HLA-G and HLA-E are claimed to play a role in establishing maternal–fetal immune tolerance and in maintaining pregnancy. The presence of polymorphism in the HLA-G gene could cause a deficient or excessive expression of the HLA-G and HLA-E molecules. These anomalies could eventually cause pregnancy losses.
Materials and methodsClinical study. A total of 90 patients were included in this study. These patients suffered spontaneous abortions between weeks 6 and 11 of pregnancy. We have analysed the most important polymorphisms of the HLA-G gene through different genetic studies and HLA-G and HLA-E expression through immunostaining in human cytotrophoblast cells from first trimester spontaneous abortions obtained by hystero-embryoscopy. Placental biopsies obtained with this technique have minimal risk of maternal contamination and provide a reliable embryo karyotype.
ResultWe found that the expression of HLA-G and HLA-E is similar between samples of normal and abnormal karyotype. In addition, we found no evidence of association between the HLA-G polymorphisms and altered expression in both abortion groups.
DiscussionThis is the first study to analyse embryonic tissue which was unable to complete its implantation successfully. We used a new technique with a minimal risk of maternal contamination which provided a reliable karyotype. Our results suggest that, neither HLA-G nor HLA-E protein expression seem responsible for spontaneous pregnancy losses in the first trimester.
Las moléculas del HLA-G y HLA-E desempeñan un papel muy importante en el establecimiento de la tolerancia inmune materno-fetal y en el mantenimiento del embarazo. La presencia de polimorfismos en el gen HLA-G podría causar una excesiva o deficiente expresión del HLA-G, y esto, afectar a la expresión del HLA-E. Estas anomalías de expresión podrían causar la pérdida del embarazo.
Materiales y métodosEstudio clínico. Un total de 90 pacientes fueron incluidas en este estudio. Estas pacientes sufrieron abortos espontáneos notificados entre la semana 6 y 11 de gestación. Analizamos los polimorfismos más importantes del gen del HLA-G mediante diferentes estudios genéticos y la expresión del HLA-G y HLA-E mediante inmunohistoquímica en las células del citotrofoblasto humano de abortos espontáneos de primer trimestre obtenidos mediante histero-embrioscopia. Con esta novedosa técnica obtenemos biopsias placentarias con el mínimo riesgo de contaminación materna y así podemos proporcionar un cariotipo embrionario con alta fiabilidad.
ResultadosEn este estudio encontramos que la expresión proteica de HLA-G y HLA-E es similar entre muestras de cariotipo normal y anormal. Además, no encontramos asociación entre los polimorfismos de HLA-G y la alteración de su expresión en ambos tipos de aborto.
DiscusiónSomos los primeros en analizar el propio tejido embrionario incapaz de terminar de forma exitosa su implantación. Nuestros resultados sugieren que, ni la expresión de la proteína del HLA-G ni del HLA-E parecen responsables de las pérdidas del embarazo espontáneo de primer trimestre.
Although the cause of pregnancy loss is multifactorial, it can be generally divided into two main causes: embryologically driven causes, mainly due to an abnormal karyotype, and maternally driven causes, which affect the endometrium and/or placental development (Li et al., 2002; Aplin, 2000). The aetiology is unknown in approximately 50% of abortion cases (Pandey et al., 2005), but it has been postulated that a proportion of these pregnancy losses may be due to immune causes.
Recognition of foreign cells is rendered by the expression of Major Histocompatibility Complex (MHC) molecules on the cell surface. During pregnancy, the maternal immune system should recognise foetal trophoblast cells as foreign if they express paternal MHC molecules. However, foetal extra-villous cytotrophoblast cells do not express the classical MHC type I molecules, HLA-A and HLA-B, and MHC type II molecules are also absent. Instead, HLA-G and HLA-E are expressed in the human cytotrophoblast, specifically in extra-villous cytotrophoblast of the placenta by them invading their way through the maternal decidua.
The different expression pattern of antigens HLA-G and HLA-E, as compared to other MHC molecules, together with the high degree of conservation of their sequences during evolution, predict a clear implication in the modulation of these molecules in immune responses, such as foeto-maternal recognition responses to maintain the semi-allogenic embryo.
