Ventilator-associated pneumonia (VAP) is one of the most common ICU-acquired infections. Preventing nasal canal colonization through an effective nasal cavity care, along with oral care seem to be an important issue.
MethodsThis single-blind randomized controlled trial was conducted on 31 patients in each intervention and control group in Alzahra and Kashani hospitals, Iran. The interventional program was considered every 8 h for 5 days. It included cleaning the nasal cavities with cotton swabs soaked in sterile normal saline, then 2 puffs of 65% sodium chloride nasal spray were used for each nostril, and finally the nasal canal was moistened with a swab dipped in Veramin gel (0.5 ml into each nostril). For the control group, routine nasal care including cleaning the outer nostrils was offered. Oral care in 2 groups was performed according to the standard protocol. Data collected through demographic and clinical questionnaire as well as modified pulmonary infection clinical scale. The chi-square and independent tests were used to determine the homogeneity of basic characteristics. Also, we estimated and compared the incidence of VAP between 2 groups by chi-square test.
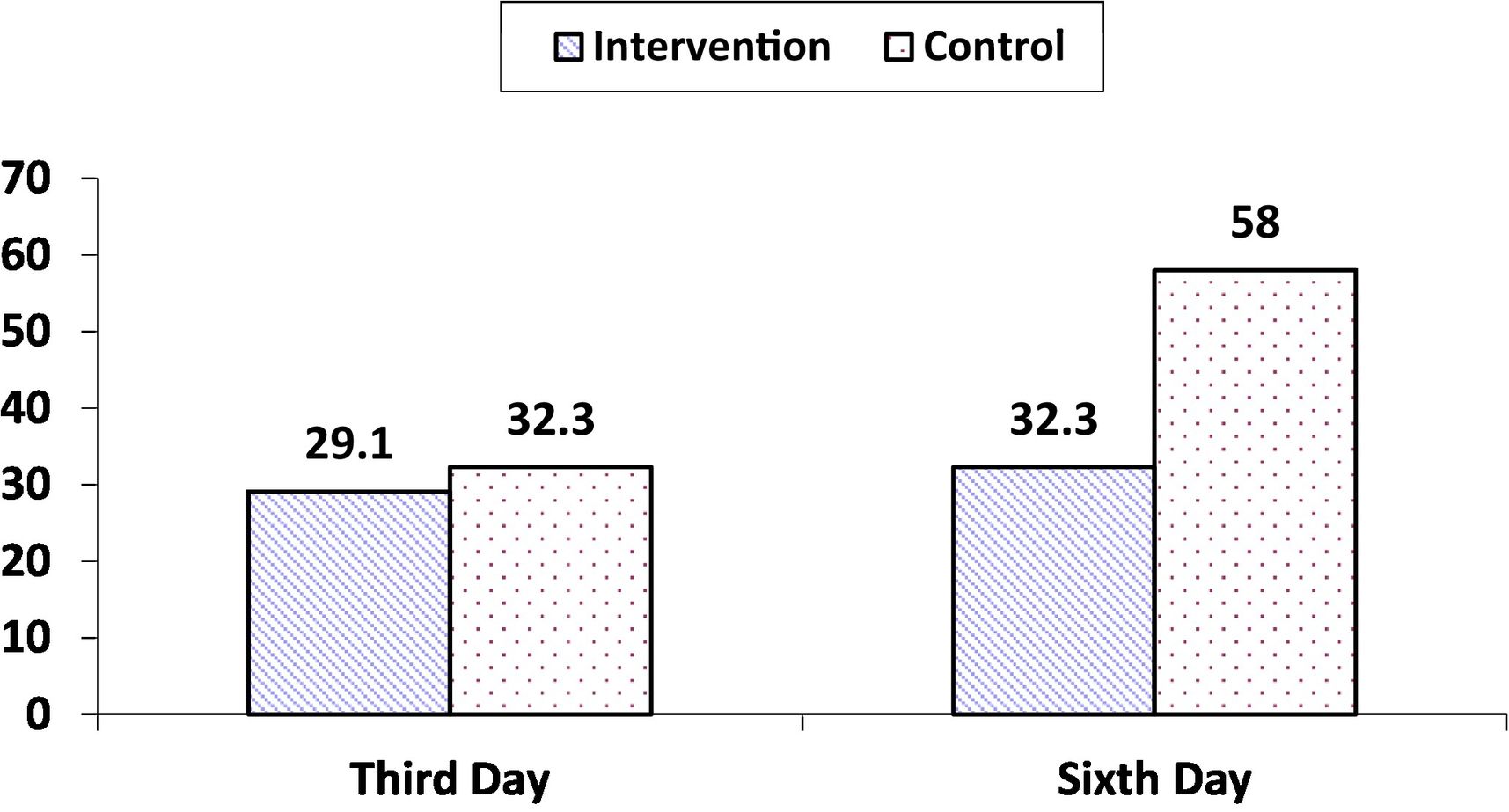
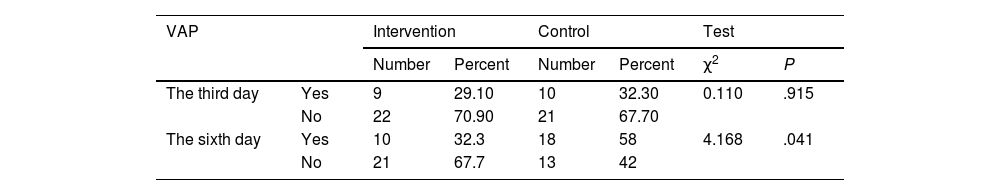
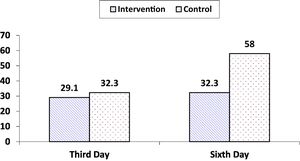
ResultsThe incidence of VAP was not statistically different in the intervention and control groups on the third day after intervention (29.1% vs. 32.3%, respectively, P = .915), while this rate on the sixth day was significantly lesser in the intervention than control (32.3 vs. 58%, P = .041).
ConclusionThe present nasal care program along with oral care is an effective strategy to prevent VAP.
