or after the administration of anabolics, selective estrogen receptor modulators or denosumab). If well tolerated orally, alendronate and risedronate are preferable. Otherwise, zoledronate is preferred. Their efficacy vs. cost-safety-convenience ratio makes aminobisphosphonates reference drugs in the field of osteoporosis.
Los aminobisfosfonatos se utilizan ampliamente en el tratamiento de la osteoporosis. Tienen gran afinidad por la hidroxiapatita, uniéndose fundamentalmente a las superficies en resorción, pero también a las superficies en formación y, en cierta medida, a las superficies en reposo. Inhiben a los osteoclastos, con lo que disminuyen las unidades de remodelación. En consecuencia, aumentan la masa ósea y reducen los concentradores de tensión. Ello disminuye el riesgo de fracturas. Si esta disminución es suficiente, pueden retirarse transitoriamente (vacaciones terapéuticas), lo que previene complicaciones graves (fractura atípica de fémur). Probablemente disminuyen la mortalidad. Pueden beneficiarse de ellos prácticamente todos los enfermos con osteoporosis en algún momento de su evolución (al comienzo del tratamiento o tras la administración de anabólicos, moduladores selectivos de los receptores estrogénicos o denosumab). Con buena tolerancia oral son preferibles el alendronato y el risedronato. En caso contrario, lo es el zoledronato. Su relación eficacia frente a coste-seguridad-comodidad los convierte en fármacos de referencia en el campo de la osteoporosis.
Aminobisphosphonates (N-BPs) are bisphosphonates (BPs) in which one of the carbon-bonded radicals (commonly designated R2) contains a nitrogen atom. This radical may consist of a linear hydrocarbon chain (alkyl BPs: alendronate, pamidronate, ibandronate) or contain a ring with a nitrogen atom (heterocyclic BPs: risedronate, zoledronate). The nature of R2 determines the ability of BP to decrease osteoclastic activity. This effect is mainly due to the inhibition of the enzyme farnesyl pyrophosphate synthetase (FPPS), and thus of the prenylation of the enzymes known as “small GTPases”, which are involved in the organisation of the cytoskeleton and the formation of the “ruffled border”1,2. The inhibition of FPPS also gives rise to the accumulation of a metabolite, Apppl, which induces apoptosis, although this is not an essential mechanism in the effect of N-BPs.
Different BPs inhibit FPPS with different intensity: zoledronate > risedronate > ibandronate > alendronate3. This order is different from that of its affinity for hydroxyapatite: zoledronate > alendronate > ibandronate > risedronate4. In any case, studies on its antiresorptive capacity in experimental animals indicate the following order: zoledronate > risedronate > ibandronate > alendronate5. In clinical practice, however, alendronate decreases bone turnover markers (BTM) and increases bone mineral density (BMD) more than risedronate6,7 because the amount per tablet is higher in the case of alendronate.
Approximately half of the dose of BP that enters the body is fixed to the bone and the other half is eliminated by the kidney. BP that attaches to bone does so preferentially on resorption surfaces, i.e., surfaces where there has already been initial osteoclast action, which should be followed later by that of others. However, they will no longer be able to develop their effect because the BP deposited there will move inwards, functionally nullifying them. It will then be released into the medium, where it can reattach to a resorption surface. Later, these resorption drug-containing surfaces are covered by new bone. BP is also fixed in its mineralization front. BP can also bind to a certain extent to quiescent surfaces (particularly zoledronate)2 and penetrate the osteocyte-canalicular system8. It will remain at all these sites until a new cycle of remodelling begins2. In this way, BPs will be eliminated slowly, over the years. The binding of BPs to hydroxyapatite is reversible, with lower affinity BPs being released more readily.
The action of BPs on osteocytes (inhibiting their apoptosis9) has also been demonstrated and even on osteoblasts (in the subperiosteum and in remodelling units [see below]), but these aspects are not well established10.
