This study aimed to characterize the prevalence as well as clinical and epidemiological features of persistent symptoms after acute COVID-19, focusing on gender-specific differences in a primary care setting.
MethodsA total of 1542 individuals with confirmed SARS-CoV-2 infection were enrolled. The study population comprised 55.77% females (mean age: 45.04 years). Risk factors for persistent COVID-19 were analyzed, revealing disparities between men and women. Symptom clusters and their prevalence were assessed over time, along with functional status using the post-COVID-19 functional status scale.
ResultsThe prevalence of persistent COVID-19 was 12.38%, with females exhibiting a 1.5 times higher risk. Females displayed a higher number of visits and persistent symptoms at 90 days, decreasing after one year. Symptom clusters varied between genders, with females experiencing more dermatological issues. Functional status analysis revealed that females had a better pre-infection status, similar status to males at 90 days, and improved status at 180-, 270-, and 365-days post-infection. Logistic regression analysis showed significant associations between persistence, gender, hospitalization, radiological abnormalities, age, and immunosuppression.
ConclusionThis study provides insights into the prevalence and clinical characteristics of persistent COVID-19 in a primary care population. Females exhibited a higher risk of persistent symptoms and displayed distinct patterns in symptom clusters and functional status compared to males. These findings contribute to a better understanding of the long-term effects of COVID-19 and highlight the importance of gender-specific considerations in post-acute care.
Determinar la prevalencia e identificar las características clínico-epidemiológicas, según perspectiva de género, en pacientes con síntomas persistentes de COVID-19 atendidos en atención primaria.
MétodosSe reclutó un total de 1542 individuos con infección SARS-CoV-2 confirmada. El 55,77% de la población estudiada eran mujeres (edad media: 45,04 años). Se analizaron los factores de riesgo de COVID-19 persistente, evidenciando diferencias entre hombres y mujeres. Se evaluaron los grupos de síntomas y su prevalencia a lo largo del tiempo, junto con el estado funcional mediante la escala de estado funcional pos-COVID-19.
ResultadosLa prevalencia de COVID-19 persistente fue 12,38%, presentando un riesgo 1,5 veces superior en la mujer. Las mujeres presentaban un mayor número de visitas y síntomas persistentes a los 90 días, que disminuían al cabo de un año. Los grupos de síntomas variaron entre géneros, las mujeres experimentaron más problemas dermatológicos. El análisis del estado funcional reveló que las mujeres tenían un mejor estado antes de la infección, un estado similar al de los hombres a los 90 días y un mejor estado a los 180, 270 y 365 días después de la infección. El análisis de regresión logística mostró asociaciones significativas entre persistencia, sexo, hospitalización, anomalías radiológicas, edad e inmunosupresión.
ConclusiónEste estudio proporciona información sobre la prevalencia y las características clínicas de la COVID-19 persistente en una población de atención primaria. Las mujeres presentaron un mayor riesgo de síntomas persistentes y mostraron patrones distintos en los grupos de síntomas y el estado funcional en comparación con los hombres. Estos hallazgos contribuyen a una mejor comprensión de los efectos a largo plazo de la COVID-19 y destacan la importancia de las consideraciones específicas de género en la atención postaguda.
COVID-19 is a multisystemic disease caused by the SARS-CoV-2 virus. Infection consists of an acute phase, and in up to 10%–20% of cases,1 a subsequent phase known as long COVID. The World Health Organization (WHO) defines long COVID as a post-infectious condition of COVID-19 characterized by symptoms persisting for at least 3 months from onset, lasting a minimum of 2 months, and not explainable by any other diagnosis.2–5 In some cases, persistent symptoms have been reported for 1–2 years.6,7 The probability of experiencing long COVID has been associated with several risk factors, any of which can pre-exist before infection, such as age, gender, pre-existing comorbidities, and prior health status, or they may be consequence of the infection, such as disease severity, initial clinical presentation, hospitalization, and the need for intensive care. The identification of long-term predictors is, then, a cornerstone for managing patient healthcare plans.5
Differences in the long-term response to SARS-CoV-2 infection have been observed between male and females, with approximately double the prevalence in females as in males, highlighting the importance of sex and gender in relation to health and disease development.8,9 Thus, in the case of males, more acute disease symptoms have been described, requiring earlier hospitalizations and leading to faster disease outcomes, in terms of both recovery or mortality.5,8,10 Females tend to receive medical attention later after the appearance the disease and have better survival rates; however, they are more likely to experience residual symptoms such as fatigue, myalgia, arthralgia, alopecia, dry eyes, ageusia, anosmia, dermatological changes, dyspnea, anxiety, and depressive episodes than males.3–5,8,10–12 Furthermore, various studies have revealed that the symptoms experienced by females impact more on their perception of their quality of life, compared to males.3,13,14
At present there is no solid evidence to support a clear relationship between the sex of the patient and the different manifestations of an infectious disease. Several authors have attempted to highlight the biological, emotional, or social factors of this relationship,13 including P. Conti et al. who reported that females appear to be physiologically less susceptible than males to experiencing complications associated with SARS-CoV-2 viral infections, due to differences in their innate immunity, steroid hormones, and factors associated with sex chromosomes.15 However, during the COVID-19 pandemic and associated confinement measures, factors such as feelings of isolation, fear of infection, and insecurity had a greater impact on females. These differences are also reported by Baum and colleagues, who suggested that females are significantly more susceptible to secondary traumatization, and this may explain the variations in post-COVID-19 symptoms between the two genders (given that the COVID-19 pandemic is considered a trigger for post-traumatic stress disorder).16 Furthermore, inherent factors related to gender in Spanish society may help elucidate the observed differences between males and females in terms of post-COVID-19 symptoms. For example, females frequently dedicate more time to care of children and elderly family members and consequently, the closure of support centers, schools and day care centers during COVID-19 lockdowns, had a greater impact on females.10,17,18
Numerous studies conducted in the pandemic and post-pandemic periods, have reported gender differences in symptom persistence among patients diagnosed with COVID-19. However, a limited number of these studies focused on non-hospitalized patients with an assessment of medium and long-term symptoms of long COVID from a gender perspective.19,20 We, therefore, still need to elucidate gender differences in non-hospitalized patients treated in primary care,4,5 in order to optimize the treatment and monitoring of patients with persistent COVID-19. This long-term disease is a major challenge for both affected individuals and healthcare professionals, and it is crucial to understand the complete natural history of the disease, underlying mechanisms and treatment options from a clinical and epidemiological perspective in both sexes. Thus, the aim of this study was to determine, from a gender perspective, the prevalence as well as clinical and epidemiological characteristics of patients with persistent COVID-19 symptoms.
