When the natural immune response does not control the replication of the SARS-CoV-2 coronavirus, it induces the production of macrophages and granulocytes with the consequent massive release of CD4 + T cells that produce IL-6 and other pro-inflammatory cytokines, resulting in lung tissue damage.1,2 Among other systemic conditions, this "cytokine storm" can cause severe pulmonary and vascular endothelial tissue damage, and consequently acute respiratory failure syndromes and prothrombotic conditions, which are the main causes of mortality.3,4
An anti-inflammatory therapy with the capacity to stop this cytokine hyperactivation, associated with anticoagulant therapy, could be key in patients with severe COVID-19 pneumonia. This challenge was faced by researchers from the University of Shanghai, intravenously infusing a suspension of mesenchymal cells (MSC), reporting rapid clinical, radiological and laboratory improvements, comparing them with those of the untreated control group5; effects attributable to the massive release of anti-inflammatory and pro-regenerative cytokines from these cells that are trapped in the pulmonary capillaries.
Worldwide, more than 30 clinical trials are being developed following this approach, four have been proposed in Spain. Due to the interest generated, it is considered appropriate to disclose the concept, the foundations and the procedure of this innovative therapy in the experimental phase, reporting the experience of what we consider to be the first case of COVID-19 treated in our country using intravenous mesenchymal stem cell therapy.
On 22nd March 2020, a member of our research team developed general malaise, fever, and unproductive cough, associated with anorexia linked to hyposmia and hypogeusia. He started home treatment with hydroxychloroquine 200 mg/8 h and azithromycin 500 mg/24 h.
The increase in symptoms, with a temperature above 39 °C, motivated his hospital admission on 26th March. The examination showed a discrete oxygenation anomaly (baseline Sat. O2 92%), very significant neutrophilic leukopenia with lymphocytosis; CRP 19.6 mg/dL, ESR 86 mg/dL; ferritin 2,512 ng/mL; increased transaminases > 3 x LSN; CKD-EPI 68 mL/min and the ions at the limit of normal. The X-ray showed a bilateral low intensity pulmonary focus suggestive of viral infection and the PCR-SARS-CoV2 confirmed the positive result. Treatment was maintained with hydroxychloroquine 200 mg/12 h, azithromycin 500 mg/24 h and paracetamol 1 g/8 h, and was supplemented with enoxaparin sodium 60 mg/day, fluid therapy and oxygen therapy through nasal prongs at 3 L/min.
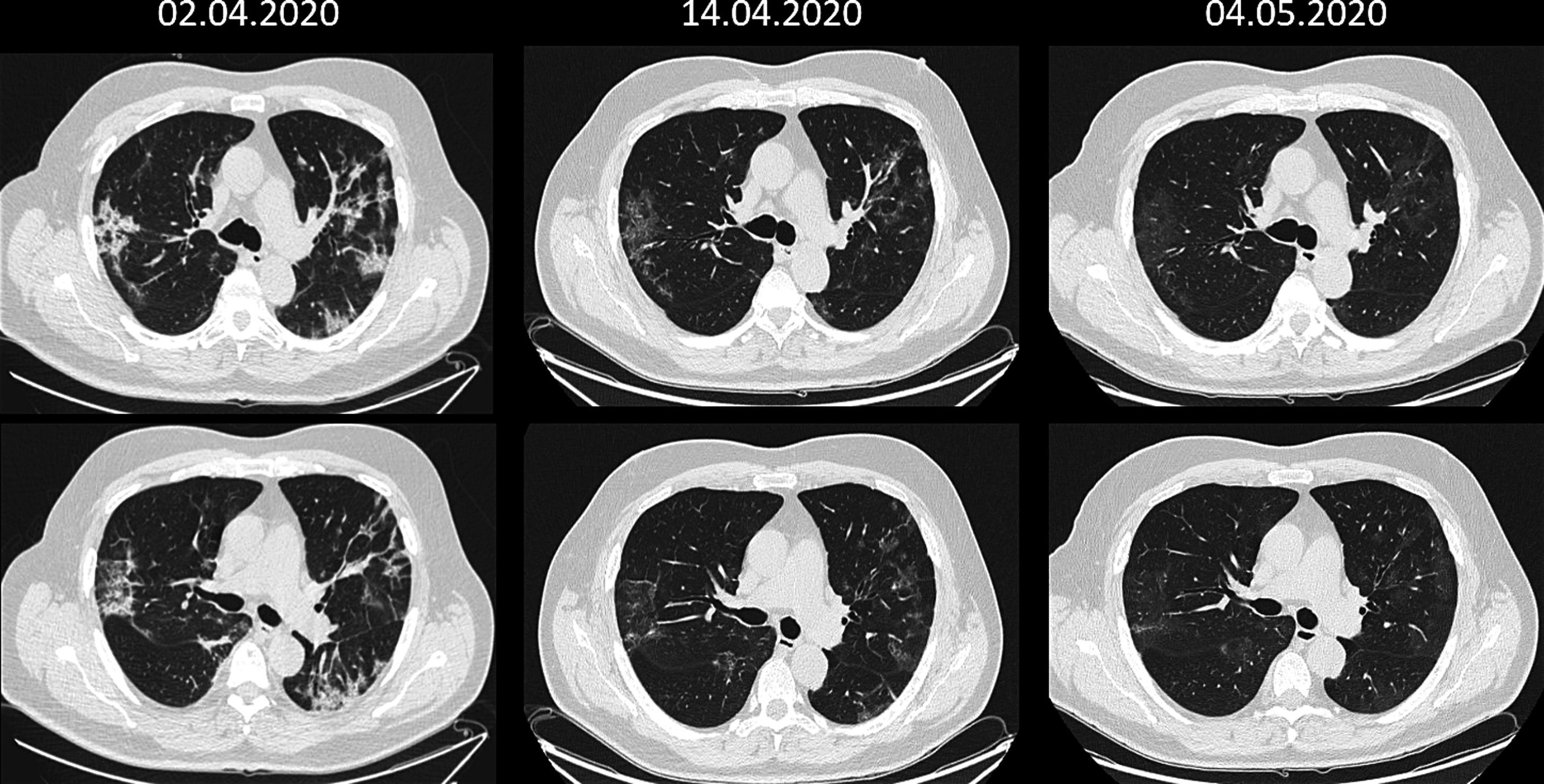