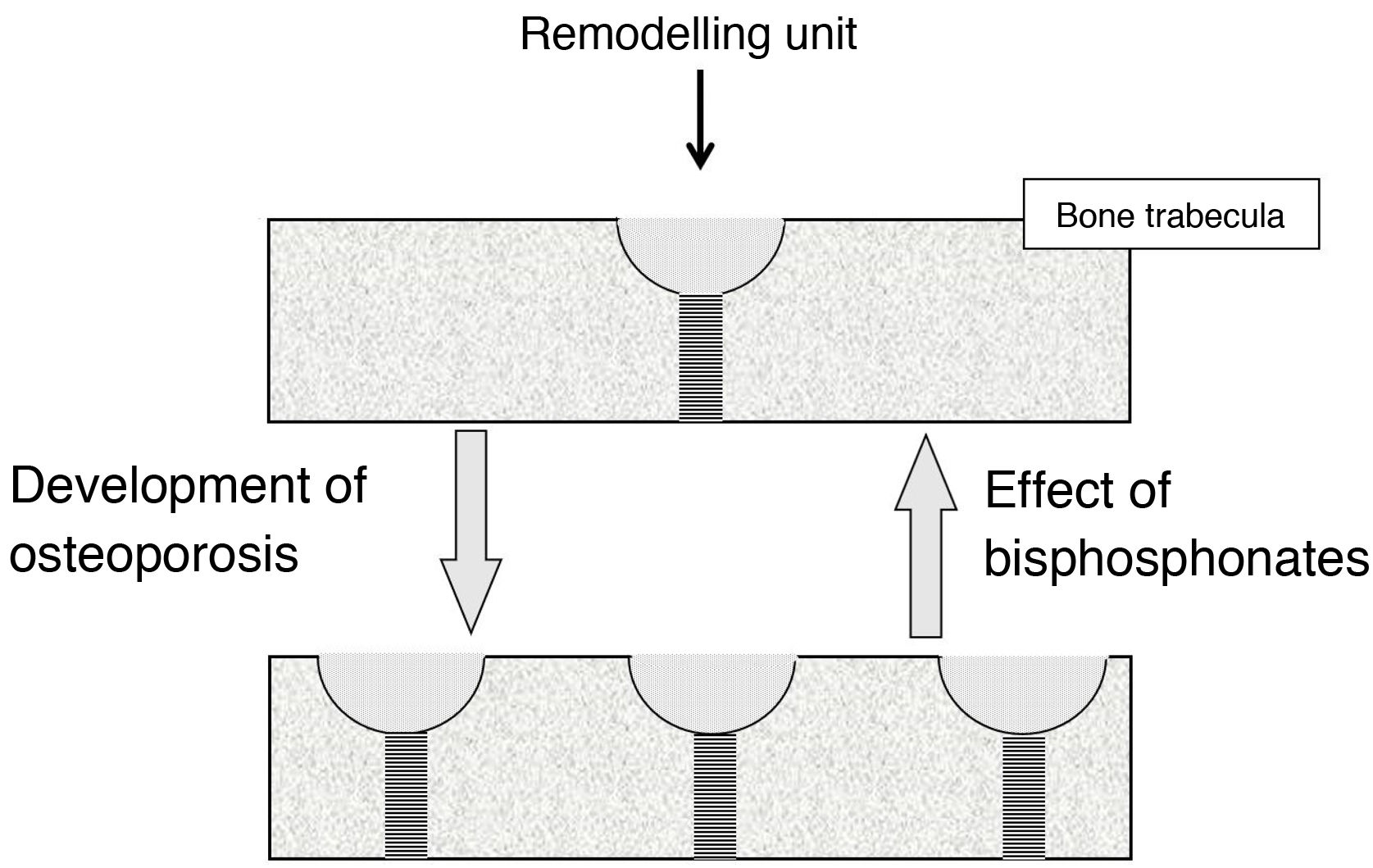
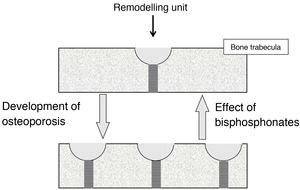
Effect on bone turnoverBone turnover is the phenomenon whereby bone is continually renewing itself. It does so because, in small foci scattered throughout the skeleton, osteoclasts act first, destroying bone, followed by osteoblasts, forming new bone. These foci constitute the remodelling units (RUs). When less bone is formed in them than is destroyed, they are said to be in negative balance. The main mechanism responsible for the development of osteoporotic fractures is the increase in bone turnover (and, therefore, the number of RUs). This increase promotes 2 types of bone loss: reversible and irreversible. The irreversible is due to a negative RU balance. Reversible is due to the fact that the opening of new RUs leads to the formation of spaces devoid of bone, which cease to exist when the unit closes. In trabecular bone, moreover, RUs weaken the bone tissue because they give rise to “stress concentrators”, points where the trabeculae are thinner.