Materials and methodsDesign and participantsThis research was a descriptive observational study, conducted with routine clinical ambispective follow-up of a cohort of subjects treated in primary care (Clinical Characterization of the Post-COVID Patient Persistent Symptoms: A Descriptive Observational Study in Primary Care).21 Data were gathered from digitized medical clinical data collection and at in-person patient visits or telephone interviews, 1 month from the onset of symptoms, followed by visit 2 (3 months), visit 3 (6 months), visit 4 (9 months) and visit 5 (12 months) respectively, as long as symptoms persisted.
The study population comprised adults from the Southern Health Area of Córdoba (Área Sanitaria Sur de Córdoba, Spain), who had laboratory-confirmed SARS-CoV-2 infection and had passed the acute phase of the disease. The data collection period included cases reported between December 2020 and May 2022. Participants were preselected in a consecutive manner based on laboratory confirmation reports. Deceased individuals or those unavailable for follow-up were excluded. A total of 1542 individuals were randomly selected to participate in the study, and invitations were extended to eligible subjects. Those who declined or could not be contacted were excluded. To achieve a 3% persistence rate with a 95% confidence level and a 1% precision, accounting for a 10% potential loss to follow-up, it was estimated that a minimum of 1157 individuals needed to be screened (calculations performed using the Ene 3.0 program).
This project was conducted in accordance with the principles of the World Medical Association’ Declaration of Helsinki (64th General Assembly, Fortaleza, Brazil, October 2013). It received authorization from the management department of the Southern Health Area of Córdoba and was approved by the Research Ethics Committee of the Reina Sofia Hospital in Córdoba (Committee reference: 5051). Verbal informed consent was a requirement for participation, and data handling procedures adhered to both the European Regulation and Organic Law 3/2018 on Data Protection.
Clinical parameters and procedureDemographic and clinical data from the acute phase were collected at the initial visit. Patients were stratified according to the risk of complications and were assessed based on the need for hospitalization and/or the presence of risk factors associated with persistent symptoms. These risk factors included age (over 60 years), cardiovascular, pulmonary, neurological diseases, chronic liver disease, diabetes mellitus, obesity, malnutrition, immunosuppression, oncological involvement, transplant history, pregnancy, and severity factors related to SARS-CoV-2. Patients were categorized as “low, moderate, or high risk” based on these criteria22 (see supplementary material for further details. Figure S1). Then, based on the post-COVID-19 risk classification, individuals were categorized according to their persistent post-infection symptoms as: 1. Asymptomatic: Individuals who showed no symptoms during the acute phase of COVID-19. 2. Acute symptoms: Patients who experienced symptoms exclusively during the acute phase. 3. Pre-existing manifestations: Those with a worsening of pre-existing symptoms associated with prior chronic conditions (e.g., exacerbation of migraine headaches in patients with a history of migraines). 4. Paradoxical manifestations: Individuals who developed new symptoms distinct from those experienced during the acute phase. 5. Sequelae: Patients exhibiting symptoms resulting from the severity of their COVID-19 infection. 6. Persistent COVID-19: Patients with symptoms that persisted over time, in accordance with the WHO definition as of March 28, 2023. Additionally, we assessed the pre-COVID-19 functional status of the subjects during the initial visit and post-COVID-19 during follow-up. We employed the post-COVID-19 functional status (PCFS) scale (accessible at https://bit.ly/3cofGaa). This scale measures the degree of progressive limitation on a scale of 0–4, where grade 0 indicates no functional limitations, grade 1 represents negligible limitations, grade 2 signifies mild limitations, grade 3 indicates moderate limitations, and grade 4 denotes severe functional limitations21,23 (see supplementary material for further details. Figure S2).
Patients who had paradoxical manifestations (after-effects or persistent COVID-19) were followed-up at subsequent visits. The duration of symptoms and functional status were examined at each visit. Follow-up ended when symptoms disappeared, and this visit was recorded as the last one. A Data Collection Notebook was completed for each participant in digital format, using Google Drive and Microsoft Office Excel.
Statistical analysisA minimum sample of 1157 people (calculated using Ene version 3.0 software) was considered necessary to achieve a persistence proportion of 3% with a precision of 1% and a 95% confidence level, accounting for a 10% potential loss.
For categorical variables, we employed absolute and relative frequencies and compared them using the Chi-squared test. To evaluate the normal distribution of quantitative variables, we applied the Kolmogorov–Smirnov test, and these were characterized using the mean and standard deviation. Subsequently, differences between data computed for both models were scrutinized using Student's t-test. Confidence intervals were established at the 95% level. To account for potential confounding variables, we utilized logistic regression analysis, with statistical significance defined as a p-value less than 0.05. All data analyses were conducted using the G-Stat 2.0 statistical software package.
ResultsStudy populationA total of 1542 individuals with confirmed SARS-CoV-2 infection were recruited; 55.77% were females (95% CI 53.25–58.28). The mean age was 45.04 years in women (95% CI 43.76–46.31) and 46.40 in men (95% CI 45.22–47.58). Overall, 958 (62.13%) were categorized as low risk, 534 (34.63%) as moderate risk, and 50 subjects (3.24%) as high risk. The number of patients hospitalized due to COVID-19 was 61 (3.96%, 95% CI 3.04–5.05) with a higher prevalence among males (42 cases, 2.72%) compared to females (19 cases, 1.23%).
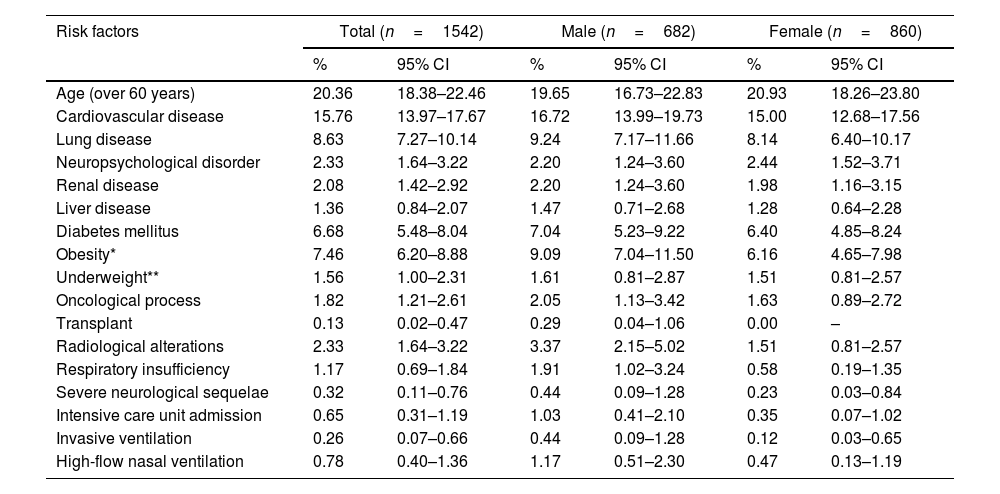
The gender-based analysis of risk factors for persistent COVID-19 revealed disparities between males and females. Particularly, 37.09% (95% CI 34.68–39.56) of the patients exhibited some risk factor, with a slightly higher frequency among men compared to women (38.27% vs 36.16%, p=0.3949). The risk of persistent COVID-19 was stratified as low in 62.13% of the sample (63.37% in females), moderate in 34.63% (34.30% in females), and high in 3.24% (2.33% in females). A detailed review of various categories revealed that obesity was more prevalent in females, whereas cardiovascular diseases, diabetes mellitus, and respiratory failure requiring oxygen were more frequently observed in males. It is also noteworthy that the need for admission to the intensive care unit was observed in 0.65% of all participants, and was more common in males (1.03%) than in females (0.35%) (Table 1).