La neumonía asociada al ventilador (NAV) es una de las infecciones adquiridas en la UCI más comunes. La prevención de la colonización del canal nasal a través de un cuidado efectivo de la cavidad nasal, junto con el cuidado oral, parece ser un tema importante.
MétodosEste ensayo controlado aleatorio simple ciego se realizó en 31 pacientes en cada grupo de intervención y control en los hospitales de Alzahra y Kashani, Irán. Se consideró el programa intervencionista cada 8 horas durante 5 días. Incluyó la limpieza de las fosas nasales con hisopos empapados en solución salina normal estéril, luego se aplicaron dos inhalaciones de cloruro de sodio al 65% en aerosol nasal por cada fosa nasal y finalmente se humedeció el canal nasal con un hisopo humedecido en gel Veramin (0,5 ml en cada uno). fosa nasal). Para el grupo de control, se ofreció atención nasal de rutina, incluida la limpieza de las fosas nasales externas. El cuidado oral en dos grupos se realizó de acuerdo con el protocolo estándar. Datos recogidos mediante cuestionario demográfico y clínico, así como escala clínica de infección pulmonar modificada. Se utilizó la prueba de chi cuadrado y la prueba independiente para determinar la homogeneidad de las características básicas. Además, estimamos y comparamos la incidencia de VAP entre dos grupos mediante la prueba de chi cuadrado.
ResultadosLa incidencia de VAP no fue estadísticamente diferente en los grupos de intervención y control al tercer día después de la intervención (29,1% vs. 32,3%, respectivamente, p = 0,915), mientras que esta tasa al sexto día fue significativamente menor en el grupo de intervención. que el control (32,3 vs. 58%, p = 0,041).
ConclusiónEl presente programa de cuidado nasal junto con el cuidado oral es una estrategia efectiva para prevenir la VAP.