BPs reduce fractures because, by inhibiting osteoclasts, they slow down bone turnover, reducing the number of RUs2. This involves a recovery of reversible bone loss, with a consequent increase in bone mass and decrease in stress concentrators (Fig. 1). BPs cannot correct irreversible loss.
The recovery of reversible bone loss translates into an increase in BMD measured by DXA of 4–6% during the first 12–18 months. Afterwards, the increase continues for another 3–5 years, with much less intensity. This second increase is due to an increase in secondary mineralisation (as osteons renew more slowly, they have more time to mineralise) and does not represent an increase in bone mass, although it may contribute to reinforcing bone resistance. Table 1 shows the increases in BMD described in the main studies of N-BP at 3 years. Studies over 10 years with alendronate11 indicate that, between 5 and 10 years, BMD continues to increase slowly in the spine, while it stabilizes in the hip. The mechanism responsible for the increase in the spine is unknown, and several hypotheses have been put forward2 (longer mineralisation, return of bone mass to its homeostatic level, some bone-forming stimulus10); an artefact component due to vertebral osteoarthritis cannot be ruled out. Maintenance in the hip would translate to a bone balance of 0 in the RUs, perhaps related to a decrease in the movement of osteoclasts, and less probably with a possible stimulating effect on osteoblasts.
Increase in bone mineral density by aminobisphosphonates compared to placebo 3 years after administration.
| Lumbar spine, % | Femoral neck, % | Total hip, % | |
|---|---|---|---|
| Alendronate | |||
| Phase III trials13a | 8.8 | 5.9 | – |
| FIT 114b | 6.2 | 4.1 | 4.7 |
| FIT 215c | 6.6 | 4.6 | 5.0 |
| Risedronate | |||
| VERT-NA17d | 6.5 | 2.8 | – |
| VERT-MN18e | 5.9 | 3.1 | – |
| HIP19f | – | 3.4 | – |
| Ibandronate | |||
| BONE21g | 2.2 | 3.4 | 4.1 |
| Zoledronate | |||
| HORIZON-PFT24h | 6.7 | 5.1 | 6.0 |
| HORIZON-RFT25i | – | 4.3 | 6.4 |
The numbers in the superscripts indicate the literature citation; the letters indicate the doses administered.
BONE: Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe; FIT: Fracture Intervention Trial; HIP: Hip Intervention Program; HORIZON: Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly; iv: intravenous route; MN: multinational; NA: North American; PFT: Pivotal Fracture Trial; RFT: Recurrent Fracture Trial; VERT: Vertebral Efficacy With Risedronate Therapy; po: per os (latin: orally).
The increase in BMD produced by BPs depends partly on their ability to decrease turnover and partly on the degree of turnover prior to their administration (the greater the number of active RUs, the greater the number of active RUs that can be closed). The latter determines that the increase in BMD in trabecular bone is greater than in cortical bone, as turnover is greater in the former. This fact, together with the closure of the stress concentrators, located in the trabecular bone, means that it is in the trabecular bone that BPs have the greatest anti-fracture efficacy. The decrease in stress concentrators explains why vertebral fractures decrease with modest increases in BMD.
The decrease in turnover produced by BPs is not unlimited2. After the initial decrease it stabilises, which contradicts the hypothesis formulated at the time that BPs could lead to a “frozen” bone, unable to renew itself.
The changes in BTMs with N-BPs were described in their main clinical trials (CTs). However, given the lack of homogeneity between them, and given that some are no longer in use, we have considered it preferable to collect the data recently published by Eastell et al.12. These authors have had access to the determination of C-terminal telopeptide of type 1 collagen (CTX) and amino-terminal propeptide of type 1 procollagen (P1NP) in the samples from the various trials and have reported the changes at the sixth month of treatment (in some cases where CTX or P1NP values were not available, they were calculated indirectly from alkaline phosphatase). Table 2 shows the decreases with N-BPs compared to the placebo group.