Risk factors for long COVID, differences between males and females enrolled patients.
| Risk factors | Total (n=1542) | Male (n=682) | Female (n=860) | |||
|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |
| Age (over 60 years) | 20.36 | 18.38–22.46 | 19.65 | 16.73–22.83 | 20.93 | 18.26–23.80 |
| Cardiovascular disease | 15.76 | 13.97–17.67 | 16.72 | 13.99–19.73 | 15.00 | 12.68–17.56 |
| Lung disease | 8.63 | 7.27–10.14 | 9.24 | 7.17–11.66 | 8.14 | 6.40–10.17 |
| Neuropsychological disorder | 2.33 | 1.64–3.22 | 2.20 | 1.24–3.60 | 2.44 | 1.52–3.71 |
| Renal disease | 2.08 | 1.42–2.92 | 2.20 | 1.24–3.60 | 1.98 | 1.16–3.15 |
| Liver disease | 1.36 | 0.84–2.07 | 1.47 | 0.71–2.68 | 1.28 | 0.64–2.28 |
| Diabetes mellitus | 6.68 | 5.48–8.04 | 7.04 | 5.23–9.22 | 6.40 | 4.85–8.24 |
| Obesity* | 7.46 | 6.20–8.88 | 9.09 | 7.04–11.50 | 6.16 | 4.65–7.98 |
| Underweight** | 1.56 | 1.00–2.31 | 1.61 | 0.81–2.87 | 1.51 | 0.81–2.57 |
| Oncological process | 1.82 | 1.21–2.61 | 2.05 | 1.13–3.42 | 1.63 | 0.89–2.72 |
| Transplant | 0.13 | 0.02–0.47 | 0.29 | 0.04–1.06 | 0.00 | – |
| Radiological alterations | 2.33 | 1.64–3.22 | 3.37 | 2.15–5.02 | 1.51 | 0.81–2.57 |
| Respiratory insufficiency | 1.17 | 0.69–1.84 | 1.91 | 1.02–3.24 | 0.58 | 0.19–1.35 |
| Severe neurological sequelae | 0.32 | 0.11–0.76 | 0.44 | 0.09–1.28 | 0.23 | 0.03–0.84 |
| Intensive care unit admission | 0.65 | 0.31–1.19 | 1.03 | 0.41–2.10 | 0.35 | 0.07–1.02 |
| Invasive ventilation | 0.26 | 0.07–0.66 | 0.44 | 0.09–1.28 | 0.12 | 0.03–0.65 |
| High-flow nasal ventilation | 0.78 | 0.40–1.36 | 1.17 | 0.51–2.30 | 0.47 | 0.13–1.19 |
CI: confidence intervals.
Visits conducted during the study periods are presented by symptomatic groups, categorized as asymptomatic, paradoxical, pre-existing, sequelae, and persistent, and the number of medical visits (Table S1). Thus, 191 patients were diagnosed with persistent COVID-19, representing a total prevalence of 12.38%. Notably, this prevalence was considerably higher in females, at 14.18%, compared to males, in whom it was 10.11%. Females exhibit a higher number of visits