Ventilator-associated pneumonia (VAP) is one of the most critical nosocomial infections among patients undergoing mechanical ventilation that can develops 48–96 h after intubation (early-onset) or after 96 h (late-onset).1 A prevalence rate of 4.8–12.6 per 1000 has been reported for VAP in different parts of Asia, and Iranian studies estimated rates of 19% and 32.2%, which in turn increases medical expenses, mortality rate, and the length of being mechanically ventilated and hospitalized in ICU.2–5 Preventive strategies included hand sanitation, bed head elevation up to 30°–45° while the patient has been placed in the supine position, oral hygiene with chlorhexidine oral solution, avoidance of gastric distention and peptic ulcer prophylaxis, prevention of deep vein thrombosis, daily assessment for sedation breaks, and daily examination of the patient's readiness to remove the endotracheal tube have previously been suggested.6 In these critically ill patients, rhinosinusitis occurs after invasive procedures like nasotracheal intubation or nasoenteric feeding tube placement.7 Also, stress-induced proteolysis can separate nectin from the epithelial receptors in the nasopharynx, and by creating a suitable environment for the colonization of bacteria in the nose, it can cause pneumonia.8 Moreover, these patients are not able to clean their own nose due to low level of consciousness. Additionally, use of steroid drugs, taking sedatives, as well as weakening of cough and swallowing reflexes and impaired ciliary movement in the respiratory system, prepares the ground for the development of pneumonia.9 Therefore, cleaning the nasal passages with a lot of dirt and germs is very important to prevent VAP. Especially if there is a tracheal tube, which causes the accumulation of secretions in the mouth and nasal cavities by hindering swallow; and as a result, bacteria move into the lower respiratory tract through the subglottic area.10 For this purpose, suctioning and cleaning the deep part of nasal vestibule with saline cotton swabs to remove accumulated secretion have been reported.11 The researchers also stated that usage of saline nasal spray may clean the nasopharynx, have antiseptic properties, moisten the nasal cavity, and liquefy secretions.11,12 On the other hand, Veramin moisturizing gel has been effective to relieve mucus dryness and improve oral health among ICU patients.13 Compared to the placebo group, this gel had 2 components, one was 100% Aloe vera jelly, which improved oral hygiene more effective than chlorhexidine,14 also reduced the formation of dental plaques like chlorhexidine.15 Another component was peppermint essential oil which showed antiseptic properties to the extent that reduced the rate of chemotherapy-induced oral mucositis.16 Therefore, we decided to use the mentioned strategies to clean the nose along with oral care and determine their effect on the incidence of VAP.
