To reach the diagnosis of giant cell arteritis (GCA), signs, symptoms, laboratory tests, imaging findings, and occasionally anatomopathological results from temporal artery biopsy are evaluated. This study describes the results of an algorithm analysis based on clinical and ultrasound evaluation of patients with suspected GCA, highlighting its diagnostic utility by contrasting its use in different clinical suspicion scenarios.
MethodProspective multicenter study evaluating patients referred with suspected GCA through a preferential circuit (fast track), grouping them according to low or high clinical suspicion of GCA. Each of these scenarios is evaluated by biopsy and ultrasound for all patients, resulting in positive, indeterminate, or negative outcomes, yielding six possible groups. Potential areas of improvement are explored, emphasizing that, following a negative or indeterminate ultrasound, 18-FDG-PET-CT could be recommended. We analyze the results and application of a diagnostic algorithm, confirming its efficiency and applicability based on whether there is high or low clinical suspicion.
ResultsSixty-nine patients (41 in the high suspicion group and 28 in the low suspicion group). There were 41 new diagnoses of GCA, 35 in the high suspicion group and 6 in the low suspicion group. Using ultrasound alone, the initial algorithm has an overall diagnostic efficiency of 72.5%, which improves to 80.5% when including 18F-FDG-PET/CT. The negative predictive value of ultrasound in patients with low clinical suspicion is 84.6%, and the positive predictive value of ultrasound in patients with high suspicion is 100%, improving sensitivity from 57.1% to 80.8% with 18F-FDG-PET/CT in this scenario. Temporal artery biopsy was performed on all patients, with no differences in sensitivity or specificity compared to ultrasound. In cases where all three tests—ultrasound, biopsy, and 18F-FDG-PET/CT—are performed, sensitivity increases to 92.3% in patients with high clinical suspicion.
ConclusionIn situations of high clinical suspicion, the algorithm provides sufficient information for the diagnosis of GCA if ultrasound is positive. A negative ultrasound is sufficient to rule out the diagnosis in the context of low clinical suspicion. 18-FDG-PET-CT may be useful in patients with high suspicion and negative or indeterminate ultrasound results.
Para llegar al diagnóstico de ACG, se evalúan signos, síntomas, pruebas de laboratorio, hallazgos de pruebas de imagen y en ocasiones los resultados anatomopatológicos de la BAT. El presente estudio describe los resultados del análisis de un algoritmo basado en la clínica y ecografía de los pacientes con sospecha de ACG, destacando su utilidad diagnóstica, al contrastar su utilización en diferentes escenarios de sospecha clínica.
MétodoEstudio prospectivo multicéntrico que evalúa mediante un circuito preferente (fast track) a los pacientes derivados con sospecha de ACG, para la realización de una ecografía de arterias temporales y axilares, agrupándose según: baja o alta sospecha clínica de ACG. Cada uno de estos supuestos es evaluado por ecografía, el resultado puede ser positiva, indeterminada o negativa, obteniendo 6 posibles grupos diferentes. Se exploran las posibles áreas de mejora, tras una ecografía negativa o indeterminada. En algunos pacientes se realiza un 18F-FDG-PET/TC. Analizamos los resultados y la aplicación de un algoritmo diagnóstico, confirmando su eficiencia y su aplicabilidad en según si hay una alta o baja sospecha clínica.
ResultadosSesenta y nueve pacientes (41 en el grupo de alta sospecha y 28 en el de baja sospecha clínica). Hubo un total de 41 diagnósticos nuevos de ACG, 35 en el grupo de alta sospecha y 6 en el de baja sospecha. El algoritmo inicial tiene una eficiencia global de diagnóstico de 72,5%, si solamente se utiliza la ecografía, que al incluir al 18F_FDG-PET/TC, mejora a 80,5%. El valor predictivo negativo de la ecografía en los pacientes que presentan una baja sospecha clínica es del 84,6% y el valor predictivo positivo de la ecografía en los pacientes con una sospecha alta es del 100%, mejorando en este escenario la sensibilidad del 57,1% a 80,8% si hacemos un 18F-FDG-PET/TC. La BAT, se realizó a todos los pacientes, sin encontrar diferencias en la sensiblidad o especificidad respecto a la ecografía. En el caso de tener las tres pruebas realizadas, ecografía, biopsia y 18F-FDG-PET/TC, la sensibilidad aumenta a 92,3% en los pacientes que presentan una alta sospecha clínica.
ConclusiónEn situaciones de alta sospecha clínica, el algoritmo proporciona suficiente información para el diagnóstico de ACG si la ecografía es positiva. En el contexto de baja sospecha clínica, una ecografía negativa es suficiente para descartar el diagnóstico. El 18-FDG-PET-TC puede ser de utilidad en aquellos pacientes con alta sospecha y ecografía negativa o indeterminada.
Giant cell arteritis (GCA) is the most common vasculitis in adulthood. Developments in imaging techniques have accelerated the diagnosis, bypassing the once mandatory requirement of a widely accepted positive temporal artery biopsy (TAB) result. In addition, they provide a better understanding of the different clinical forms of presentation and the extent of the disease.1,2
To arrive at an accurate diagnosis of GCA, assessment is based on symptoms, signs, laboratory tests, imaging findings or histopathology of the patient.3–5 The classical diagnostic approach includes superficial TAB, considered the gold standard, and other imaging tests, and a pre-test clinical score in the context of clinical suspicion helps and increases the cost-effectiveness of the diagnostic process.6
The utility, reproducibility and sensitivity to change to expert, Doppler mode, temporal and axillary artery ultrasound of (TAAUS) as a diagnostic tool in order to avoid TAB is widely accepted.7–9 However, 18F-fluorodeoxyglucose-labelled positron emission computed tomography (18F-FDG-PET/CT) may help in cases where the signs/symptoms or TAAUS is negative or indeterminate.10–12
The recent ACR/EULAR 2022 classification criteria strongly support the relevance of imaging tests in the classification of GCA within the vasculitis group.13 The classification criteria are often considered as diagnostic criteria, but, depending on the clinical profile, they may complicate strict compliance, as GCA is a very heterogeneous disease. On the other hand, it should be noted that, in clinical practice, reaching a diagnosis of certainty in individual patients requires the use of additional clinical criteria beyond the classification criteria, and interpretation is sometimes based on the clinical experience of the practitioner.