in all time periods, as expected. Statistical significance in the difference in symptom persistence between genders was observed at the 90-day mark (p=0.0160) and in subsequent visits (6, 9, 12 months), while gender-based differences persist but do not reach statistical significance (p=0.6800, 0.6195, 0.2467). The number of asymptomatic patients increases (92.93%) after 1 year of follow-up. Consequently, the prevalence of persistent patients decreases to 6.29% after one year (see Table S1 for further details).
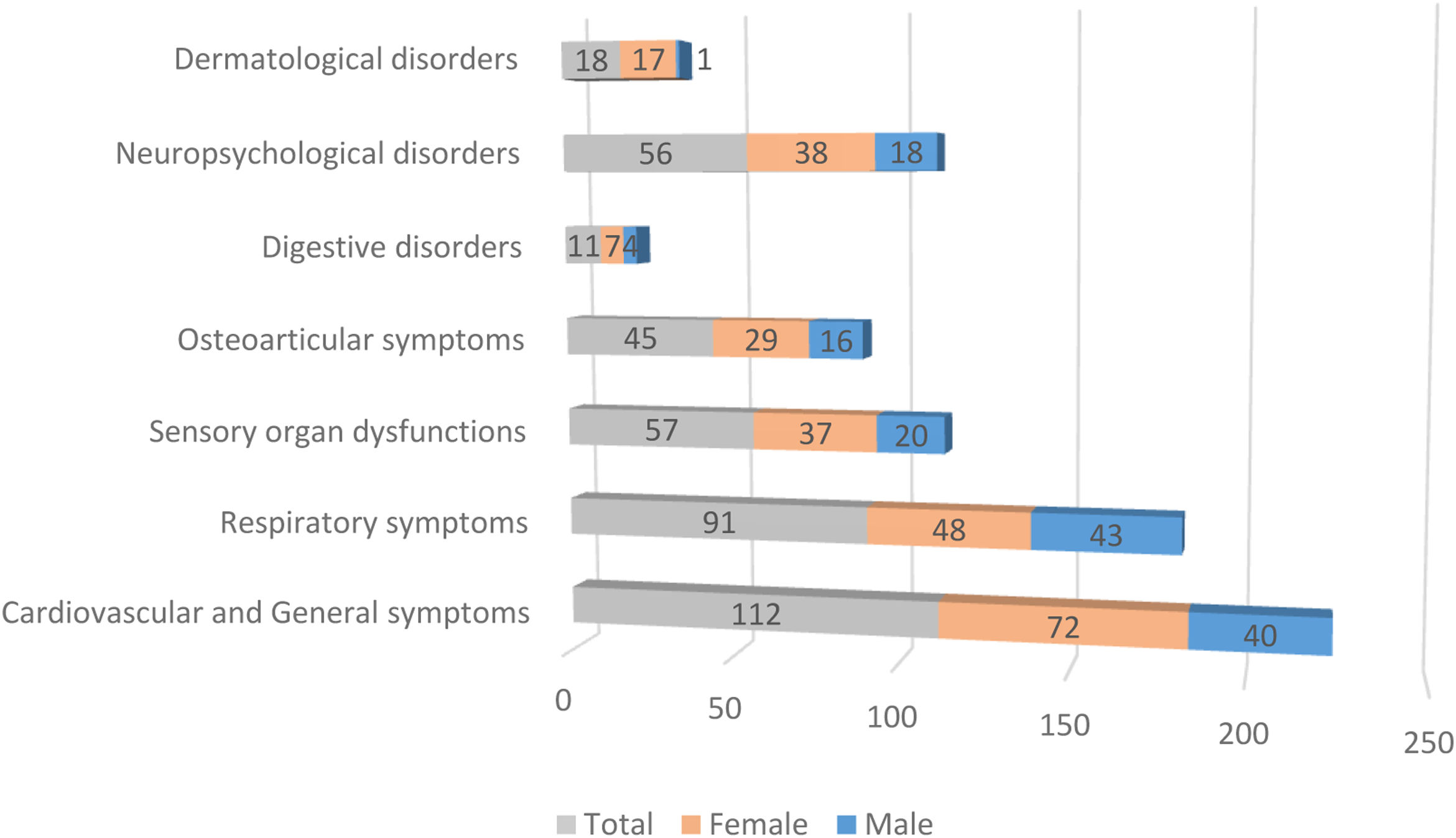
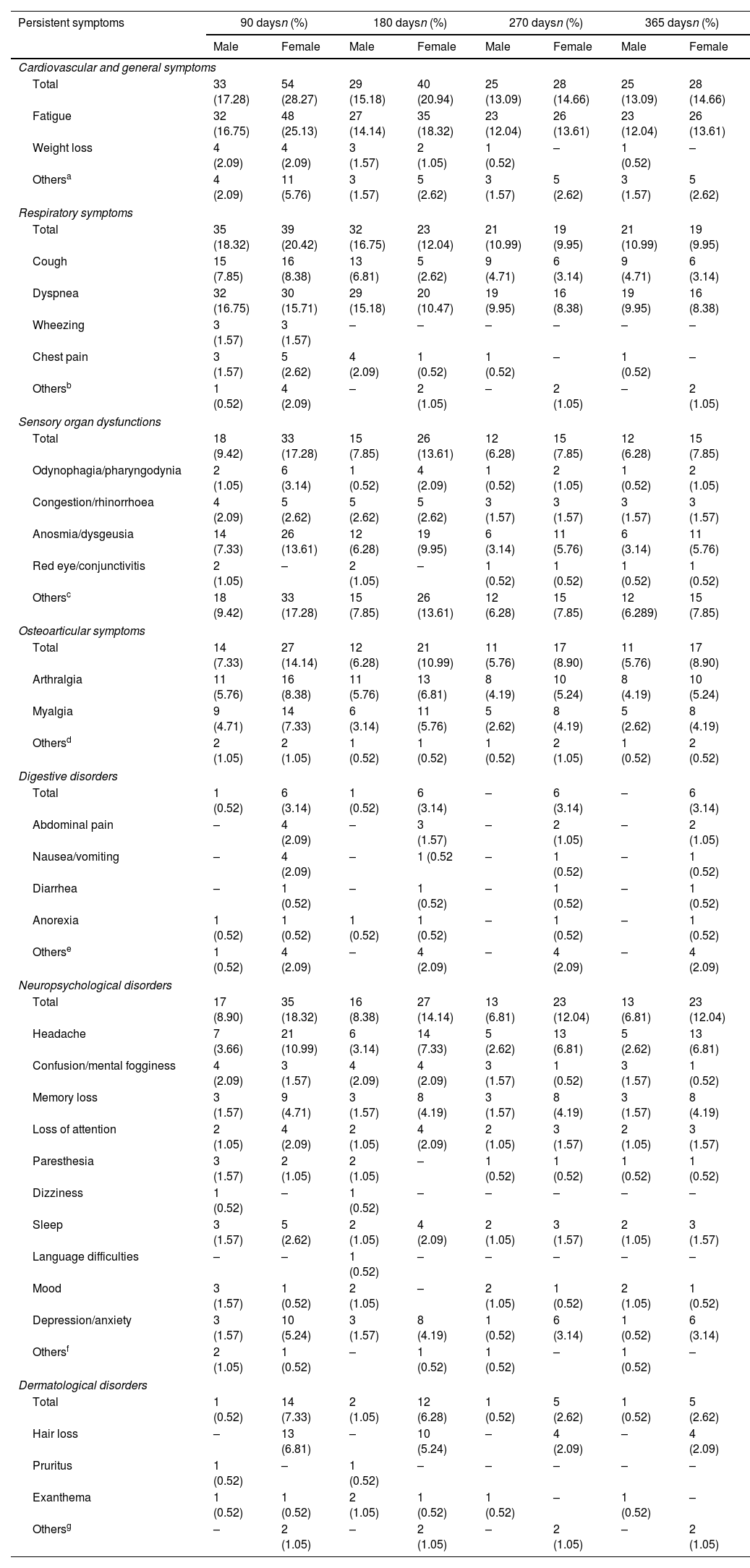
Fig. 1 illustrates the differences in prevalence of symptom clusters between male and female patients; there were more females in the symptomatic groups. The differences observed in dermatological symptoms were notable, females showed a 94% higher prevalence, while the rest of the symptom clusters showed a difference of about 65%. A detailed analysis of the symptoms (see Table 2) revealed that at 90 days, females presented significantly more persistent respiratory compared to males (p=0.0106). In contrast, dyspnea was significantly more frequent in males (p=0.0020). As indicated, females also exhibited more dermatological symptoms in general (p=0.0133), alopecia being the most prevalent complaint (p=0.0050). However, in the group of patients with persistent symptoms at 180 days, males exhibited more respiratory symptoms (p<0.0001), predominantly cough (p=0.0008) and dyspnea (p<0.0001), while the females presented digestive symptoms (p=0.0426) and persistent alopecia problems (p=0.0146). At 270 days, females remained the majority with global persistent symptoms (p=0.0489), while males have the most respiratory symptoms (p=0.0153), especially cough (p=0.0449) and dyspnea (p=0.0133). Finally, the largest cohort of patients with long COVID symptoms 1 year after the initial visit comprised predominantly females (p=0.0489), and respiratory symptoms were more frequent in males (p=0.0153), particularly cough (p=0.0449) and dyspnea (p=0.0133) (see Table 2 for further details).