Effect of aminobisphosphonates on P1NP and CTX 6 months after administration.
| P1NP, % | CTX, % | |
|---|---|---|
| Alendronate | ||
| Phase III trials | 32.9 | 26.3 |
| FIT 1 | 29.5 | 21.9 |
| FIT 2 | 30.4 | 24.0 |
| Risedronate | ||
| VERT-NA | 30.5 | 24.4 |
| VERT-MN | 31.2 | 24.9 |
| HIP | 34.0 | 23.9 |
| Ibandronate | ||
| BONE | 30.0 | 23.9 |
| Zoledronate | ||
| HORIZON-PFT | 34.2 | 41.2 |
| HORIZON-RFT | 28.7 | 23.0 |
BONE: Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe; CTX: C-terminal telopeptide of collagen type 1; FIT: Fracture Intervention Trial; HIP: Hip Intervention Program; HORIZON: Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly; MN: multinational; NA: North American; PFT: Pivotal Fracture Trial; P1NP: amino-terminal propeptide of procollagen type 1; RFT: Recurrent Fracture Trial; VERT: Vertebral Efficacy With Risedronate Therapy.
Source: Eastell et al.12
The relationship between these changes and the decrease in fractures leads the authors to conclude that these changes explain a large proportion of the decrease in vertebral fractures (>50%), but not in non-vertebral or hip fractures. This is consistent with the previously mentioned idea of a greater effect of BPs on vertebral fractures.
Demonstration of the anti-fracture effect of bisphosphonates and approval of their useOral BPs have poor intestinal absorption (<1%), which is interfered with by food binding to Ca++ and other cations present in food. They should therefore be administered on an empty stomach, waiting half an hour (one hour in the case of ibandronate) before ingestion. In addition, they can cause irritation of the upper gastrointestinal mucosa, so after taking them the patient should remain in an upright position to facilitate transit through the oesophagus.
AlendronateIt was the first BP approved for the treatment of osteoporosis (1995). Initially administered at 10 mg/day, this was later changed to 70 mg/week, which decreases the likelihood of gastrointestinal discomfort.
Even though it had shown efficacy in a double CT showing a reduction in vertebral fractures13, Merck designed a second CT – the Fracture Intervention Trial (FIT) – to further refine the drug’s characteristics. It was made up of 2 substudies (FIT 1 and FIT 2), published in 199614 and 199815. The first of these was conducted in patients with vertebral fracture, both of which required a BMD at the femoral neck (FN) ≤ 0.68 g/cm2 (Hologic QDR-2000). When the NHANES III study was published, it was found that this BMD corresponded to a T-score of –1.6. Many patients, therefore, did not have densitometric osteoporosis. This created confusion in the interpretation of the results. Finally, data from patients with vertebral fracture or T-score ≤ −2.5 in FN of FIT 1 and 2 together16 were published, showing a significant decrease in radiographic and clinical vertebral fractures, hip fracture and total clinical fractures. The latter was already significant at 12 months. Table 3 shows the results. In 2012, the FDA approved a 70 mg effervescent formulation.
Effect of aminobisphosphonates on the different types of fractures (RR or HR).
| Morphometric vertebral fractures | Non-vertebral fractures | Hip fractures | Clinical fractures | Clinical vertebral fractures | Wrist fractures | |
|---|---|---|---|---|---|---|
| Alendronate | ||||||
| Phase III CT13a | 0.52 (0.28−0.95) | 0.79 (0.52−1.22) | – | – | – | – |
| FIT 114b | 0.53 (0.41−0.68) | 0.80 (0.63−1.01) | 0.49 (0.23−0.99) | 0.72 (0.58−0.90) | 0.52 (0.31−0.87) | – |
| FIT 215c | 0.56 (0.39−0.80) | 0.88 (0.74−1.04) | 0.79 (0.43−1.44) | 0.86 (0.73−1.01) | – | 1.19 (0.87−1.64) |
| FIT 1 + 2,T < −2.5 or vertebral fracture16d | 0.52 (0.42−0.66) | 0.64 (0.51−0.80) | 0.47 (0.26−0.79) | 0.70 (0.59−0.82) | 0.55 (0.36−0.82) | 0.70 (0.49−0.98) |
| Risedronate | ||||||
| VERT-NA17e | 0.59 (0.43−0.82) | 0.60 (0.39−0.94) | – | – | – | – |
| VERT-MN18f | 0.51 (0.36−0.73) | 0.67 (0.44−1.04) | – | – | – | – |
| Total HIP19g | – | 0.8 (0.7−1.0) | 0.7 (0.6−0.9) | – | – | – |
| 70−79 yrs. | – | – | 0.6 (0.4−0.9) | – | – | – |
| ≥ 80 yrs. | – | – | 0.8 (0.6−1.2) | – | – | – |
| Ibandronate | ||||||
| BONE21h | ||||||
| Arm 2.5 mg/d | 0.38 (0.25−0.59) | – | – | – | 0.51 (p = 0.01) | – |
| Intermittent arm | 0.50 (0.34−0.74) | – | – | – | 0.52 (p = 0.01) | – |
| Zoledronate | ||||||
| HORIZON PFT24i | 0.30 (0.24−0.38) | 0.75 (0.64−0.87) | 0.59 (0.42−0.83) | 0.67 (0.58−0.77) | 0.23 (0.14−0.37) | – |
| HORIZON RFT24j | 0.54 (0.32−0.92) | 0.73 (0.55−0.98) | 0.70 (0.41−1.19) | 0.65 (0.50−0.84) | – | – |
The numbers in the superscripts indicate the literature citation; the letters indicate the doses administered.