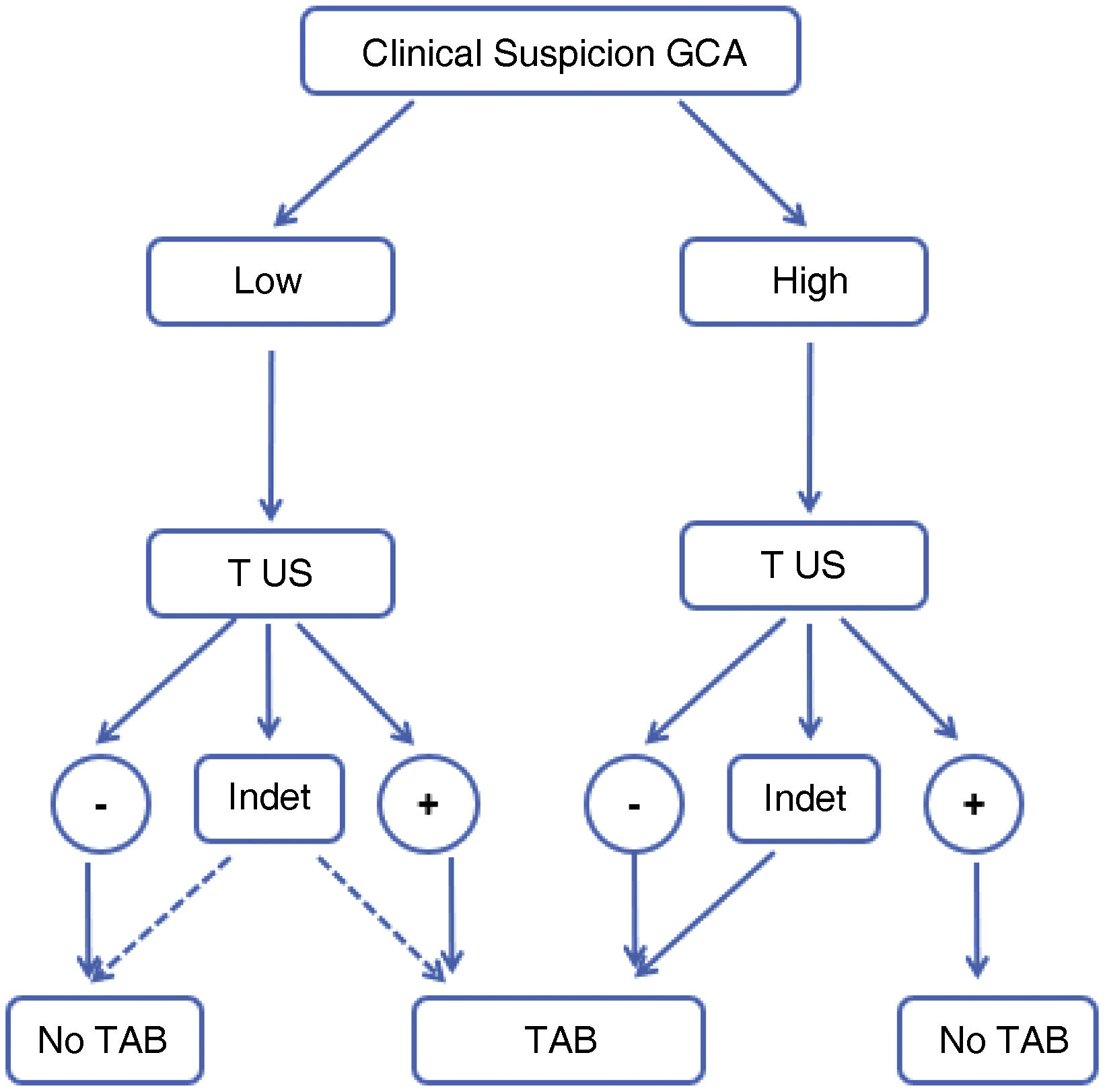
The present study describes the results of the analysis of a previously published algorithm (Fig. 1),14 highlighting its diagnostic utility, contrasting its use in different scenarios of clinical suspicion and exploring areas for improvement, classifying those patients with negative or indeterminate TAAUS who may benefit from subsequent 18F-FDG-PET/CT.
Original algorithm proposed for the diagnosis of GCA in daily clinical practice. High suspicion is defined as the presence of any of the following clinical manifestations: 1)exclusive head symptoms (recent onset headache, jaw claudication or visual disturbances); 2)with polymyalgia rheumatica, according to EULAR/ACR 2012 criteria; 3)unspecific febrile syndrome, once infectious causes have been ruled out and screening for neoplasms is negative; 4)ictus, with no relevant cardiovascular history or findings of atherogenic aetiology after targeted study. Low suspicion: patients with suspicion due to symptoms other than those mentioned in the previous section (e.g., headache screening, anaemia, elevated acute phase reactants).14
GCA: giant cell arteritis; US: temporal and axillary arteries ultrasound; INDET: “indeterminate” ultrasound result, defined as halo sign in 1 or 2 or less of the 8 arteries examined; NO TAB: do not perform temporal artery biopsy; PET-CT: 18F-FDG-PET/CT;– : negative result; +: positive result.