Physical and psychological symptoms at follow-up, their evolution over time and differences between male and female patients.
| Persistent symptoms | 90 daysn (%) | 180 daysn (%) | 270 daysn (%) | 365 daysn (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Cardiovascular and general symptoms | ||||||||
| Total | 33 (17.28) | 54 (28.27) | 29 (15.18) | 40 (20.94) | 25 (13.09) | 28 (14.66) | 25 (13.09) | 28 (14.66) |
| Fatigue | 32 (16.75) | 48 (25.13) | 27 (14.14) | 35 (18.32) | 23 (12.04) | 26 (13.61) | 23 (12.04) | 26 (13.61) |
| Weight loss | 4 (2.09) | 4 (2.09) | 3 (1.57) | 2 (1.05) | 1 (0.52) | – | 1 (0.52) | – |
| Othersa | 4 (2.09) | 11 (5.76) | 3 (1.57) | 5 (2.62) | 3 (1.57) | 5 (2.62) | 3 (1.57) | 5 (2.62) |
| Respiratory symptoms | ||||||||
| Total | 35 (18.32) | 39 (20.42) | 32 (16.75) | 23 (12.04) | 21 (10.99) | 19 (9.95) | 21 (10.99) | 19 (9.95) |
| Cough | 15 (7.85) | 16 (8.38) | 13 (6.81) | 5 (2.62) | 9 (4.71) | 6 (3.14) | 9 (4.71) | 6 (3.14) |
| Dyspnea | 32 (16.75) | 30 (15.71) | 29 (15.18) | 20 (10.47) | 19 (9.95) | 16 (8.38) | 19 (9.95) | 16 (8.38) |
| Wheezing | 3 (1.57) | 3 (1.57) | – | – | – | – | – | – |
| Chest pain | 3 (1.57) | 5 (2.62) | 4 (2.09) | 1 (0.52) | 1 (0.52) | – | 1 (0.52) | – |
| Othersb | 1 (0.52) | 4 (2.09) | – | 2 (1.05) | – | 2 (1.05) | – | 2 (1.05) |
| Sensory organ dysfunctions | ||||||||
| Total | 18 (9.42) | 33 (17.28) | 15 (7.85) | 26 (13.61) | 12 (6.28) | 15 (7.85) | 12 (6.28) | 15 (7.85) |
| Odynophagia/pharyngodynia | 2 (1.05) | 6 (3.14) | 1 (0.52) | 4 (2.09) | 1 (0.52) | 2 (1.05) | 1 (0.52) | 2 (1.05) |
| Congestion/rhinorrhoea | 4 (2.09) | 5 (2.62) | 5 (2.62) | 5 (2.62) | 3 (1.57) | 3 (1.57) | 3 (1.57) | 3 (1.57) |
| Anosmia/dysgeusia | 14 (7.33) | 26 (13.61) | 12 (6.28) | 19 (9.95) | 6 (3.14) | 11 (5.76) | 6 (3.14) | 11 (5.76) |
| Red eye/conjunctivitis | 2 (1.05) | – | 2 (1.05) | – | 1 (0.52) | 1 (0.52) | 1 (0.52) | 1 (0.52) |
| Othersc | 18 (9.42) | 33 (17.28) | 15 (7.85) | 26 (13.61) | 12 (6.28) | 15 (7.85) | 12 (6.289) | 15 (7.85) |
| Osteoarticular symptoms | ||||||||
| Total | 14 (7.33) | 27 (14.14) | 12 (6.28) | 21 (10.99) | 11 (5.76) | 17 (8.90) | 11 (5.76) | 17 (8.90) |
| Arthralgia | 11 (5.76) | 16 (8.38) | 11 (5.76) | 13 (6.81) | 8 (4.19) | 10 (5.24) | 8 (4.19) | 10 (5.24) |
| Myalgia | 9 (4.71) | 14 (7.33) | 6 (3.14) | 11 (5.76) | 5 (2.62) | 8 (4.19) | 5 (2.62) | 8 (4.19) |
| Othersd | 2 (1.05) | 2 (1.05) | 1 (0.52) | 1 (0.52) | 1 (0.52) | 2 (1.05) | 1 (0.52) | 2 (0.52) |
| Digestive disorders | ||||||||
| Total | 1 (0.52) | 6 (3.14) | 1 (0.52) | 6 (3.14) | – | 6 (3.14) | – | 6 (3.14) |
| Abdominal pain | – | 4 (2.09) | – | 3 (1.57) | – | 2 (1.05) | – | 2 (1.05) |
| Nausea/vomiting | – | 4 (2.09) | – | 1 (0.52 | – | 1 (0.52) | – | 1 (0.52) |
| Diarrhea | – | 1 (0.52) | – | 1 (0.52) | – | 1 (0.52) | – | 1 (0.52) |
| Anorexia | 1 (0.52) | 1 (0.52) | 1 (0.52) | 1 (0.52) | – | 1 (0.52) | – | 1 (0.52) |
| Otherse | 1 (0.52) | 4 (2.09) | – | 4 (2.09) | – | 4 (2.09) | – | 4 (2.09) |
| Neuropsychological disorders | ||||||||
| Total | 17 (8.90) | 35 (18.32) | 16 (8.38) | 27 (14.14) | 13 (6.81) | 23 (12.04) | 13 (6.81) | 23 (12.04) |
| Headache | 7 (3.66) | 21 (10.99) | 6 (3.14) | 14 (7.33) | 5 (2.62) | 13 (6.81) | 5 (2.62) | 13 (6.81) |