On 31 March the patient was still suffering from severe symptoms with dyspnoea and abnormal laboratory values: Hb 12 g/dL; leukopenia with very significant lymphocytosis; elevated transaminases and alkaline phosphatase; D-dimer 307 µg/L (<230 Ref.), ferritin 2,283 ng/mL and CRP 9.5 mg/dL. Computed tomography (CT) showed a new ground glass peripheral bronchopneumonic image at the base of the upper lobe of the left lung and persistence of peripheral involvement of the right lung parenchyma and vascular thickenings, more evident than in previous radiographs.
Given that our research team has extensive experience in cell therapy, on 2nd April, with prior authorization and under the control of the Spanish Agency of Medicines and Medical Devices, we infused intravenously a dose of 80 × 10 E6MSC of allogeneic bone marrow (1 × 10 E6MSC/kg of weight) in suspension of 100 cc saline solution administered at a rate of 40 drops/min. The MSCs were isolated and cultured under the correct manufacturing standards for clinical application (GMP) at the Institute of Molecular Biology and Genetics of Valladolid.
We did not experience any complications or adverse effects. At 24 hours, the patient was afebrile with generalized improvement in symptoms and biochemical values, with persistent difficulties in intake due to hyposmia and hypogeusia, but which improved notably after 48 hours. From the fifth day all biochemical parameters were within normal range and the clinical symptoms related to the coronavirus had disappeared, maintaining the loss of appetite and tiredness. CT showed a clear improvement in the right pulmonary focus and the left lung abnormality. Hospital discharge was granted on 8th April, maintaining anticoagulant treatment for one month.
PCR-SARS-CoV-2 became negative as of 6th April and after a month the leukocyte formula and the rest of laboratory values were normal, showing negative IgM antibodies (0.7) and positive IgG (8.5 +++) against SARS-CoV-2. Lung CT scan was almost normal (see CT Fig. 1) and complete lung function tests were strictly normal: FVC 4.35 L (97% ref.), FEV1 3.64 L (105% ref.), index 70%, TLC 6.10 L (90% ref.), DLCO 25.7 mL/min/mmHg (95% ref.).
Even assuming the role that natural immunity might have played, with the beneficial effects of hydroxychloroquine being disputed, we speculate that the immunomodulatory and pro-regenerative effect of intravenous administration of high-dose expanded MSC may have been primarily responsible for the favourable clinical, biological and radiological course of the case. The clinical trials already underway should provide quality data that allow us to advance in the knowledge of this innovative therapeutic proposal.
Authorization of treatment with Cell TherapyAuthorization for Compassionate Use from the Spanish Agency of Medicines and Medical Devices (AEMPS).
Informed consentInformed consent was obtained from the patient.
Please cite this article as: Soler Rich R, Rius Tarruella J, Melgosa Camarero MT. Abordaje terapéutico del SARS-CoV-2 (COVID-19) mediante células mesenquimales de médula ósea alogénica expandidas. Conceptos a propósito de un primer caso en España. Med Clin (Barc). 2020;155:318–319.