BONE: Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe; CT: clinical trial; FIT: Fracture Intervention Trial; HIP: Hip Intervention Program; HORIZON: Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly; HR: hazard ratio; iv: intravenous route; MN: multinational; NA: North American; PFT: Pivotal Fracture Trial; RFT: Recurrent Fracture Trial; RR: relative risk; VERT: Vertebral Efficacy With Risedronate Therapy; po: per os (latin: orally).
It was the second BP approved by the FDA (year 2000) for the treatment of osteoporosis. It was initially administered at a dose of 5 mg/day. Later it was changed to a weekly regimen of 35 mg, with less gastrointestinal discomfort.
Procter & Gamble based the development of the drug on the Vertebral Efficacy With Risedronate Therapy (VERT) studies, one North American (VERT-NA)17 and one multinational (VERT-MN)18, published in 1999 and 2000, respectively. Women with vertebral fractures and T-score < −2.0 were included. The chosen doses were 2.5 and 5 mg/day, although the first was discontinued after an initial phase. Lower doses than those used with alendronate were chosen because preclinical studies indicated that risedronate was more potent. Vertebral and non-vertebral fractures significantly decreased (Table 3). The decrease in the incidence of vertebral fractures in both trials was seen as early as 12 months.
Subsequently, to assess the effect on hip fracture, the Hip Intervention Program study was designed19. The doses used were 2.5 or 5 mg/day. For economic reasons, the initial design was modified, and the patients were divided into two groups: one aged 70−79 years and the other aged 80 years and over. The description of both is complex, but essentially the first was characterized by significant osteoporosis and the second by certain risk factors for hip fracture. The incidence of this fracture decreased in the first group, but not in the second (Table 3).
In 2007 the FDA approved a 75 mg formulation designed to be taken as one tablet 2 consecutive days once a month, and in 2008 a 150 mg formulation designed to be taken as one tablet monthly. Finally, in 2010 it approved a preparation with a special pharmaceutical formulation (“gastro-resistant”), designed to minimize the interaction of risedronate with food and to favour its absorption. In this preparation, 35 mg risedronate is combined in each tablet with 100 mg EDTA (which chelates Ca++ and other di- or trivalent cations from food and intestinal contents). In addition, the tablet has a pH-sensitive enteric coating, which prevents its disintegration in the stomach and delays its dissolution down to the small intestine. Increases its bioavailability and allows the drug to be administered before or after breakfast20.
IbandronateIt was developed with the idea of administering a BP with long intervals between doses. The FDA had approved its daily oral administration in 2003. In 2004, the Hoffmann-La Roche-sponsored BONE21 study was published, comparing patients treated with 2.5 mg/day; patients treated with 20 mg every other day for a total of 12 doses, in 3-month cycles; and patients assigned to placebo. The incidence of vertebral fractures –morphometric and clinical– was significantly reduced in the first 2 groups (Table 3). Non-vertebral fractures did not decrease. Subsequently, the MOBILE22, study, with BMD as the outcome measure, found that 150 mg administered once a month orally is not only not inferior, but superior to 2.5 mg/day, with the FDA approving this regimen in 2005. Later, the DIVA23 study compared daily oral administration with intravenous administration (2 mg/2 months or 3 mg/3 months) and found the latter to be superior. The FDA approved intravenous ibandronate in 2006.