| Confusion/mental fogginess | 4 (2.09) | 3 (1.57) | 4 (2.09) | 4 (2.09) | 3 (1.57) | 1 (0.52) | 3 (1.57) | 1 (0.52) |
| Memory loss | 3 (1.57) | 9 (4.71) | 3 (1.57) | 8 (4.19) | 3 (1.57) | 8 (4.19) | 3 (1.57) | 8 (4.19) |
| Loss of attention | 2 (1.05) | 4 (2.09) | 2 (1.05) | 4 (2.09) | 2 (1.05) | 3 (1.57) | 2 (1.05) | 3 (1.57) |
| Paresthesia | 3 (1.57) | 2 (1.05) | 2 (1.05) | – | 1 (0.52) | 1 (0.52) | 1 (0.52) | 1 (0.52) |
| Dizziness | 1 (0.52) | – | 1 (0.52) | – | – | – | – | – |
| Sleep | 3 (1.57) | 5 (2.62) | 2 (1.05) | 4 (2.09) | 2 (1.05) | 3 (1.57) | 2 (1.05) | 3 (1.57) |
| Language difficulties | – | – | 1 (0.52) | – | – | – | – | – |
| Mood | 3 (1.57) | 1 (0.52) | 2 (1.05) | – | 2 (1.05) | 1 (0.52) | 2 (1.05) | 1 (0.52) |
| Depression/anxiety | 3 (1.57) | 10 (5.24) | 3 (1.57) | 8 (4.19) | 1 (0.52) | 6 (3.14) | 1 (0.52) | 6 (3.14) |
| Othersf | 2 (1.05) | 1 (0.52) | – | 1 (0.52) | 1 (0.52) | – | 1 (0.52) | – |
| Dermatological disorders | ||||||||
| Total | 1 (0.52) | 14 (7.33) | 2 (1.05) | 12 (6.28) | 1 (0.52) | 5 (2.62) | 1 (0.52) | 5 (2.62) |
| Hair loss | – | 13 (6.81) | – | 10 (5.24) | – | 4 (2.09) | – | 4 (2.09) |
| Pruritus | 1 (0.52) | – | 1 (0.52) | – | – | – | – | – |
| Exanthema | 1 (0.52) | 1 (0.52) | 2 (1.05) | 1 (0.52) | 1 (0.52) | – | 1 (0.52) | – |
| Othersg | – | 2 (1.05) | – | 2 (1.05) | – | 2 (1.05) | – | 2 (1.05) |
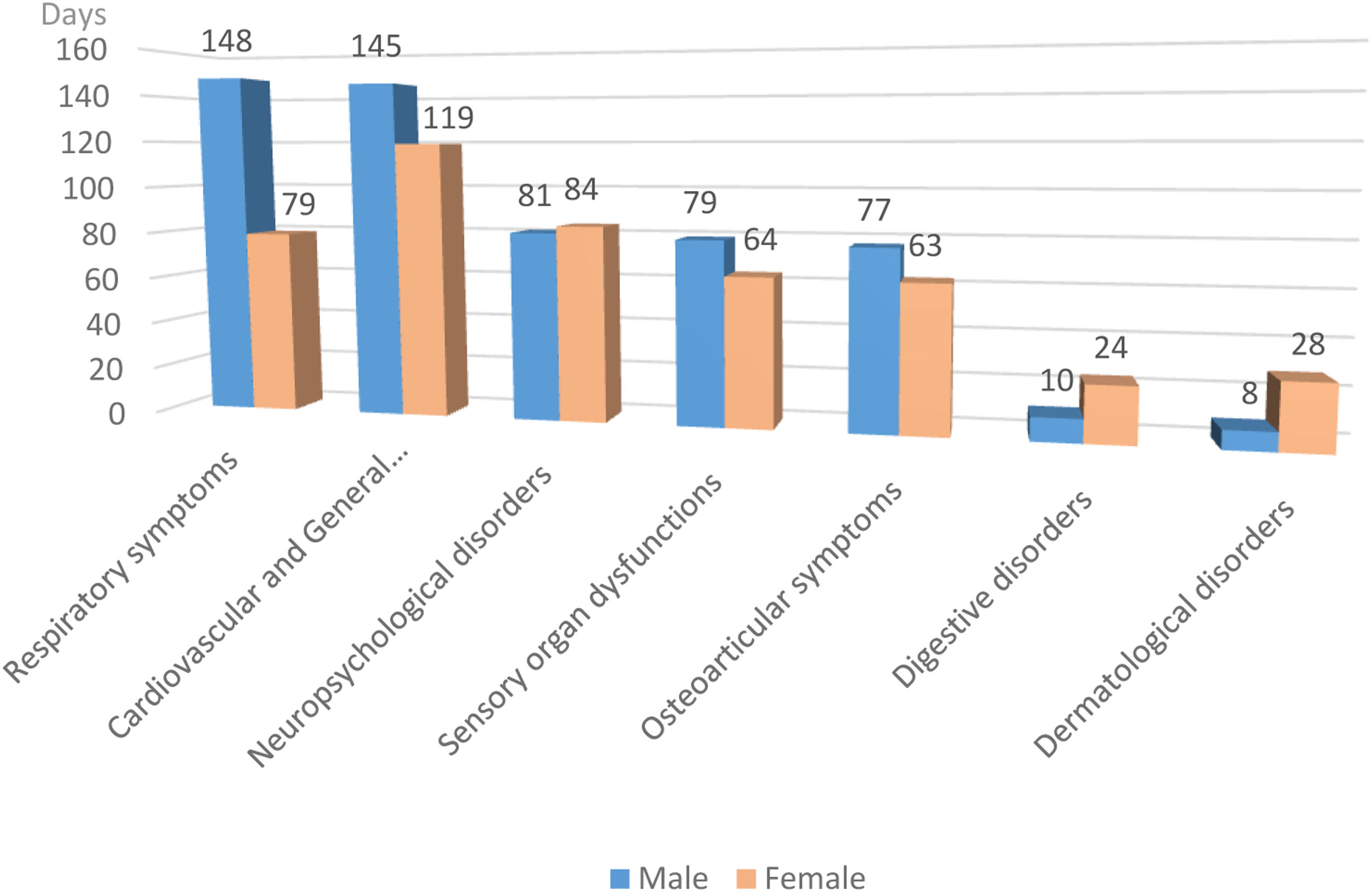
Fig. 2 shows that the duration of symptoms is similar in both genders, with the exception of respiratory, digestive and dermatological symptoms, with significance found only in the respiratory group (p=0.0009).
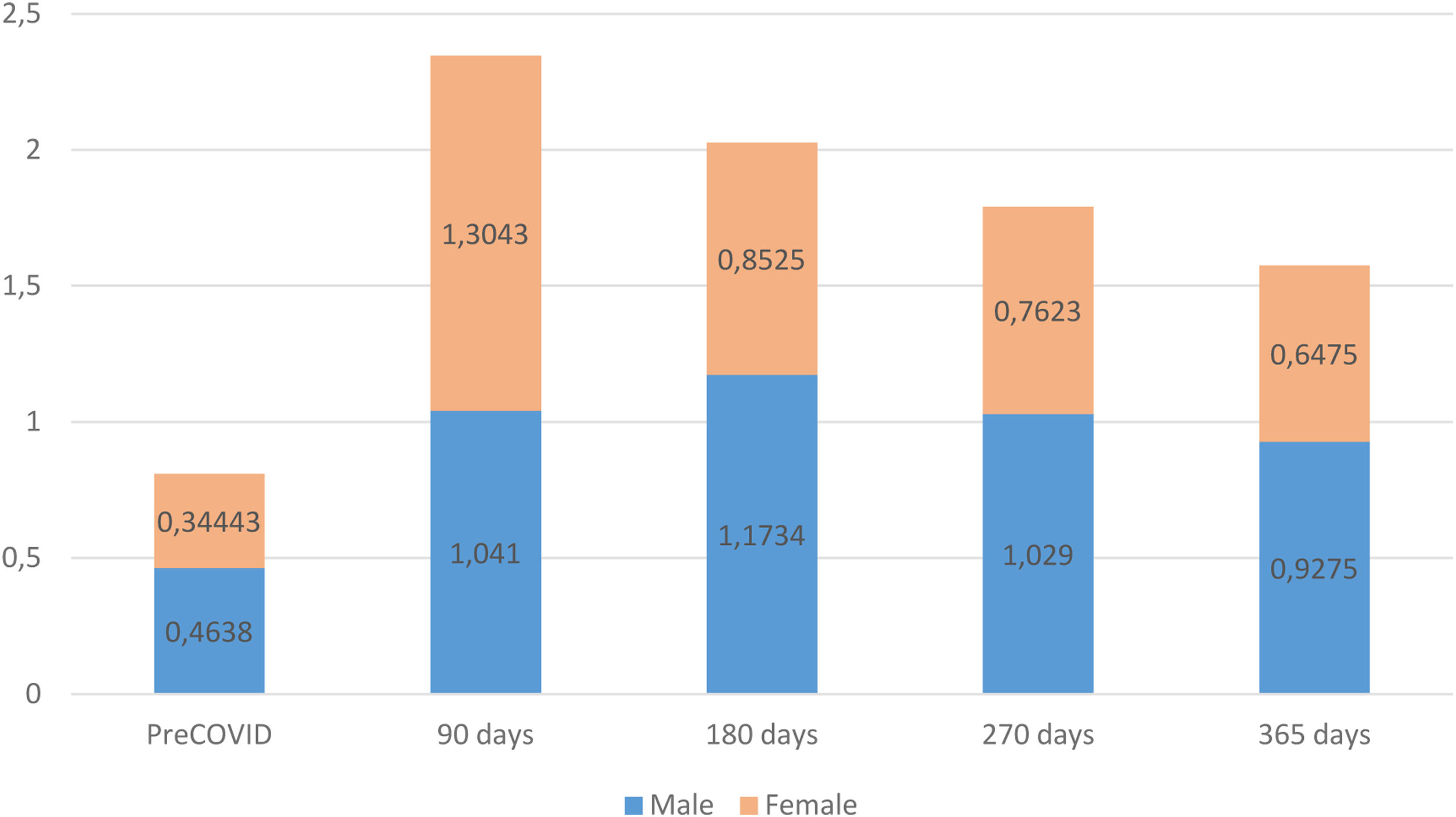
The assessment of patients’ functional status, measured with the post-COVID-19 functional status (PCFS) scale, revealed that, prior to COVID-19, the male group exhibited a worse functional status than females. However, at the time of the diagnosis of persistent COVID-19, females displayed a greater functional decline. Both male and female groups improved their functional status during the monitoring period but, starting at 180 days after the acute phase of the infection, males again exhibited a higher degree of functional impairment than females, a trend that persisted until the end of the follow-up (Fig. 3).
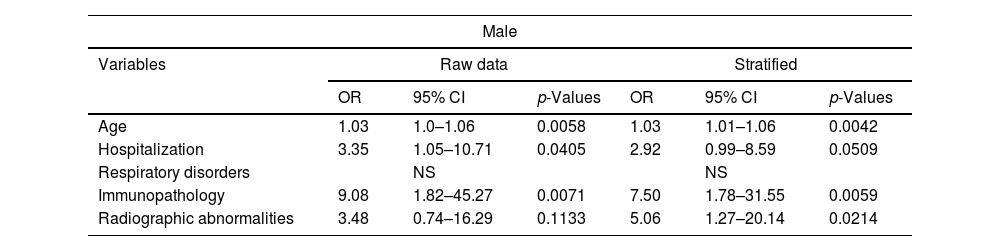
The data represent an analysis of variables, including their odds ratios (OR), 95% confidence intervals (CI), and p-values. The analysis was conducted for both the unadjusted data and the stratified data. These results present the odds ratios, confidence intervals, and p-values for each variable in both the raw and stratified analyses. Multivariate logistic regression analysis modeled the occurrence of persistence by sex, stratified by age, and risk factors considered for developing persistent COVID-19. The Chi-squared test yielded a p-value of <0.0001, revealing that persistence was statistically significantly associated with female gender, patients who had been hospitalized for COVID-19, significant radiological abnormalities post-hospitalization, and a history of lung disease. For the male gender, significance emerged with hospitalization, radiological abnormalities, age, and immunosuppression (Table 3).