Prospective study, conducted between September 2019 and January 2023, in three hospitals in the metropolitan area of Barcelona.
Ethical aspectsThe protocol was approved by the centre’s Ethics Committee with code: PR313/19; ISC 19/66. The study was conducted according to the standards of Good Clinical Practice and subject to the ethical principles of the Declaration of Helsinki.
PopulationConsecutive patients referred for suspected GCA who agreed to participate were included. Patients could come from specialised outpatient clinics, hospital outpatient clinics or hospital inpatients. The different services involved in the referral were family medicine, internal medicine, rheumatology, neurology and ophthalmology. The fast-track diagnostic circuit was activated, which guaranteed the completion of a TAAUS within 24–48working hours.
VariablesData were collected in an electronic case report form for subsequent analysis. All patients underwent history-taking, laboratory tests with acute phase reactants, ESR, CRP, and CBC, TAAUS in grey scale and Doppler mode, and superficial TAB. The doses and days of glucocorticoid (GC) treatment before the different complementary examinations were recorded, and a review was performed at 6months to confirm the definitive diagnosis. 18F-FDG-PET/CT was requested at medical discretion, in cases of diagnostic doubt or as a study of the extent of vascular involvement in GCA. The 18F-FDG-PET/CT result was interpreted according to the semi-quantitative visual criterion (0–3) of uptake in relation to the liver.15
The gold standard for the diagnosis of GCA was medical judgment, taking into account the TAB result or the presence of large vessel vasculitis on 18F-FDG-PET/CT.
Vascular Doppler ultrasound was performed in the common temporal arteries, their parietal and frontal branches and bilaterally in the axillary arteries (total 8 arteries) according to protocol by expert technicians (P.E., P.M., C.M. and H.C) with more than 10years of experience and accredited certification using high-end ultrasound scanners. The final diagnosis was made on the basis of medical judgement; the doctors involved in the decision making were P.E., P.M., J.N. and H.C.
Patients were grouped into two major groups, with low or high clinical suspicion of GCA, and within these two groups, they were grouped according to the Doppler TAAUS result (positive, indeterminate or negative), making a total of 6 diagnostic groups. The following definitions were used to classify patients into the corresponding groups.
- -
High clinical suspicion. Presence of any of the following clinical manifestations: 1)exclusive head symptoms (recent onset headache, jaw claudication or visual disturbances); 2)with polymyalgia rheumatica, according to EULAR/ACR 2012 criteria16; 3)unspecific febrile syndrome, once infectious causes had been ruled out and screening for neoplasms was negative; and 4) stroke, with no relevant cardiovascular history or findings of atherogenic aetiology after a targeted study.
- -
Low clinical suspicion. Patients with suspicion due to symptoms other than those mentioned in the previous section (e.g., headache screening, anaemia, elevated acute phase reactants).
- -
Positive TAAUS. Presence of halo sign, established by OMERACT, in 3 or more of the 8 arteries examined.4
- -
Indeterminate TAAUS. Halo sign in 1 or 2 of the 8 arteries explored.
- -
Negative TAAUS. No pathological findings.
For statistical analysis, the results of the descriptive variables are presented with measures of central tendency. Sensitivity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV) and overall test efficiency, positive (LR+) and negative (LR–) likelihood ratio of Doppler TAAUS and 18F-FDG-PET/CT, with respect to definitive diagnosis, were calculated. Also, in a complementary, post hoc manner, the Southend Probability Pretest Score was calculated for our cohort. This index is based on Bayesian probability and has proven to be effective in stratifying referrals to fast-track clinics. Initially, a PreTest Probability Score (PTPS) is calculated, where the patient may have a low (LR), intermediate (IR) or high risk (HR) probability of GCA, while ruling out diagnoses that may be mistaken for a diagnosis of LCA.6 Ultrasound findings are then interpreted in their clinical context. TAAUS can be positive or negative, leading to a diagnosis of probable, possible, doubtful or unlikely GCA.6
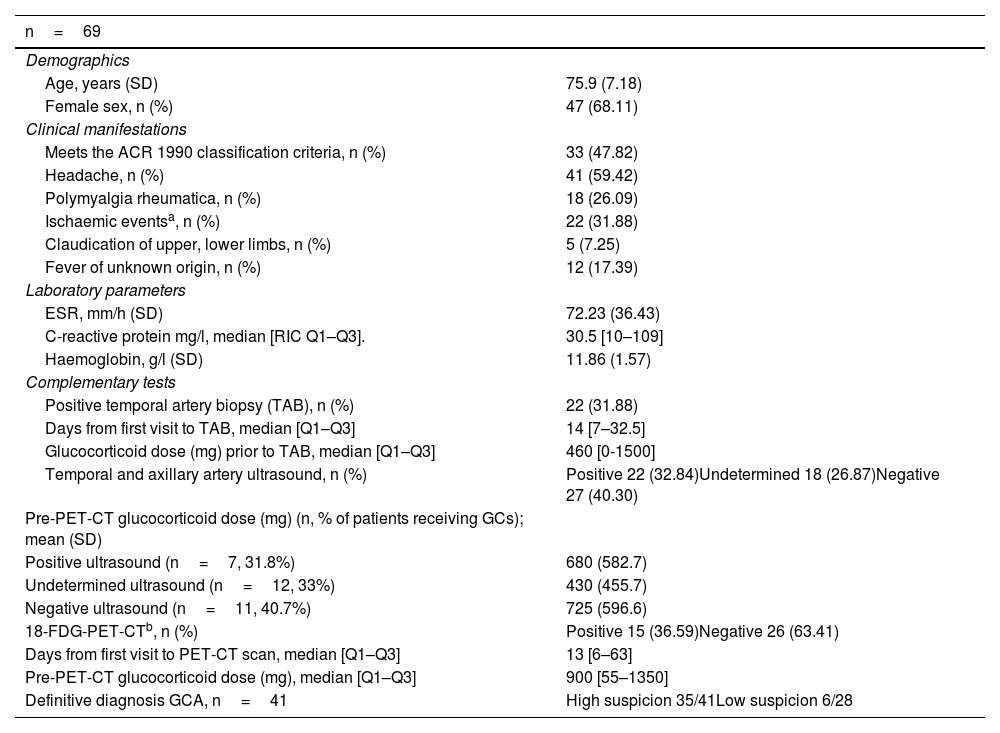
ResultsThe clinical-demographic characteristics of the 69 patients are described in Table 1. Headache was the most common referral symptom (59%). While TAB was positive in 21 (30.43%) of the patients, the definitive diagnosis of GCA was made in 41 patients (59.42%). The mean age was 75.9 (SD: 7.2) years, with no significant difference between the different study groups. GCs were initiated in 36.2% of cases, with no significant difference between positive, indeterminate or negative TAAUS groups.