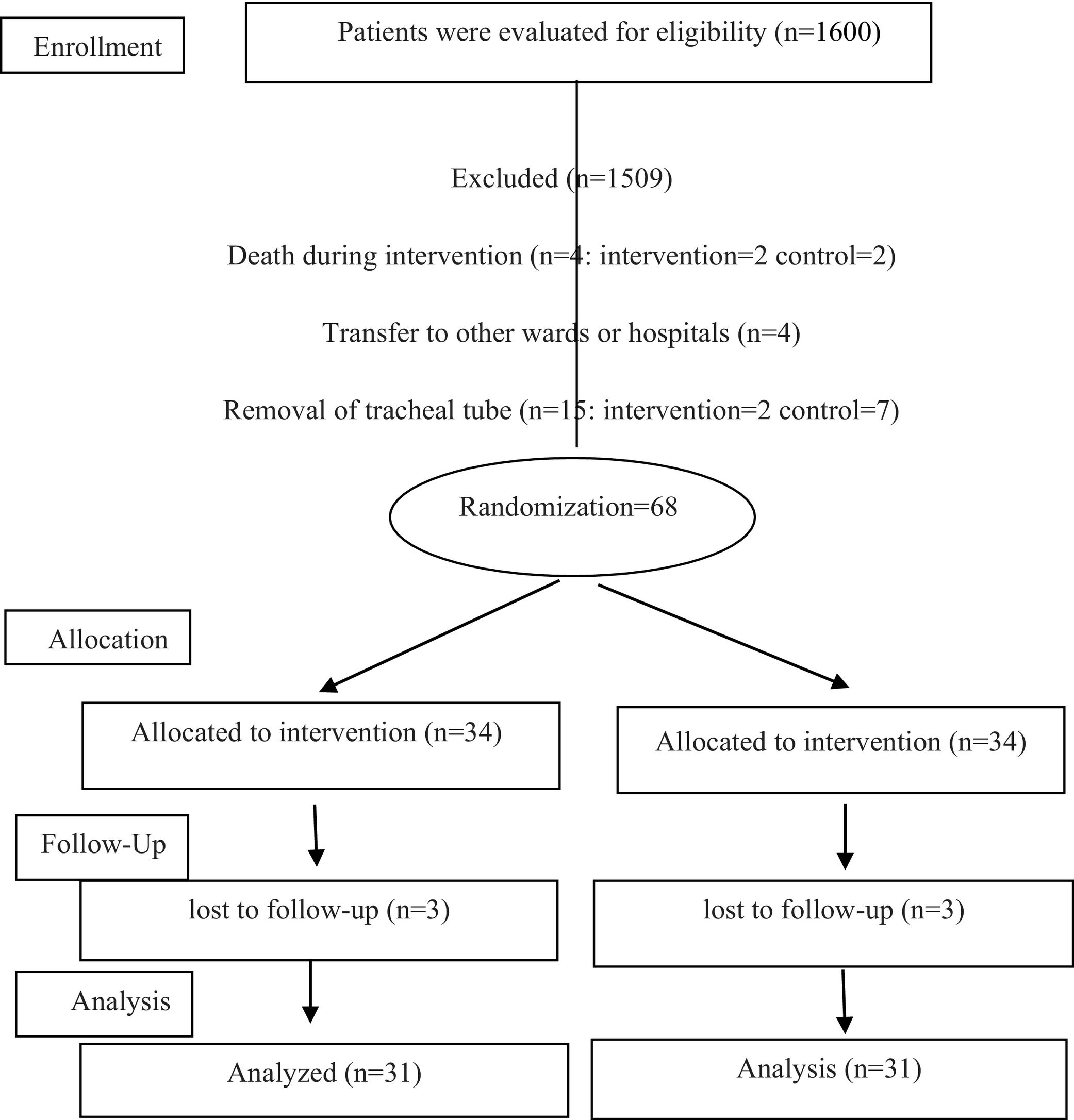
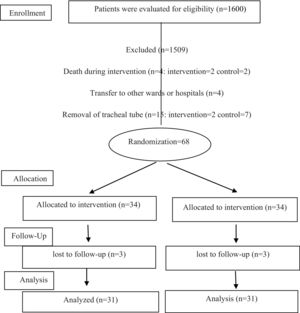
Materials and methodsThis study is a single-blind randomized controlled trial (with the registration number IRCT20201112049362N1) conducted from December 21, 2020 to July 23, 2021 in Alzahra and Kashani Hospitals, Isfahan, Iran. The Ethics Committee of Isfahan University of Medical Sciences approved this trial (IR.MUI.RESEARCH.REC1399.426). Ninety-one patients were selected by convenient sampling method from all hospitalized patients with tracheal tube receiving mechanical ventilation who met the inclusion criteria, and finally the intervention was performed on 31 patients in each intervention and control group with an attrition rate of 10% using the following formula of N = (z1-α/2 + z1-β)2 [p1(1 − p1) + p2(1 − p2)]/(p1 − p2)2. z1-α/2 is a confidence coefficient of 95%, i.e., 1.96, z1-β is a confidence coefficient of 80%, i.e., 0.84, p1 and p2 represent the incidence of VAP in control and intervention which estimated to be 50% and 20%, respectively.3,17 Inclusion criteria were 21–75 years of age, being intubated within 24 h, not having pneumonia before the intervention, not taking immunosuppressive drugs, not being diagnosed with adult respiratory distress syndrome (ARDS) and immune deficiency diseases, no severe septal deviation, nasal polyps, sinusitis, high intracerebral pressure, and non-participation of the samples in another study. Patients were excluded after death, removal of the tracheal tube, and transfer to other wards or hospitals before the end of the intervention. Allocation ratio was 1:1 at the individual level that was achieved by randomization using QMinim Online Minimization®1 by an independent person. Allocation of patients into 2 groups with a minimum difference between them was developed by the software; thus, the homogeneity between subjects was also obtained, while confounding variables were managed. The desired data for entering the software included age, APACHE IV, gender, educational level, hospitalization cause, cigarette smoking, feeding route, and consciousness level (GCS score). This process continued in the same way until the sample size was completed. After randomly assigning the patients to experimental or control group, the scheduled nose care program was carried out during 5 days for intervention group as follows: cleaning the nasal cavity with cotton swabs soaked in sterile normal saline, using 65% sodium chloride nasal spray, 2 puffs for each nostril separately, smearing the inside of the nose with a swab dipped in Veramin gel (0.5 ml into each nostril) every 8 h for 5 days.11,13,18 For the control group, staff