Comparative analysis of logistic regression model prevalence of the study population between male and female patients.
| Male | ||||||
|---|---|---|---|---|---|---|
| Variables | Raw data | Stratified | ||||
| OR | 95% CI | p-Values | OR | 95% CI | p-Values | |
| Age | 1.03 | 1.0–1.06 | 0.0058 | 1.03 | 1.01–1.06 | 0.0042 |
| Hospitalization | 3.35 | 1.05–10.71 | 0.0405 | 2.92 | 0.99–8.59 | 0.0509 |
| Respiratory disorders | NS | NS | ||||
| Immunopathology | 9.08 | 1.82–45.27 | 0.0071 | 7.50 | 1.78–31.55 | 0.0059 |
| Radiographic abnormalities | 3.48 | 0.74–16.29 | 0.1133 | 5.06 | 1.27–20.14 | 0.0214 |
| Female | ||||||
|---|---|---|---|---|---|---|
| Variables | Raw data | Stratified | ||||
| OR | 95% CI | p-Values | OR | 95% CI | p-Values | |
| Age | NS | NS | ||||
| Hospitalization | 5.85 | 1.13–30.30 | 0.0351 | 7.46 | 1.64–33.79 | 0.0091 |
| Respiratory disorders | 2.24 | 1.03–4.86 | 0.0410 | 1.97 | 1.03–3.74 | 0.0387 |
| Immunopathology | NS | NS | ||||
| Radiographic abnormalities | 16.14 | 1.17–221.06 | 0.0372 | 18.37 | 1.86–180.97 | 0.0126 |
NS: not significant (p>0.05); OR: odds ratio.
The aim of our study was to characterize the prevalence as well as clinical and epidemiological characteristics of patients experiencing persistent symptoms after an acute episode of COVID-19. We focused on individuals who visited primary care, employing a gender-specific differential analysis. Our results were compared to previously documented data, establishing a basis for comparative evaluation. Our study reveals that the prevalence of persistent COVID-19 was 12.38% and females had a 1.5 times higher risk of being diagnosed with long COVID (14.18%) compared to males (10.11%). This relationship between being female and the persistence of symptoms, remained consistent throughout the follow-up period and is consistent with the results shown in other studies. Furthermore, our analysis is in line with studies conducted in hospitalized patients, although the difference between both genders was greater.4,5,11 Thus, Huang et al. describes a prevalence of 68% at 6 months post-infection,24 aligning with our results that show a decline in prevalence at 1 year (49%). Fernández-de-las-Peñas et al. describes persistent COVID-19 in up to 60% of hospitalized survivors at 8 months post-infection.13 The increase in prevalence among hospitalized patients compared to outpatients was documented by Chen et al. (0.54% vs 0.34%).7
Although the group of patients with persistent symptoms was the most numerous, our study also acquired data from patients who presented other symptoms, such as paradoxical symptoms, pre-existing conditions, and sequelae; the latter were the least numerous and were only observed in patients followed-up for 28–90 days. Although the sample of patients with persistent COVID-19 presenting paradoxical or pre-existing symptoms is limited, we observed that the female group is more susceptible to experiencing these symptoms concomitantly with long COVID. The results also showed that the difference between males and females remained constant over the study period, and in most cases, patients recovered fully from their symptoms, a finding that may be influenced by the fact that this study did not include patients who required hospitalization.