General patient characteristics.
| n=69 | |
|---|---|
| Demographics | |
| Age, years (SD) | 75.9 (7.18) |
| Female sex, n (%) | 47 (68.11) |
| Clinical manifestations | |
| Meets the ACR 1990 classification criteria, n (%) | 33 (47.82) |
| Headache, n (%) | 41 (59.42) |
| Polymyalgia rheumatica, n (%) | 18 (26.09) |
| Ischaemic eventsa, n (%) | 22 (31.88) |
| Claudication of upper, lower limbs, n (%) | 5 (7.25) |
| Fever of unknown origin, n (%) | 12 (17.39) |
| Laboratory parameters | |
| ESR, mm/h (SD) | 72.23 (36.43) |
| C-reactive protein mg/l, median [RIC Q1–Q3]. | 30.5 [10–109] |
| Haemoglobin, g/l (SD) | 11.86 (1.57) |
| Complementary tests | |
| Positive temporal artery biopsy (TAB), n (%) | 22 (31.88) |
| Days from first visit to TAB, median [Q1–Q3] | 14 [7–32.5] |
| Glucocorticoid dose (mg) prior to TAB, median [Q1–Q3] | 460 [0-1500] |
| Temporal and axillary artery ultrasound, n (%) | Positive 22 (32.84)Undetermined 18 (26.87)Negative 27 (40.30) |
| Pre-PET-CT glucocorticoid dose (mg) (n, % of patients receiving GCs); mean (SD) | |
| Positive ultrasound (n=7, 31.8%) | 680 (582.7) |
| Undetermined ultrasound (n=12, 33%) | 430 (455.7) |
| Negative ultrasound (n=11, 40.7%) | 725 (596.6) |
| 18-FDG-PET-CTb, n (%) | Positive 15 (36.59)Negative 26 (63.41) |
| Days from first visit to PET-CT scan, median [Q1–Q3] | 13 [6–63] |
| Pre-PET-CT glucocorticoid dose (mg), median [Q1–Q3] | 900 [55–1350] |
| Definitive diagnosis GCA, n=41 | High suspicion 35/41Low suspicion 6/28 |
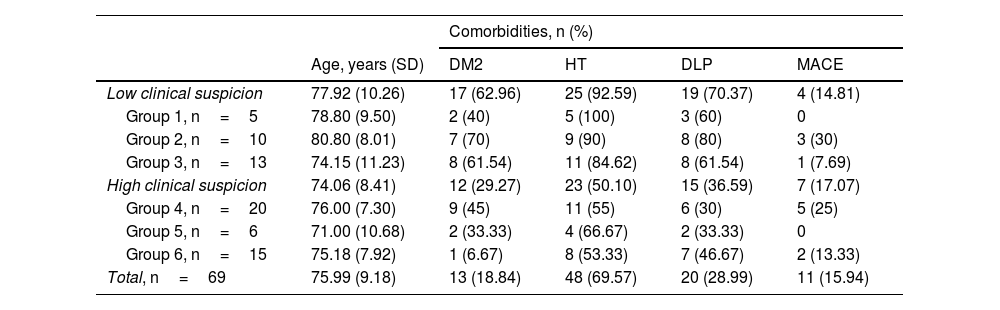
There was a significant difference (p<0.05) between the groups with high and low clinical suspicion of GCA, with the prevalence of type2 diabetes mellitus, hypertension and dyslipidaemia being higher in the group with low clinical suspicion of GCA, but not for history of major cardiovascular event (Table 2).