ZoledronateIt was selected for development by Ciba-Geigy in 1987, at a time when intravenous administration was a novelty. It based its development on the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) trial, the Pivotal Fracture Trial (PFT) and the Recurrent Fracture Trial (RFT). The first24 selected patients with a T-score in FN ≤ −2.5, with or without vertebral fractures, or a T-score of ≤ −1.5 together with at least 2 mild vertebral fractures or a moderate vertebral fracture. Patients were stratified into 2 groups: group 1 (for vertebral fracture assessment) could not receive any other anti-osteoporotic medication, while group 2 (for hip fracture assessment together with group 1) could receive calcitonin, raloxifene, hormone replacement therapy or tibolone. Administration of 5 mg iv annually for 3 years decreased the incidence of morphometric vertebral, hip, non-vertebral, clinical vertebral and any clinical fractures (Table 3). A slight increase in the incidence of atrial fibrillation was observed. This work was published in 2007 and the FDA approved the use of zoledronate that same year.
The HORIZON RFT25 was conducted in patients with hip fracture. A reduction in clinical fractures (significant at 12 months), clinical vertebral fractures and non-vertebral fractures was confirmed. Hip fractures were not significantly reduced (Table 3). An unexpected finding was a 28% decrease in mortality in the zoledronate arm (p = 0.01).
Recently, an EC26 has been performed in osteopenic women over 65 years of age involving the administration of 4 infusions of 5 mg zoledronate at 18-month intervals. Overall, fragility fractures were significantly reduced (hazard ratio [HR] 0.63), and specifically symptomatic, morphometric vertebral and non-vertebral fractures. Mortality showed a decreasing trend (HR 0.65; p = 0.08).
Comparative studiesNo head-to-head clinical trials have been conducted comparing different N-BPs. In contrast, several network meta-analyses have been carried out, without reaching uniform results27–30. An observational study published in 2007 concluded that risedronate reduced the risk of hip and non-vertebral fractures more than alendronate31, but another conducted 2 years later using the same methodology failed to prove this32. Given the greater bioavailability of gastroresistant risedronate, it is worth noting that a recent retrospective real-world study comparing it with other oral BPs has found it to reduce osteoporotic fractures to a greater extent (17% at any site; 29% at the spine)33.
Adverse reactions and beneficial effectsAdverse reactionsThe adverse reactions of BPs have been extensively reviewed in other publications. We will only make some comments regarding the atypical femur fracture (AFF) and osteonecrosis of the jaw (ONJ), as they have influenced the development of the “drug holidays” concept.
Atypical femur fractureNumerous papers have been published on its association with BPs, with information that is not always consistent. We believe that the results of the recent study by Black et al.34 on almost 200,000 women should be taken as a reference at this stage. They are collected in Table 4.
Epidemiology of atypical fracture of the femur. Comparison of provoked atypical femur fractures and prevented fragility fractures during bisphosphonate administration.
| Increase in incidence with administration time | |||||
|---|---|---|---|---|---|
| Time | Reference(< 0.25A) | From 0.25 yrs. < 3 yrs. | From 3 yrs. < 5 yrs. | From 5 yrs. < 8 yrs. | ≥ 8 yrs. |
| Adjusted RH | – | 2.54 (0.79−8.4) | 8.86 (2.79−28.20) | 19.88 (6.32−62.49) | 43.5 (13.70−138.15) |
| Absolute no. AFF/105 person-years | 0.07 | 0.56 | 2.54 | 6.06 | 13.30 |
| Decrease in incidence with time of administration | ||||
|---|---|---|---|---|
| Time | Reference (<0.25 yrs.) | >0.25−1.25 yrs. | >1.25−4 yrs. | >4 yrs. |
| Adjusted RH | – | 0.52 (0.37−0.72) | 0.21 (0.13−0.34) | 0.26 (0.14−0.48) |
| Absolute No. AFF/105 person-years | 4.50 | 1.81 | 0.62 | 0.47 |
| Fragility fractures prevented by AFF occurred (Caucasian population) | ||
|---|---|---|
| After 3 years | After 5 years | |
| Hip fractures | 75 | 36 |
| Clinical fractures | 270 | 170 |
AFF: atypical femur fracture.
Source: Black et al.3,4
The determining mechanisms of AFF are not exactly known. They seem related to BP interference in stress fracture repair processes. The use of glucocorticoids and femoral bowing are risk factors.