cleaned the outer nostrils by tap water as routine nasal care. Oral care in test and control groups was performed according to the standard protocol as follows: opening the patient's mouth, deep mouth and throat suctioning, brushing the internal and external surfaces of teeth and gums with circular movements for 2 min with an electric toothbrush and antibacterial solution (2% chlorhexidine) each 8 h. Brushing all surfaces of tongue and palate with back and forth movements, use a swab instead of a toothbrush in cases of bleeding gums, using moisturizing gel to care for the oral mucosa, using mouth moisturizer to mucous membranes, debris separation, moistening the entire surface of the tongue, lubricate the lips with vaseline.19 An informed consent was taken of first-degree relatives. The patients in the intervention and control groups were examined for VAP by the researcher at 8 am on the third day and the sixth day after the intervention (the fourth and seventh day after intubation). Data collection tool contained demographic and clinical questionnaire as well as modified pulmonary infection clinical scale. Demographic and clinical questionnaire included age, gender, level of education, reason for hospitalization, level of consciousness, underlying disease, history of smoking, nutritional status, types of medications, date and time of intubation, which were extracted from the medical records of patients. To measure the rate of VAP, we used the modified clinical pulmonary infection scale (MCPIS). This scale includes 5 measures of body temperature, lung secretions, white blood cell count, the ratio of po2 to fio2 in milliliters of mercury, and chest radiography; and a score of 0–2 has been considered for each criterion. While the maximum score is 10, a score of 5 or more than 5 indicates VAP. We used the Persian version of this scale which its reliability had previously assessed through internal homogeneity and Cronbach's alpha that was 91%.4 The researcher determined the score of this scale on the third and sixth days after intervention at 8 am.
Statistical analysisThe data were analyzed using the SPSS statistical software package (Version 21.0, Armonk, NY, USA: IBM Corp), and P < .05 was considered significant. The normality of the data was also examined through the Kolmogorov–Smirnov test. The homogeneity of the demographic and clinical characteristics of the 2 groups were analyzed by the chi-square and independent sample t-tests. The incidences of VAP in 2 groups on the third and sixth days after intervention were determined and compared by the chi-square test.
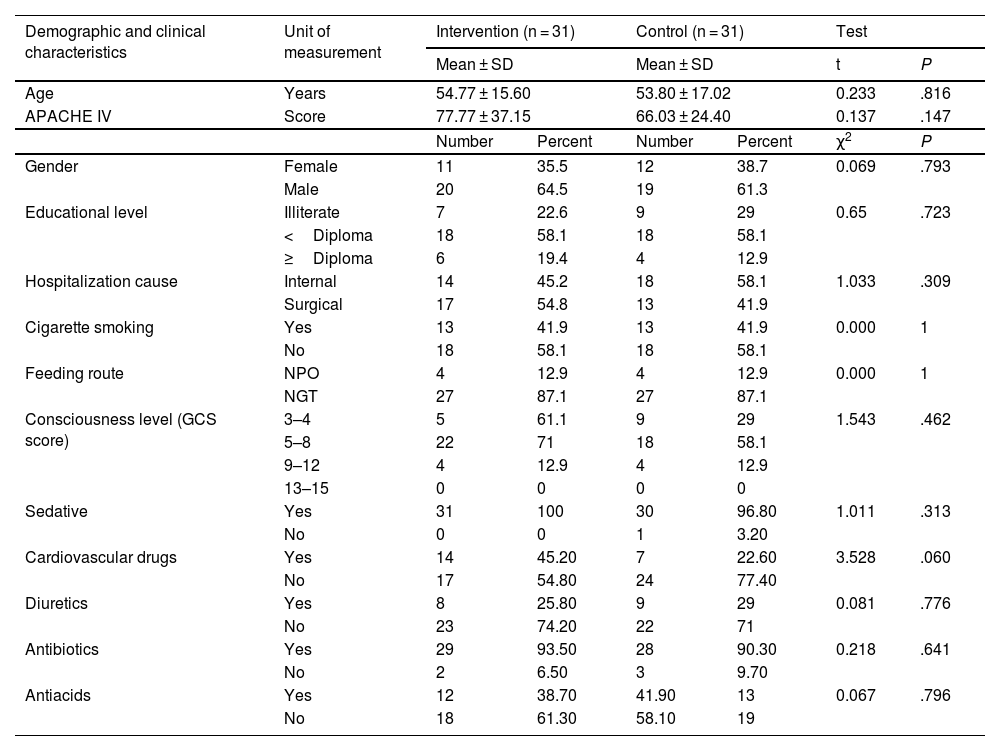