Cardiovascular risk factors present at baseline visit.
| Comorbidities, n (%) | |||||
|---|---|---|---|---|---|
| Age, years (SD) | DM2 | HT | DLP | MACE | |
| Low clinical suspicion | 77.92 (10.26) | 17 (62.96) | 25 (92.59) | 19 (70.37) | 4 (14.81) |
| Group 1, n=5 | 78.80 (9.50) | 2 (40) | 5 (100) | 3 (60) | 0 |
| Group 2, n=10 | 80.80 (8.01) | 7 (70) | 9 (90) | 8 (80) | 3 (30) |
| Group 3, n=13 | 74.15 (11.23) | 8 (61.54) | 11 (84.62) | 8 (61.54) | 1 (7.69) |
| High clinical suspicion | 74.06 (8.41) | 12 (29.27) | 23 (50.10) | 15 (36.59) | 7 (17.07) |
| Group 4, n=20 | 76.00 (7.30) | 9 (45) | 11 (55) | 6 (30) | 5 (25) |
| Group 5, n=6 | 71.00 (10.68) | 2 (33.33) | 4 (66.67) | 2 (33.33) | 0 |
| Group 6, n=15 | 75.18 (7.92) | 1 (6.67) | 8 (53.33) | 7 (46.67) | 2 (13.33) |
| Total, n=69 | 75.99 (9.18) | 13 (18.84) | 48 (69.57) | 20 (28.99) | 11 (15.94) |
DLP: dyslipidaemia; DM2: diabetes mellitus type 2; HT: hypertension; MACE: major adverse cardiovascular event.
In the low clinical suspicion group (n=28), there were 6 patients with GCA (21.43%), whose diagnosis was reached by TAB (n=2), positive 18F-PET/CT for large vessel vasculitis (n=2) and clinical criteria, due to their good response to GCs (n=2) (Table 3). In the group with high clinical suspicion (n=41), 85.36% had a definitive diagnosis of GCA (n=35). Patients with high suspicion of GCA and another diagnosis at follow-up were distributed as follows: non-arteritic ischaemic optic neuropathy (n=1), hypertensive/diabetic retinopathy (n=1), chondrocalcinosis (n=1), corticoid-resistant polymyalgia rheumatica (n=1), post-COVID headache (n=1), sepsis (n=1), and none of them had a positive TAAUS (indeterminate TAAUS n=2, negative TAAUS n=4).
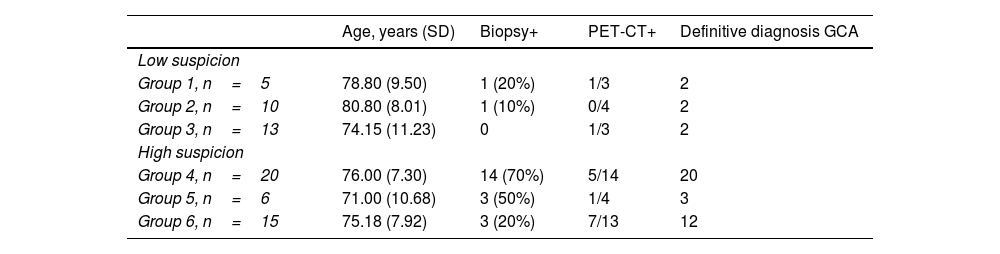
Results of biopsy and 18F-FDG-PET/CT and diagnosis of GCA, by groups.
| Age, years (SD) | Biopsy+ | PET-CT+ | Definitive diagnosis GCA | |
|---|---|---|---|---|
| Low suspicion | ||||
| Group 1, n=5 | 78.80 (9.50) | 1 (20%) | 1/3 | 2 |
| Group 2, n=10 | 80.80 (8.01) | 1 (10%) | 0/4 | 2 |
| Group 3, n=13 | 74.15 (11.23) | 0 | 1/3 | 2 |
| High suspicion | ||||
| Group 4, n=20 | 76.00 (7.30) | 14 (70%) | 5/14 | 20 |
| Group 5, n=6 | 71.00 (10.68) | 3 (50%) | 1/4 | 3 |
| Group 6, n=15 | 75.18 (7.92) | 3 (20%) | 7/13 | 12 |
Group 1: low suspicion/positive US. Group 2: low suspicion/indeterminate ultrasound. Group 3: low suspicion/negative ultrasound. Group 4: high suspicion/positive ultrasound. Group 5: high suspicion/undetermined ultrasound. Group 6: high suspicion/negative ultrasound.
Of the patients with a definitive diagnosis of GCA, but negative or indeterminate TAAUS (n=18), TAB was positive in 7 patients (38.9%). Patients with negative or indeterminate TAAUS and negative TAB (n=11) underwent 18F-FDG-PET/CT, confirming involvement by the presence of large vessel vasculitis in 72.73% (n=8).