Some functions performed by HLA-G include the suppression of the cytolytic and cytotoxic activity of NK cells and Tc lymphocytes (CD8) (Rouas-Freiss et al., 1997; Kapasi et al., 2000; Contini et al., 2003), the alteration of local cytokine secretion towards a compatible physiological situation with implantation (Rieger et al., 2002; Loke and King, 2000), and the possible participation in the regulation and stabilisation of the superficial HLA-E expression across the leader peptide. HLA-G encodes the leader peptide linked to the HLA-E molecule, a necessary process for its superficial expression and stability. Both, the leader peptide and the HLA-G molecule are very important for the HLA-E expression (Lee et al., 1998a; Lemaoult et al., 2004).
Surprisingly, there are relatively very few studies into HLA-G expression and abortion, this being the most common disorder in pregnancy. Some authors have observed a lower HLA-G expression in cytotrophoblast (Emmer et al., 2002) and serum (Athanassakis et al., 1999; Pfeiffer et al., 2000; Alegre et al., 2007) in recurrent spontaneous abortion (RSA) in comparison with pregnancies to term. However, there is still some controversy as to the role of these molecules in maintaining pregnancy. In fact, other authors have found no differences in the expression patterns of HLA-G and HLA-E in trophoblast between RSA and voluntary pregnancy interruption (VPI) (Bhalla et al., 2006) or the expression levels of HLA-G between chromosomally normal RSA and RSA with trisomy 16 (Patel et al., 2003). Similarly, it has been shown that the soluble HLA-G levels in serum between RSA and alive newborns are similar (Gonzalez et al., 2010).
From a genetic perspective, certain polymorphisms have been associated with abortion in relation to the HLA-G gene (Pfeiffer et al., 2001; Aldrich et al., 2001; Ober et al., 2003; Hviid et al., 2004a; Abbas et al., 2004; Tripathi et al., 2004; Xue et al., 2007; Zhu et al., 2010). Interestingly, some polymorphisms have functional consequences as they can affect the transcriptional regulation of mRNA (Ober et al., 2006; Hiby et al., 1999; O’Brien et al., 2001; Rousseau et al., 2003; Hviid et al., 2003; Larsen and Hviid, 2009) and the superficial expression of the protein level of HLA-G (Ober et al., 1998; Rebmann et al., 1999, 2001; Rizzo et al., 2005; Chen et al., 2008; Mendes-Junior et al., 2010; Gonzalez et al., 2010). On the other hand, some authors have reported no association between determined polymorphisms in peripheral blood and risk of abortion (Sipak-Szmigiel et al., 2007, 2008; Yan et al., 2006; Mendes-Junior et al., 2007), and others have found no association between determined polymorphisms and the HLA-G expression in either peripheral blood or term placenta (Hviid et al., 2004b). Therefore, it is not very likely that one polymorphism will be capable of producing pregnancy loss. This is due to the strong linkage disequilibrium that exists in the region of the HLA-G (Ober et al., 1996), which favours that different loci tend to inherit in block. For this reason, some authors believe that the association between a given haplotype of HLA-G and spontaneous recurrent miscarriages is possible (Berger et al., 2010).
Regarding HLA-E, it is noteworthy that it contains similar genetic and molecular characteristics to those of HLA-G (O’Callaghan and Bell, 1998). The tissue surface expression is restricted to extra-villous cytotrophoblast and needs a leader peptide from other HLA molecules. It also has low polymorphism and some of them can affect its expression and functions (Lee et al., 1998b). It performs the immunosuppression functions of NK cells and T CD8+ lymphocytes (Borrego et al., 1998; Braud et al., 1998). Nonetheless, it is thought that HLA-E is also especially involved in maintaining pregnancy, although some authors found no differences in their expression between RSA and VPI (Bhalla et al., 2006).
Therefore, in this study we aimed to determine the presence of polymorphisms in the HLA-G gene in samples of extremely highly purified trophoblast tissue of spontaneous abortions in both chromosomally normal and abnormal embryos and its association with the local expression of HLA-G and HLA-E in cytotrophoblast tissue.
Materials and methodsPopulationThis study was approved by the Institutional Review Board at the Instituto Valenciano de Infertilidad (IVI Valencia).
After obtaining the signed informed consent form a total of 90 patients were included in the study. These patients reported spontaneous abortion between gestation weeks 6 and 11 with embryos stopped between week 4 and week 9.5 of gestational age, observed following morphological visualization at the time of hystero-embryoscopy.