The results of our study, indicating a higher prevalence of persistent symptoms in females, contrast with earlier studies published in 2020 and 2021.24 However, these results are consistent with more recent studies.1,3,21 This discrepancy probably derives from the fact that initial studies were mainly conducted in hospitalized patients, in which the gender balance appears to be the opposite to that of patients who did not require hospitalization.
Confirmed cases of patients who have experienced an acute infection of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) have shown a significant impact on physical health. With this in mind, we conducted an analysis of the functional status of people affected by persistent COVID-19, focusing on gender-related perspectives. To quantitatively assess the pre-infection functional status and its evolution during the persistence of symptoms, we utilized the post-COVID-19 functional status (PCFS) scale. This scale assigns a value of 0 to the absence of symptoms and 5 to patient mortality, involving a wide range of routine tasks and activities.
Our observations revealed that, pre-infection, females showed a better functional status compared to males, while in the first 90 days after infection, the status of both was similar. However, after this initial period, our study indicated that the functional status of females at 180, 270 or 365-days post-infection was again better than that of males, and similar to their pre-infection status. Thus, our results confirm current evidence that female patients exhibit lower risk of severe acute infection and lower mortality compared to male patients, who experience a broad spectrum of post-COVID-19 symptoms. These symptoms encompass various aspects, including physical, emotional, and cognitive factors, and health-related quality of life.4,11,13 Also, we observed that obesity is a strong risk factor for persistent COVID-19 in males and that it plays an important role in the development of other chronic diseases, since obesity correlates with worse health status, as has been described in previous studies.7,25 The data collected in our study revealed that the prevalence of symptoms had no significant relationship with patient gender, except for respiratory symptoms, which were nearly two fold more prevalent in males. Additionally, females experienced a higher number of symptoms across all evaluated categories, including dermatological, neuropsychological, digestive, musculoskeletal, sensory organs, general, and cardiovascular symptoms. These results are consistent with those of Maglietta et al.4 in their meta-analysis to determine factors associated with hospitalization for COVID-19. Maglietta et al. also demonstrated a significant association between fatigue and mental health issues in females in their study, which motivated us to examine these symptoms in our own study. We observed that neuropsychological symptoms, such as depression or anxiety, memory disturbances and headaches, were more common in females and persisted over time, with a limited improvement over the follow-up period. Fatigue, together with dyspnea, were the most prevalent persistent symptoms in our series, which is in line with the results of Maglietta et al.
On the other hand, although males had a higher tendency to exhibit persistent dyspnea, our study indicates that females experienced a more pronounced persistence of respiratory issues overall. The study also concludes that females presented a higher number of symptoms related to dermatological problems, especially those associated with alopecia. These results align with those reported by Fernández-de-las-Peñas et al. highlighting the potential risk of females for developing certain post-COVID-19 symptoms, such as fatigue, dyspnea, or dermatological symptoms.11
Most of the prolonged COVID-19 symptoms, including dyspnea, fatigue and depressive symptoms, appeared at the same incidence in patients at 12–24 weeks and for 24 weeks after recovery, independent of acute COVID-19 severity.3
Our study revealed that females experienced olfactory dysfunction with almost twice as often as males (13.61% and 7.33%, respectively), which is consistent with earlier studies that showed a higher prevalence of sensory organ dysfunction associated with viral infections caused by SARS-CoV-2 or other pathogens, such as influenza or Epstein-Barr virus.26 Gastrointestinal issues showed a much lower prevalence in males (0.52% vs 3.14% in females), consistent with a recent study determining the cumulative prevalence of prolonged gastrointestinal symptoms in survivors of both mild and severe COVID-19.27
Finally, the comparison of prevalence in the study population between male and female patients revealed that women show a significant association between persistent COVID-19 and a history of lung disease, hospitalization during the acute phase, as well as notable radiological abnormalities at discharge. On the other hand, men show an association with hospitalization and significant radiological abnormalities at discharge, but also with advanced age and immunosuppression; however, there is no association with a history of pulmonary disorders.
Strengths and limitationsTo the best of our knowledge, no similar studies have been conducted in primary care with such a large sample size as ours. Our results are consistent with other studies conducted outside of primary care. Furthermore, in this study, we examined a substantial population over an extended period of 1 year, including both hospitalized and non-hospitalized patients. Thus, we have been able to obtain relevant conclusions from a gender perspective about the frequency and prevalence of the variables investigated. The observational design was a limitation of our work, and inhibits the exploration of a causal relationship. Another limitation was that the pre- and post-functional status scale used to measure pre- and post-functional status was not validated. The ambispective telephone follow-up introduces the possibility of memory bias, and the lack of validation for the PCFS scale is acknowledged; nevertheless, our experience may provide evidence for its application in post-COVID-19 care.
ConclusionOur results reveal that the prevalence of COVID-19 is higher in females than in males, as supported by literature data. Moreover, females show a greater symptom load in all systems studied, characterized by a longer course and more severe functional decline. In contrast, males tend to develop more risk factors and experience a more severe form of the disease. These results suggest that healthcare needs to adapt to significant differences in disease manifestation based on sex. Consequently, it crucial that the treatment and follow-up of these patients should consider gender-specific manifestations. These results not only demonstrate the importance of personalized care, but they also highlight the need for increased attention to gender differences in the persistence of COVID-19 symptoms and their functional impact.
Authors’ contributionsAraceli Rodríguez Onieva: conceived and designed the analysis, collected the data, contributed data or analysis tools, wrote and reviewed the paper. Celina Angélica Soto Castro: collected the data, wrote and reviewed the paper. Verónica García Morales: collected the data, wrote and reviewed the paper. María Aneri Vacas: collected the data, wrote and reviewed the paper. Antonio Hidalgo Requena: conceived and designed the analysis, collected the data, contributed data or analysis tools, performed the analysis, wrote and reviewed the paper.
FundingThis work has received financial support from the Progreso y Salud Foundation in the 2021 call for Research and Innovation Projects in Primary Care, Regional Hospitals, and CHARES of the public health system in Andalucia. Project code: AP-0202-2021-C2-F2.
Conflict of interestThe authors declare no conflict of interest in relation to the research, authorship and publication of this article.
The authors acknowledge Dr. Laura Hidalgo (Medical Science Consulting, Valencia, Spain) for medical writing support received under the PUBLIbeCAs programme. We also thank the AP-0202-2021-C2-F2 project, funded in the 2021 call for research and innovation projects in the field of Primary Care, Regional Hospitals and High-Resolution Hospital Centres of the Andalusian public health system.