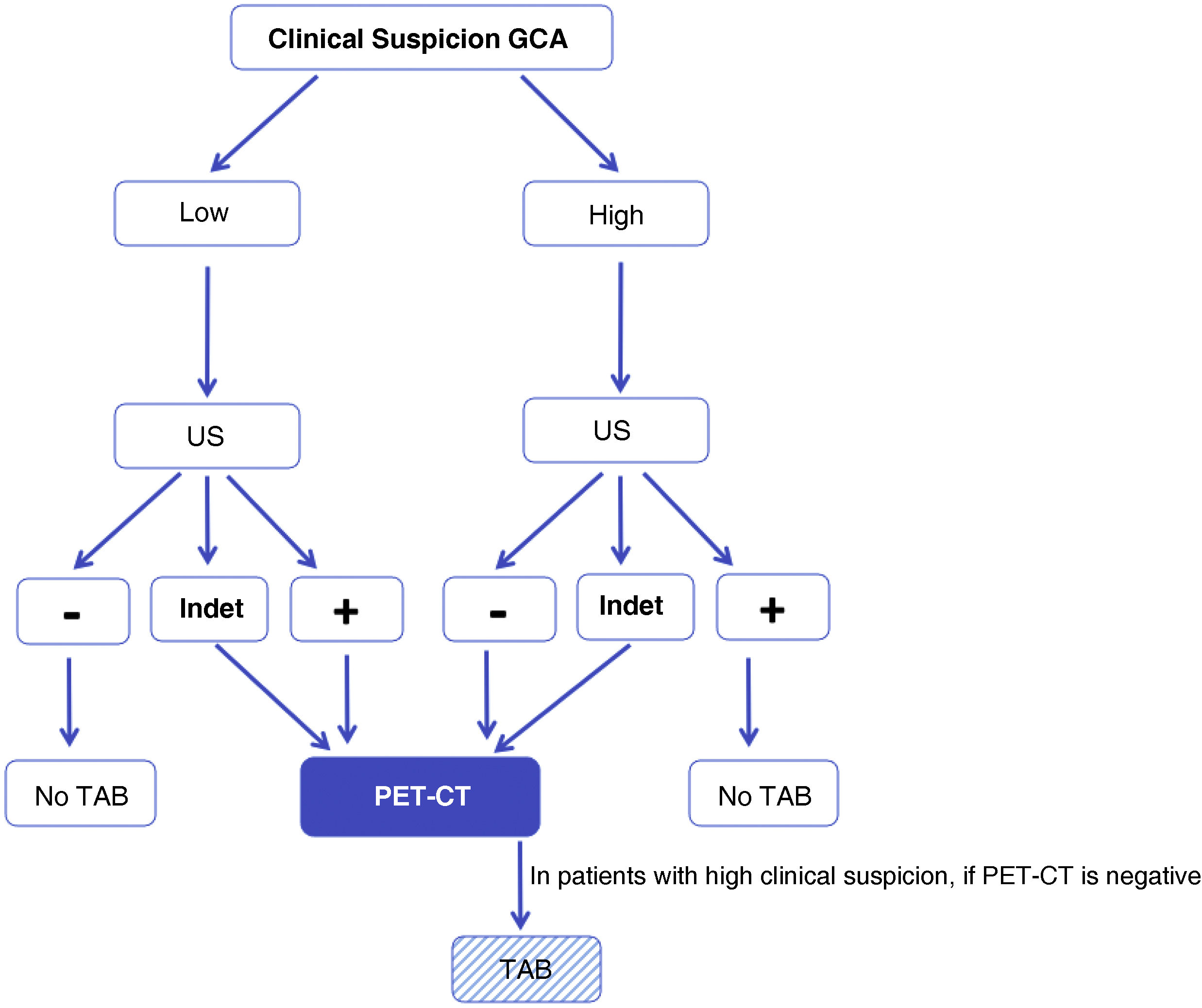
The design of our study, in which we included patients with low clinical suspicion and positive TAAUS (group1, n=5) and patients with high clinical suspicion and negative TAAUS (group6, n=15), forces us to accept, a priori, false positives (n=3) and false negatives (n=12), respectively (Tables 3 and 4). However, when analysing the results, our new algorithm (Fig. 2) shows an overall sensitivity of 77.4% and specificity of 90%, with a diagnostic efficiency for TAAUS of 72.5% and for PET-CT of 69.98% (true positives +true negatives/total patients). Focusing on groups 3 and 4, representing low suspicion and negative TAAUS (n=13) and high suspicion and positive TAAUS (n=20), respectively, the specificity of our algorithm rises to 100% and the sensitivity rises to 86.36%. Of the patients with high clinical suspicion and negative TAAUS (n=15), 13 underwent 18F-FDG PET/CT, which was positive for large vessel vasculitis in 7 cases (53.85%), leading to the diagnosis of GCA, extracranial phenotype.
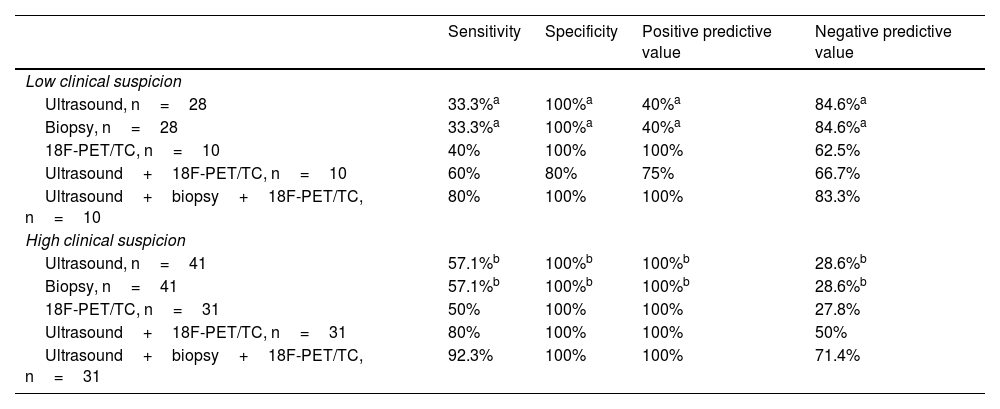
Results of sensitivity, specificity, positive predictive value and negative predictive value in the different low and high clinical suspicion scenarios of GCA.
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
|---|---|---|---|---|
| Low clinical suspicion | ||||
| Ultrasound, n=28 | 33.3%a | 100%a | 40%a | 84.6%a |
| Biopsy, n=28 | 33.3%a | 100%a | 40%a | 84.6%a |
| 18F-PET/TC, n=10 | 40% | 100% | 100% | 62.5% |
| Ultrasound+18F-PET/TC, n=10 | 60% | 80% | 75% | 66.7% |
| Ultrasound+biopsy+18F-PET/TC, n=10 | 80% | 100% | 100% | 83.3% |
| High clinical suspicion | ||||
| Ultrasound, n=41 | 57.1%b | 100%b | 100%b | 28.6%b |
| Biopsy, n=41 | 57.1%b | 100%b | 100%b | 28.6%b |
| 18F-PET/TC, n=31 | 50% | 100% | 100% | 27.8% |
| Ultrasound+18F-PET/TC, n=31 | 80% | 100% | 100% | 50% |
| Ultrasound+biopsy+18F-PET/TC, n=31 | 92.3% | 100% | 100% | 71.4% |
Although the values for sensitivity (S), specificity (S), positive predictive value (PPV) and negative predictive value (NPV) are equal, the patients were not: those positive for ultrasound (2), 1 was confirmed by TAB and 1 by 18F-FDG-PET/CT; those positive for Table (2),1 had positive ultrasound and 1 undetermined.