They underwent hystero-embryoscopic biopsies to selectively take only embryo tissue for the karyotyping analysis. Briefly, a Hamou examination and contact hysteroscope III with a Hopkins forward-oblique 30° telescope, 2.9mm in diameter and 30cm length, and a Bettocchi single-flow operating sheath, size 3.8mm, provided with a 5-Fr channel for semi rigid instruments (Karl Storz GmbH, Tuttlingem, Germany) was used. Normal saline was used as the distending medium. The hysteroscope was gently introduced into the uterine cavity without cervical dilatation. The prominence of the gestational sac was located. A small hole was made in the gestational sac wall using a 5-Fr biopsy spoon forceps (Karl Storz GmbH). The scope was introduced gradually in the extracelomic and amniotic cavities. Direct embryo and chorion biopsies were taken and placed in saline solution. This technology allows sample to be obtained with a minimal risk of mother-tissue contamination (Ferro et al., 2003).
A total of 99 embryo cytotrophoblast samples were recovered from 90 patients because 9 of them presented two gestational sacs, and it was possible to obtain a sample from both sacs. These cytotrophoblasts samples were used to determine embryo karyotype and the genetic studies about on HLA-G polymorphisms and HLA-G immunostaining analysis. Additionally maternal deciduas were employed to perform the genetic studies about the heteroparentality of the trophoblast sample.
To accomplish this study, the possible known reasons for pregnancy loss were analyzed and discarded in the normal karyotype cases, such as chromosomal anomalies in the progenitors or the presence of uterine or endocrine anomalies or antiphospholipid syndrome in the patient (Beydoun and Saftlas, 2005). Samples with chromosomal embryo anomalies were used as reference samples.
Gene polymorphismsAfter karyotyping 99 cytotrophoblast samples, a total of 57 samples from 55 patients were included for the polymorphisms analysis.
The samples were randomly selected and attempts were made to include a similar number of patients in each group: 30 samples with abnormal karyotype and 27 samples with normal karyotype.
DNA extraction was done with organic solvents by the DNA isolation kit (Gentra System. Puregene TM, USA).
The genetic studies were conducted to evaluate the heteroparentality of the trophoblast sample and the non-contamination with maternal decidua by analysing the generated fragments by a PCR multiplex with different combinations of polymorphic marker-type microsatellites CA (data not shown).
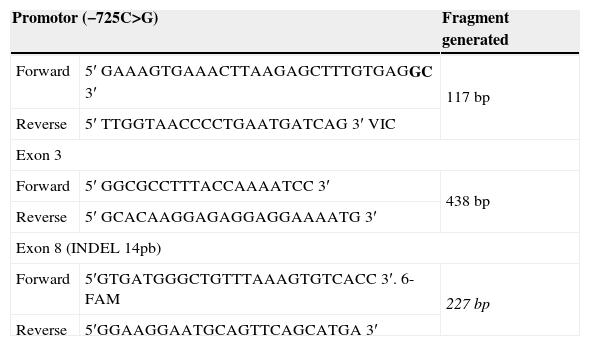
For the analysis of polymorphisms of greater interest use the following techniques and the primers shown in Table 1.
Primers used in the genetic analysis of the HLA-G.
| Promotor (−725C>G) | Fragment generated | |
|---|---|---|
| Forward | 5′ GAAAGTGAAACTTAAGAGCTTTGTGAGGC 3′ | 117 bp |
| Reverse | 5′ TTGGTAACCCCTGAATGATCAG 3′ VIC | |
| Exon 3 | ||
| Forward | 5′ GGCGCCTTTACCAAAATCC 3′ | 438 bp |
| Reverse | 5′ GCACAAGGAGAGGAGGAAAATG 3′ | |
| Exon 8 (INDEL 14pb) | ||
| Forward | 5′GTGATGGGCTGTTTAAAGTGTCACC 3′. 6-FAM | 227 bp |
| Reverse | 5′GGAAGGAATGCAGTTCAGCATGA 3′ | |
For the genetic analysis of −725C>G polymorphism in the promoter region, a PCR was done to amplify the genetic fragment of interest, while the RFLP (restriction fragment length polymorphism) technology employed a StuI restriction enzyme. The PCR product was digested when the C allele was present, i.e. when polymorphism not present. The PCR products were visualised in an automatic electrophoretic system (ABI Prism 3130, Applied Biosystem, CA, USA) and by the GenneMapper 4.0 program (Applied Biosystems, CA, USA).
