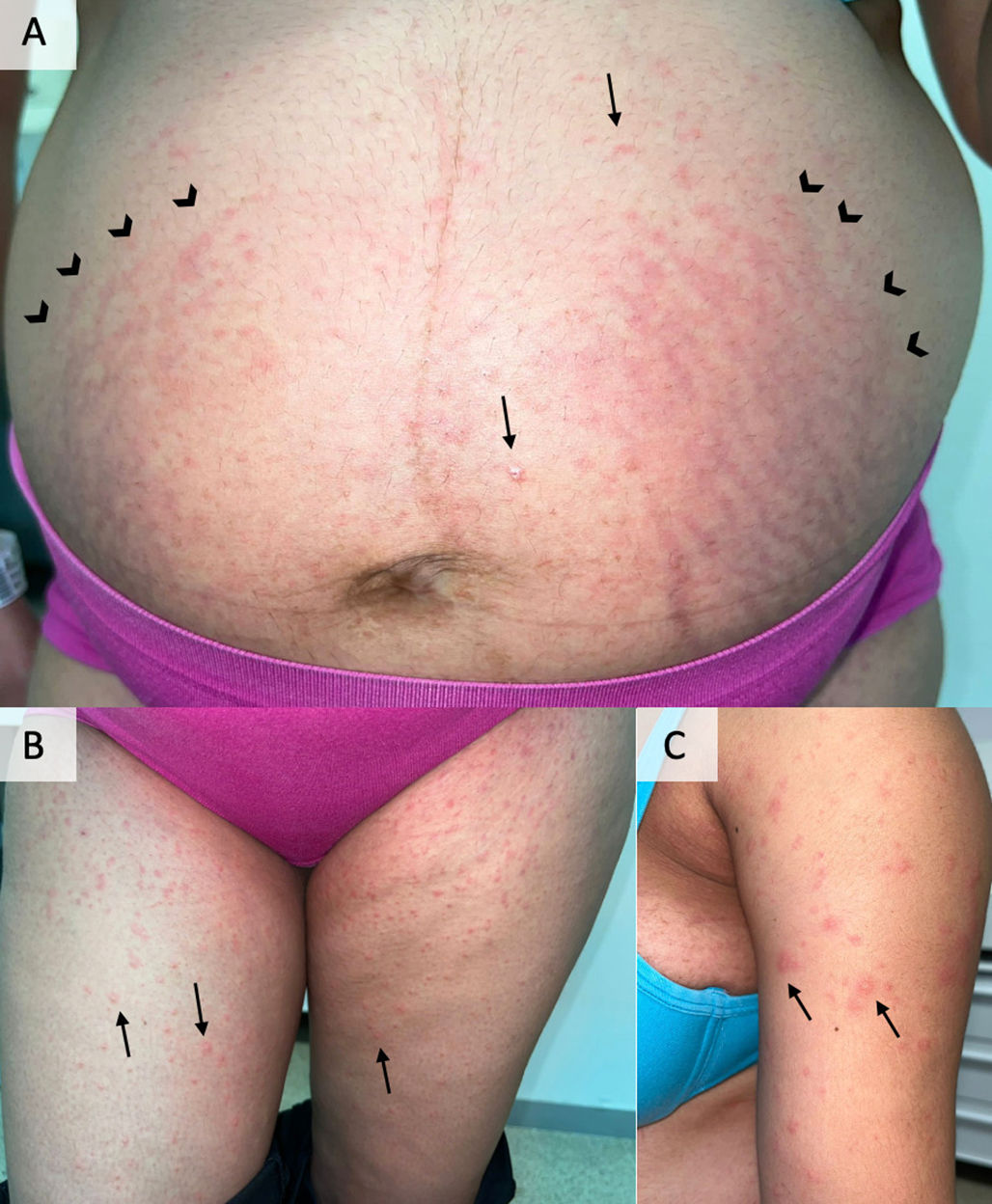
A 34-week gestational tercigestus woman consulted for a 2-days history of multiple erythematous and shiny papules (black arrows in Fig. 1) of generalized distribution, predominantly on the abdomen (Fig. 1A), lower limbs (Fig. 1B) and upper limbs (Fig. 1C) roots. The great affectation at the level of the abdominal distension striae (arrowheads in Fig. 1A) and the respect of the periumbilical area were striking. A diagnosis of polymorphic eruption of pregnancy was made. She took topical betamethasone and cetirizine 10mg/12 hours with complete resolution of the symptoms in 10 days and no recurrence of the lesions in a subsequent pregnancy.
Presentation of skin lesions at the time of consultation. They were erythematous and shiny papules (black arrows) of abdominal distribution (panel a), lower limb root (panel b) and upper limb root (panel c). As can be seen in panel a, the respect of the periumbilical area and the predominant involvement of the abdominal distension striae (arrowheads in panel a) were notorious.
Polymorphic eruption of pregnancy is an inflammatory condition characterized by the presence of erythematous papules, clinically pruritic, and with predominantly abdominal involvement beginning with gestational distension striae. The diagnosis is clinical, and it is essential to differentiate it from gestational pemphigoid, which, although it also usually affects the third trimester, typically presents as tense blisters that affect the periumbilical region. Treatment is mainly symptomatic, with topical corticosteroids and systemic antihistamines. Its prognosis is excellent and there is no increased risk of recurrence in future pregnancies. Knowledge of this entity is essential for every doctor, thus avoiding diagnostic maneuvers and aggressive treatments.
AuthorshipAll authors had access to the data and played a role in writing this manuscript.
Author contributions- -
Miguel Mansilla-Polo, Blanca Novillo-Del Álamo and Daniel Martín-Torregrosa managed clinical treatment and procedures, contributing to the development of this paper.
- -
Carlos Abril-Pérez contributed to the writing and supervision of this paper.
- -
Rafael Botella-Estrada supervised the work.
- This article has no funding source.
- Oral and written consent was obtained to publish this image.
EthicsProcedures followed here were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983. We have not use patients' names, initials, or hospital numbers.
FundingNo specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statementThe authors have declared no conflicts of interest.