Modified algorithm proposed for the diagnosis of GCA in daily clinical practice. High suspicion is defined as the presence of any of the following clinical manifestations: 1)exclusive head symptoms (recent onset headache, jaw claudication or visual disturbances); 2)with polymyalgia rheumatica, according to EULAR/ACR 2012 criteria; 3)unspecific febrile syndrome, once infectious causes have been ruled out and screening for neoplasms is negative; 4)ictus, with no relevant cardiovascular history or findings of atherogenic aetiology after directed study. Low suspicion: patients with suspicion due to symptoms other than those mentioned in the previous point (e.g., headache screening, anaemia, elevated acute phase reactants).
GCA: giant cell arteritis; ECHO: ultrasound of temporal and axillary arteries; INDET: ultrasound result “indeterminate”, defined as halo sign in 1 or 2 or less of the 8 arteries examined; NO TAB: do not perform temporal artery biopsy; PET-CT: 18F-FDG-PET/CT;– : negative result; +: positive result.
Table 4 describes the sensitivity, specificity, PPV and NPV of our cohort. TAAUS is compared in the different clinical scenarios; in patients who underwent PET/CT, the combination of PET/CT and TAAUS is compared, and finally the results are analysed using the three techniques: ultrasound, TAB and PET/CT. The negative likelihood ratio for TAAUS in the diagnosis of GCA in patients with low clinical suspicion is 67%.
In the low clinical suspicion group, no difference was found for NPV between TAAUS and TAB. Combining imaging techniques in this group improved the sensitivity for diagnosis (33% vs. 60%). The NPV was lower due to a false positive (fever of unknown origin with positive TAAUS).
In the high clinical suspicion group, TAAUS showed a PPV of 100%, as did TAB or positive PET-CT. Combining imaging techniques in this group improved the sensitivity for diagnosis (57.1% vs. 80%), helping to detect those patients with mainly extracranial GCA.
Applying the PTPS to our population, we found that 19 (27.53%) had LR, 21 (30.43%) IR and 29 (42.03%) HR, of which the diagnosis of GCA was made in 4.35% (3), 17.39% (12) and 36.23% (25) of each group, respectively. Whereas, with the proposed algorithm, 28 (40.58%) were classified as low suspicion and 41 (59.42%) as high clinical suspicion of GCA, diagnosing GCA in 8.69% (6) and 50.72% (35), respectively.
DiscussionHigh-resolution Doppler TAAUS is widely available and is considered an essential tool in units caring for patients with a suspected diagnosis of GCA.10 A favourable result allows, according to the new ACR/EULAR 2022 criteria, to classify a patient with GCA.13 Recently, Molina-Collada et al.17 described the first external validation study of these classification criteria for the diagnosis of patients with suspected GCA, demonstrating adequate performance in supporting clinical diagnosis and improved diagnostic accuracy compared with the 1990 ACR GCA classification criteria. In addition, Narvaez et al.18 published data showing that the new classification criteria are more sensitive than the old ACR criteria in real-life settings in different clinical phenotypes, including patients with cranial and extracranial GCA.
This paper shows the results of both TAAUS and TAB for the initial diagnostic approach and PET/CT at the physician's discretion in order to further study or confirm the presence of large vessel vasculitis. We can see that our algorithm, which starts from clinical suspicion and the TAAUS result, in a fast track clinic, reinforces the importance of a positive TAAUS for the diagnosis of GCA, emphasising cautious interpretation and critical analysis, recalling the following principles: a)the importance of taking into account the clinical context of the patient6,10,19; b)counting the number of affected arteries,20 and c)lastly, the examiner’s learning curve.21–23 In cases where TAAUS is indeterminate or negative, other diagnostic tests, such as 18F-FDG-PET/CT or TAB, should be extended.
In line with recent EULAR/ACR recommendations, which advise the use of imaging tests in patients with suspected GCA,10 the proposed algorithm, in the context of high suspicion and positive TAAUS, TAB is not essential to confirm the diagnosis. Our results (Table 4) show that TAB adds little independently, given that in both the low and high clinical suspicion groups the NPV and PPV are identical to those of TAAUS (84.6% and 100%, respectively), thus avoiding an invasive procedure with the well-known connotations of TAB, as well as the segmental nature of the disease, responsible for false-negative results,24 compared to a point-of-care test in less than 24–28hours.
On the other hand, the symptoms proposed by our original algorithm, of high and low clinical suspicion, only partially fulfilled the objective in the rest of the groups, and when in doubt due to an indeterminate TAAUS, glucocorticoids should be administered, while the diagnosis is confirmed or ruled out, and it is here where 18F-FDG-PET/CT is of special interest, and may be useful in those patients with a high suspicion and negative or indeterminate ultrasound, as mentioned above.
Moreel et al.25 have recently published a systematic review and meta-analysis on the diagnostic performance of combined PET/CT, TAAUS and MRI in GCA. Their results support that the combination of TAAUS of temporal arteries and large vessels, together with PET/CT, demonstrates outstanding accuracy in the diagnosis of GCA. Large vessel ultrasound in addition to temporal arteries and PET/CT evaluation of cranial arteries in addition to large vessels increased sensitivity (91% vs. 80% for TAAUS [p<0.0001] and 82% vs. 68% for PET/CT [p=0.07], respectively), without decreasing specificity. The authors emphasise that the choice between PET/CT and TAAUS depends on the context, experience, clinical status and hospital resources.