* Exon 3 polymorphismFor the genetic analysis of the polymorphism in exon 3, a PCR was done to amplify the genetic fragment of interest, with sequencing done by the BigDye terminator v3.1 sequencing kit in a 3700 DNA analyzer (Applied Biosystem, CA, USA). The obtained sequences were analysed by the SeqScape software, version 2.6, and were compared to the reference sequence of the gene obtained from the following address: http://www.ensembl.org/Homo_sapiens/Gene/Sequence?g=ENSG00000204632. Information of the codons considered more interesting was acquired (e.g., codons 93, 110 and 130).
* INDEL 14-bp polymorphismThe INDEL 14-bp variant was analysed by PCR and the PCR products were visualised by an automatic electrophoretic analysis system (ABI Prism 3130) and with the GenneMapper 4.0 program (Applied Biosystem, CA, USA). The following were found: a fragment of 211bp for the samples that were homozygous for the deletion of 14bp; a 227bp fragment for the samples that were homozygous for the insertion of 14bp; two fragments of 211bp and 227bp for the heterozygous samples.
ImmunostainingIn order to detect HLA-G in the embryonic cytotrophoblast samples, 24 patients and 27 samples were employed. The samples for this technology were randomly selected and attempts were made to include a similar number of patients in each group: 13 abnormal karyotype samples and 14 normal karyotype samples. We believe that this is a sufficiently representative number to determine the HLA-G expression, especially as other studies have included a similar number of samples from different abortion types (Patel et al., 2003; Bhalla et al., 2006; Emmer et al., 2002; Rabreau et al., 2000; Menier et al., 2003; Honig et al., 2005; Hviid et al., 2004b).
Trophoblast cells were cryo-embedded in an OCT compound (Sakura. Finetek Europe, B.V), snap-frozen in liquid nitrogen and stored at −80°C until thin cryosections (5μm) were obtained. HLA immunostaining quantification was done with the Vectastain Elite ABC Kit (Vector Laboratory, UK). An MEM-G/9 monoclonal antibody (ABCAM; ab7758) was utilised at a dilution of 1:500 for HLA-G detection and 4D12 (MBL, K0215-3) at a dilution of 1:100 for HLA-E detection. A biotinylated secondary antibody and Avidin Biotin Complex (ABC) plus DAB were used as the detection system. Tissues were counterstained with haematoxylin. To test immunostaining specificity, serial of cytotrophoblast was used in the absence of a primary antibody as negative control.
All the sections were evaluated quantitatively in order to grade the intensity of the HLA-G expression by five researchers for internal consistency purposes. The pattern of expression and the intensity of staining were evaluated as follows: 1 no expression; 2 weak expression; 3 moderate expression; 4 high expression.
Statistical analysisThe statistical analysis was performed by the Statistical Package for Social Sciences 19.0 (SPSS Inc., Chicago, USA).
A chi-square test was carried out for the statistical analysis of the presence of polymorphisms and haplotypes. A non-parametric Mann–Whitney U and Kruskal–Wallis test was used for the statistical analysis of the HLA-G expression in polymorphisms and haplotypes, where p values<0.05 were considered statistically different.
To assess any possible differences found among the different observers of immunostaining, a quadratic Kappa index was utilised.
ResultsKaryotype analysis and population characteristicsWith the 90 interventions and 99 samples of trophoblast, it was possible to obtain chromosome results in 95 samples of trophoblast (95.95%), since in 4.1% (4/99) of cases existed lack of fetal growth. Among the 95 analyzed trophoblasts have to that 28.4% (27/95) had a normal karyotype and 71.6% (68/95) had abnormal karyotypes, being the most frequent autosomal trisomies (58.8%). The chromosomal constitution of the pregnancy was based on the analysis of the trophoblast cells. Only in 7.3% of the cases discrepancies between embryo and trophoblast cells were observed (embryos normal and trophoblast abnormal), these cases were considered as abnormal karyotype as it can be the cause of the miscarriage.
The analyzed samples showed that 54.7% of the pregnancies had a masculine karyotype and 45.2% had a feminine one. The average age of the patients was 34.1±3.1 years.