Osteonecrosis of the jawIt is very rare in patients treated with oral BP for osteoporosis. Chiu et al.35 reported a cumulative incidence that went from 0.04% in the first year of treatment to 2.14% at 8 years. It usually follows a dentoalveolar manipulation. Inhibition of bone remodelling has been linked to its pathogenesis. A possible antiangiogenic effect of BPs is also considered36, with trophic impairment in oral mucosa. Predisposing factors are poor oral hygiene and the use of glucocorticoids. Discontinuation of treatment a few months before dental surgery is thought to be useless, but some advocate it because of the theoretical effect of the drug on the mucosa. More invasive surgical procedures should be avoided.
Beneficial effectsThe most important is a decrease in mortality, not definitively confirmed yet, but supported by numerous studies. Lower mortality from cancer (myeloma, breast, colon), cardiovascular disease and pneumonia37 seem to contribute to this.
Relationship of BPs with anabolic drugsSequential treatmentBone-forming drugs after bisphosphonatesAdministration of BP before teriparatide attenuates its effect on BMD increase38, particularly in the hip, where it may transiently decrease slightly. However, the VERO39 study showed that attenuation does not result in a decrease in the anti-fracture effect. The STRUCTURE study shows that prior administration of BP attenuates the efficacy of romosozumab in increasing BMD but does not prevent it40. It can be concluded that the administration of bone-forming agents after PB should not be ruled out when considered appropriate.
BP after bone-forming agentsAfter withdrawal of any bone forming agent, an antiresorptive agent should be administered. The efficacy of N-BPs is widely demonstrated41–43.
CombinationsThe effect of the combination of BP with PTH on BMD has been studied, but not on fractures. The combination can be considered from the beginning of the treatment, or by adding teriparatide to previously established BP treatment. In relation to the first possibility, a first study44 showed that the combination of teriparatide with alendronate did not improve the effects of the former. However, a later one with zoledronate45 concluded that the combination provides a favourable outcome, at least for the first 6 months. Regarding studies in which teriparatide is added to previous BP treatment, negative46 and positive47 results have again been reported.
It could be concluded that, in general, the association of BP and bone-forming agents is not indicated but should not be ruled out if it is considered potentially useful in a specific situation.
Usefulness and interest of drug holidaysShortly after BPs were introduced on the market, questions were raised as to whether they would have a limited effect duration and whether they would have to be discontinued after a few years of administration. Two studies have addressed this issue. The first is the FLEX study48, FIT extension, in which women who had received alendronate for 5 years were randomized to continue alendronate or go on placebo for another 5 years. The second is an extension of the HORIZON PFT49, in which women who had received zoledronate for 3 years were randomized to continue zoledronate or switch to placebo for another 3 years. In both, it is found that patients who continue with the drug develop fewer vertebral fractures (clinical in the first case [relative risk 0.45; 0.24−0.85], morphometric in the second [odds ratio 0.51; 0.26−0.95]). An post hoc analysis also noted a beneficial effect on non-vertebral fractures50.
In both studies it was observed that there is a sub-population of patients where after 5 (alendronate) or 3 years (zoledronate) of treatment the BP may be discontinued because the patient has a low fracture risk, and the treatment does not further decrease this risk. Once discontinued, the low-risk status is maintained for a certain period of time, as the BP continues to act inside the bone. Later, as this is eliminated, the risk of fracture increases again, and the treatment must be reintroduced. The interval of time without treatment is known as “drug holidays”.
The possibility of suspending BP in certain patients without increasing the incidence of fractures is an enormous advantage, due to the fact already mentioned that its prolonged administration favours the occurrence of AFF and ONJ.
The criteria for establishing a “drug holiday” regimen have not been precisely defined, but logically they must be derived from what was observed in the FLEX study and the HORIZON extension. It could be said that there are 2 essential criteria and 2 recommended criteria. According to the first48,49, a “drug holiday” regimen should not be established if the patient has a hip T-score < −2.5 or if the patient experiences a fracture during treatment. According to the latter, it is advisable to avoid drug holidays if the patient is over the age of 75 or predominantly has vertebral or hip fractures (in addition, of course, to other risk factors such as glucocorticoid treatment)51,52.