ResultsThe CONSORT diagram of the present trial is displayed in Fig. 1. The mean age of patients in intervention and control groups were 54.77 ± 15.60 and 53.80 ± 17.02, respectively. Acute Physiology and Chronic Health Evaluation (APACHE) IV scores2 of the patients in intervention and control groups were 77.77 ± 37.1 and 66.03 ± 24.40, respectively. Majority of the patients were men, with a Glasgow Coma Scale (GCS) score of 5–8, being fed through NGT, and were not smoking. Also, they were hospitalized because of surgical reasons and their education level was lesser than a high school diploma. Sedatives and antibiotics are the most frequently prescribed medications for 2 groups. The patients' demographic and clinical characteristics between 2 groups were not significantly different. Also, the chi-square test did not reveal significant differences between the 2 groups in terms of type of medication used (Table 1). The incidence of VAP on the third day of intervention was not significantly different between the 2 groups. But, the rates on the sixth day in the intervention and control groups were 32.3 and 58%, respectively, which is not comparable (P = .041) (Table 2, Fig. 2).
Patients' demographic and clinical characteristics.
| Demographic and clinical characteristics | Unit of measurement | Intervention (n = 31) | Control (n = 31) | Test | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | t | P | ||||
| Age | Years | 54.77 ± 15.60 | 53.80 ± 17.02 | 0.233 | .816 | ||
| APACHE IV | Score | 77.77 ± 37.15 | 66.03 ± 24.40 | 0.137 | .147 | ||
| Number | Percent | Number | Percent | χ2 | P | ||
| Gender | Female | 11 | 35.5 | 12 | 38.7 | 0.069 | .793 |
| Male | 20 | 64.5 | 19 | 61.3 | |||
| Educational level | Illiterate | 7 | 22.6 | 9 | 29 | 0.65 | .723 |
| <Diploma | 18 | 58.1 | 18 | 58.1 | |||
| ≥Diploma | 6 | 19.4 | 4 | 12.9 | |||
| Hospitalization cause | Internal | 14 | 45.2 | 18 | 58.1 | 1.033 | .309 |
| Surgical | 17 | 54.8 | 13 | 41.9 | |||
| Cigarette smoking | Yes | 13 | 41.9 | 13 | 41.9 | 0.000 | 1 |
| No | 18 | 58.1 | 18 | 58.1 | |||
| Feeding route | NPO | 4 | 12.9 | 4 | 12.9 | 0.000 | 1 |
| NGT | 27 | 87.1 | 27 | 87.1 | |||
| Consciousness level (GCS score) | 3–4 | 5 | 61.1 | 9 | 29 | 1.543 | .462 |
| 5–8 | 22 | 71 | 18 | 58.1 | |||
| 9–12 | 4 | 12.9 | 4 | 12.9 | |||
| 13–15 | 0 | 0 | 0 | 0 | |||
| Sedative | Yes | 31 | 100 | 30 | 96.80 | 1.011 | .313 |
| No | 0 | 0 | 1 | 3.20 | |||
| Cardiovascular drugs | Yes | 14 | 45.20 | 7 | 22.60 | 3.528 | .060 |
| No | 17 | 54.80 | 24 | 77.40 | |||
| Diuretics | Yes | 8 | 25.80 | 9 | 29 | 0.081 | .776 |
| No | 23 | 74.20 | 22 | 71 | |||
| Antibiotics | Yes | 29 | 93.50 | 28 | 90.30 | 0.218 | .641 |
| No | 2 | 6.50 | 3 | 9.70 | |||
| Antiacids | Yes | 12 | 38.70 | 41.90 | 13 | 0.067 | .796 |
| No | 18 | 61.30 | 58.10 | 19 | |||
APACHE IV: Acute Physiology and Chronic Health Evaluation IV, NPO: Non per oral, NGT: Naso gastric tube, GCS: Glasco coma score.