Although the initial algorithm proposed by the research group14 did not perform badly, we have learned and proved that, in the presence of unexpected ultrasound data, such as low suspicion and positive TAAUS, or high suspicion and negative TAAUS, a PET-CT scan should be requested and histopathological confirmation with a TAB should be assessed, so we propose the diagnostic algorithm shown in Fig. 2.
Sebastian et al.6 published an algorithm that has been shown to be effective in stratifying fast-track clinic referrals. This approach has a much higher sensitivity, specificity, PPV and NPV for TAAUS, overall and in all categories, than what has been reported to date, with a sensitivity of 97%, specificity of 97% and accuracy of 97% in the total population, compared to previous work with sensitivity between 81.8 and 91.6% and specificity between 77 and 95.83%.7,19,22 When the PTPS was applied to our population, the diagnosis of GCA was reached in 4.35% of the LR group, 17.39% in the IR group and 36.23% in the HR group. Whereas, with the proposed algorithm, GCA was diagnosed in 8.69% in the low-suspicion group and 50.72% in the high-risk group. Both approaches, PTPS and the proposed algorithm, stratify some patients as clear positives and rule out clear negatives, leaving a number of patients with intermediate clinical (PTPS) or indeterminate TAAUS (proposed algorithm) in need of further testing and clinical re-evaluation. PTPS domains, such as demographic, laboratory and clinical, in which each variable is weighted differently (e.g. female, older and with shorter duration of symptoms), increase the probability of diagnosis.
Our algorithm and the Fast Track Clinic project presented in this prospective study have several strengths. One of them is to draw two opposing scenarios of clinical suspicion, with TAB and therapeutic approach based on clinical and ultrasound findings, which occasionally includes 18F-FDG-PET/CT as a complementary test to confirm or rule out the diagnosis. Secondly, the work allows the staging of a real clinical practice routine, describing the different reasons for referral according to the clinical phenotype of a very heterogeneous disease, which until recently has been underdiagnosed in cases involving only large vessels. This has allowed the profile and diagnostic algorithm to evolve as the use of PET/CT has become more widespread. Finally, we would like to emphasise that this is a multicentre study, representative of our geographical area, in which the Doppler TAAUS technicians are accredited experts with a very long experience in grey scale and Doppler mode scanning.
The project also has some limitations, including the lack of homogeneity of the 6 different diagnostic groups, a factor due to the rare nature of GCA. Also, this project was conducted during the critical phase of the COVID-19 pandemic, which is why some patients with low suspicion refused to participate in the study to avoid travelling and TAB. To overcome this limitation, it was decided to place a minimum of 5 patients per group, and then continued as in normal clinical practice.
In a cohort with low clinical suspicion, we recommend the use of TAAUS as sufficient if the result is negative. If TAAUS is positive, we recommend initiating treatment and assessing each case individually when requesting PET-CT and/or TAB, preferring PET/CT, given its greater sensitivity.
In a cohort of high clinical suspicion, the use of TAAUS and its positive result are sufficient for the diagnosis of GCA. Taking into account that a group of patients with high clinical suspicion may have an indeterminate or negative TAAUS (n=21) and still detect GCA (n=15), we recommend the use of PET-CT as a second imaging test, to rescue diagnoses of extracranial GCA.
TAB will always confirm the diagnosis, but TAAUS in the correct clinical context also seems to be sufficient. PET-CT is useful in both low and high clinical suspicion, but be aware that, due to cost, radiation and possible availability, in cases of low clinical suspicion its NPV is moderate (62.5%), and in the case of high suspicion we recommend it when few1–2 or no arteries are found to be affected in the TAAUS.
In conclusion, we would like to emphasise that the design of the algorithm and its application is not against the classic gold standard for this disease, namely, TAB. The final objective is to validate this algorithm and to reinforce the role of Doppler TAAUS as minimally invasive and accessible imaging technique that allows a broad assessment of disease activity in different vessels with improved sensitivity and a lower false negative rate compared to TAB.
The definitive diagnosis of GCA requires a comprehensive multidisciplinary approach involving different specialties due to the varied phenotypes of presentation. Additionally, we should acknowledge the improvement in new diagnostic techniques in recent years. The combination of all of them (imaging tests, such as vascular Doppler TAAUS and PET/CT), together with clinical evaluation, has led us today to an improvement in diagnostic accuracy and to be able to initiate treatment quickly, avoiding serious and irreversible cardiovascular complications.
Ethical considerationsThe study described has been conducted in accordance with the Code of Ethics of the World Medical Association, Declaration of Helsinki.
Informed consent was obtained from each patient and all ethical procedures were followed.
The privacy rights of human subjects have been honoured. Approval has been obtained from the Clinical Research Ethics Committee (PR313/19; ISC 19/66).
FundingThis work received financial support from the Societat Catalana de Reumatologia through the Kern grant 2018-2019, for individual and collaborative research projects: “Proof of concept of the algorithm for the application of ultrasound in the diagnosis of giant cell arteritis”, presented by Dr Patricia Moya and Dr Paula Estrada.
Conflict of interestThe authors declare that they have no conflicts of interest.
We want to thank the Societat Catalana de Reumatologia, for awarding a competitive grant to PE and PM to conduct this project. Also, the Department of Medicine of the University of Barcelona and the Autonomous University of Barcelona.