The threshold of −2.5 T, despite being the first to be identified as discriminating between those who needed to continue with treatment and those who did not, has been widely debated, and a proposal has been made to raise it to −2.0 or −1.5. The arguments are of a speculative nature and are mainly based on the idea that there is an inverse relationship between the T-score and the risk of fracture. Against this theoretical disagreement stands the evidence provided by the FLEX and the HORIZON extension showing that with values above −2.5 the patient no longer benefits from the treatment (but the cost, discomfort and side effects persist). In this respect it is worth mentioning the recent study by Black et al.53, according to which vertebral fractures decrease with BMD increases in total hip of 1.4%, non-vertebral fractures with increases of 2.1% and hip fractures with increases of 3.2%, figures which can be compared with those shown in Table 1 for the different N-BP.
The optimal drug holiday duration is not well established. It should vary according to the BP (with those with lower adherence they should be shorter) and the evolution of BMD and BTM (although the latter is poorly defined). It has been pointed out that it could be one year for risedronate, 2–3 for alendronate and 3–5 for zoledronate.
In relation to drug holidays, one more comment should be made regarding zoledronate. After the first extension of the HORIZON-PFT, a second extension was carried out for another 3 years, during which some patients continued to be treated and others were not54. No significant differences were observed in BMD and BTM between the 2 groups (the sample was already too small to assess fractures). It was concluded that 9 years of treatment is not superior to 6 years of treatment. Two conclusions could be drawn from this: a) holidays would be virtually obligatory after 6 years of zoledronate, and b) 6 injections cover practically a decade of treatment.
A number of observational studies have tried to add information about drug holidays, but on the whole – probably because of the biases involved – they have not provided anything significant55–58.
Initial and long-term treatment with bisphosphonatesIndicationBPs may be indicated in all patients with osteoporosis at some point in their condition. As part of a sequential treatment, they can be used when withdrawing bone-forming agents, as well as when withdrawing SERMs in women initially treated with them. In all other patients, oral BPs will be the drug of choice if there are no problems that contraindicate this route, particularly if their age is – as a reference point – below 75 years59. If it is advisable to avoid the oral route or the patient is older, it is preferable to start treatment with an injectable antiresorptive agent, in which case zoledronate (with anti-fracture efficacy similar to its alternative, denosumab) could also be used24,60. This approach is consistent with that contained in the recent version of the SEIOMM Guidelines59.
Long-term treatmentOral BPsIts administration must be maintained for the first 5 years of treatment unless there is a contraindication. Subsequently, the establishment of drug holidays should be considered. If they are accepted, after them the treatment is reintroduced, and another vacation period may be considered later. In this way, the patient can stay in BP-holidays-BP cycles, etc., for the rest of his/her life. If the patient does not meet the criteria for a holiday, treatment is maintained, with the possibility of a holiday being assessed every 2–3 years. If after 10 years of treatment it is still not eligible for a holiday, the risk of complications (AFF) should be assessed. If this is high, temporary substitution of BP with teriparatide could be considered. In any case, the evidence we have after 10 years is practically non-existent.
ZoledronateThe establishment of drug holidays after 3 years of treatment should be assessed with this drug. If the patient is treated for 6 years, it is believed that drug holidays can be established after that, in any case, due to what was previously mentioned. If the risk of osteoporotic fracture is considered to be high, teriparatide could be administered temporarily before returning to zoledronate.
ConclusionDuring the last 25 years, N-BPs have been the most widely used drugs in the treatment of osteoporosis. With an anti-resorptive mechanism of action, its efficacy is beyond any doubt. Their great appetite for hydroxyapatite allows a type of therapeutic regimen that is only possible with them (“drug holidays”). They are useful both in initial treatment and in long-term treatment, sometimes as part of sequential therapeutic formulas. All this, together with their good tolerance, points to a bright future for them.
FundingThis work has been funded with a grant from Theramex Healthcare Spain. Theramex has had no involvement in the writing of the manuscript.
Conflict of interestsJ. González Macías has received funding for conferences and honoraria for presentations or chairing sessions from Lilly, UCB-Amgen, Menarini, Theramex and Gedeon Richter. He has participated in clinical trials sponsored by UCB-Amgen and FAES.
JM Olmos has received travel grants and speaking fees from UCB-Amgen, Eli Lilly, Stada, Gedeon-Richter, and Grünenthal. He has participated in clinical trials sponsored by UCB-Amgen and FAES and has served on advisory boards for UCB, Stada, and Gedeon-Richter.