This intervention decreased the incidence of VAP on the sixth day after 5-day intervention compared to control among patients undergoing mechanical ventilation. The first part of this scheduled care was cleaning the nasal cavity with cotton swabs soaked in sterile normal saline. In this field, Wei et al. compared 3 nasal care and concluded that the new atomizing nasal cleaning is an effective way.11 In fact, in group A where only suctioning was used, bacteria surveyed on the surface of mucus and total incidence of VAP was 36.76%. In group B, researcher used the cotton swabs dipped in saline after suctioning the secretions, and the incidence of VAP became 30.24%, while nasal cavity was not completely cleaned. Because the tracheal catheters prevented the researcher to clean the dead corner of nasopharynx easily and consequently secretions were accumulated. In group C, spraying along the nasogastric tubes and tracheal catheters cleaned the deep part of nasopharynx and reduced the occurrence of VAP, due to reduction of bacteria colonization in the dead corner of nasopharynx. As well, their seawater nasal spray was rich in minerals that had bactericidal or antiviral properties. Furthermore, their physiologic seawater spray could moisten nasal cavity, dissolve nasal secretions, also reduce pathogenic microorganisms and bacteria accumulation. Finally, the rate of VAP became 20.38%.20 Therefore, the second part of present care was use of nasal spray comprising 65% normal saline. Likewise, Awad et al. concluded that nasal spray reduced bacterial colonization and improved the secretion score by moisturizing the nose, dissolving of secretions, and stimulation of mucociliary clearance. Also, it had anti-inflammatory actions by decreasing substances like prostaglandins, leukotriene, and histamine.21 Similarly, Ozturan et al. suggested that the routine use of saline sprays together with oral care is necessary to provide the nasal hygiene. Because it is an economical and painless procedure that could dissolve secretions, and stimulate mucociliary clearance.18 The third part of our care program was the use of Veramin gel to moisturize the nose, as Atshi et al. showed that Veramin gel is significantly effective in relieving dry mouth, preventing the formation of dental plaque, and improving oral health.13 Considering the similarity of the villi and the structure of the mouth and nose, it seemed logical to use smearing the inside of the nose with a swab dipped in Aloe vera–Peppermint gel (0.5 ml into each nostril) every 8 h for 5 days.
We surveyed the simultaneous effects of 3 nasal care accompanied by oral care which have been rarely studied. Moreover, moisturizing the nose with Veramin gel has not been reported previously. However, obtained findings are the result of a master's thesis that was faced with time constraints; therefore, we were not able to follow up longer to get and present the results after a longer period of time. Also, the small sample size was another limitation. Thus, further studies with larger sample sizes and a longer follow-up time are suggested.
Authors contributionAF: Drafting the work, the acquisition of data, provide the questionnaire, implementation of care plan for patients, revising the work critically for important intellectual content, final approval of the version to be published.
SG: The conception and design of the work, revising the work critically for important intellectual content, final approval of the version to be published, investigate the accuracy of questionnaire, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
MS: Revising the work critically for important intellectual content, final approval of the version to be published, investigate the accuracy of questionnaire, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
VA: Revising the work critically for important intellectual content, final approval of the version to be published, investigate the accuracy of questionnaire, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Registration number in case of a clinical trial and where it is registered (name of the registry and its UR): IRCT20201112049362N1
Ethical Standards DisclosureThe Ethics Committee of Isfahan University of Medical Sciences approved this trial (IR.MUI.RESEARCH.REC1399.426). An informed consent was taken of first-degree relatives. For the control group, staff cleaned the outer nostrils by tap water as routine nasal care. Oral care in test and control groups was performed according to the standard protocol. An informed consent was taken of first-degree relatives.
We acknowledge of Vice-chancellor of Research and Technology of Isfahan University of Medical Sciences (399423) for the financial support.