Of all the patients included in this study, 16.7% (15/90) were considered recurrent abortions, defined as ≥3 spontaneous pregnancy losses before 20 weeks of gestation, and 83.3% (75/90) were classified as spontaneous isolated abortions. Also, 23.3% (21/90) of gestations were naturally conceived, whereas 76.7% (69/90) were achieved by assisted reproduction technology (ART).
Polymorphisms in normal and abnormal cytotrophoblast samplesAll the samples showed heteroparental information, therefore demonstrating the absence of maternal contamination (results not shown).
Of the 57 samples of trophoblast genetically analysed for the HLA-G gene polymorphisms, 47.4% (27/57) had an embryonic normal karyotype and 52.6% (30/57) an abnormal karyotype.
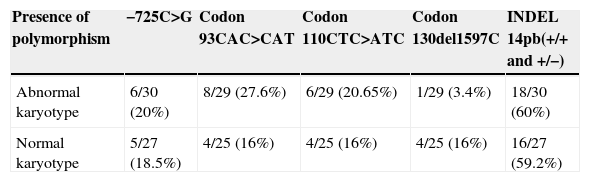
No significant differences were observed for the presence of the different polymorphisms (−725C>G, X2: 0.02; p=0.88) (codon 93, CAC>CAT, X2: 1.04; p=0.30) (codon 110, CTC>ATC, X2: 0.19; p=0.65) (codon 130, del1597C, X2: 2.5; p=0.11) (INDEL 14 pb, X2: 0.003; p=0.95) between abortions of normal and abnormal karyotypes. No increased incidence of the polymorphisms in the normal karyotype samples was found (Table 2).
Presence of polymorphism in the HLA-G gen.
| Presence of polymorphism | −725C>G | Codon 93CAC>CAT | Codon 110CTC>ATC | Codon 130del1597C | INDEL 14pb(+/+ and +/−) |
|---|---|---|---|---|---|
| Abnormal karyotype | 6/30 (20%) | 8/29 (27.6%) | 6/29 (20.65%) | 1/29 (3.4%) | 18/30 (60%) |
| Normal karyotype | 5/27 (18.5%) | 4/25 (16%) | 4/25 (16%) | 4/25 (16%) | 16/27 (59.2%) |
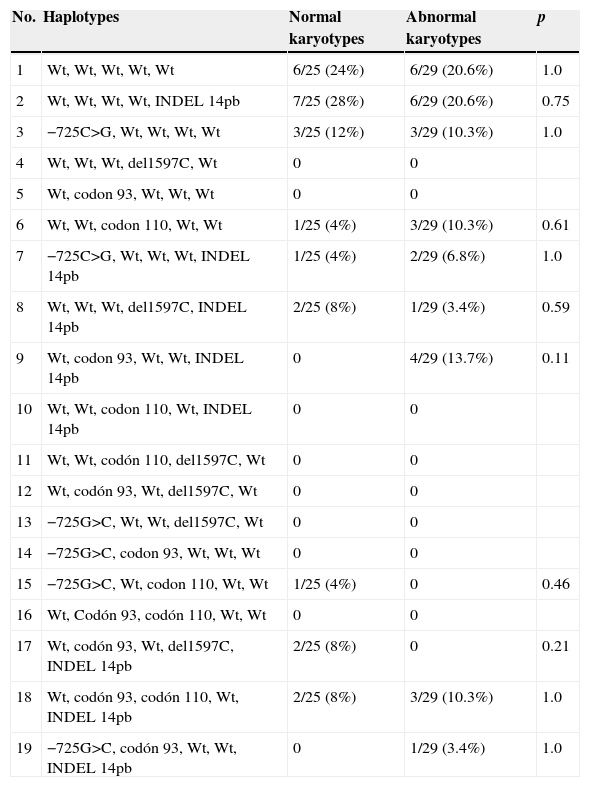
After detect afore mentioned polymorphisms in the HLA-G gene, a series of possible haplotypes was generated (see Table 3 for details).
Haplotypes generated of HLA-G.
| No. | Haplotypes | Normal karyotypes | Abnormal karyotypes | p |
|---|---|---|---|---|
| 1 | Wt, Wt, Wt, Wt, Wt | 6/25 (24%) | 6/29 (20.6%) | 1.0 |
| 2 | Wt, Wt, Wt, Wt, INDEL 14pb | 7/25 (28%) | 6/29 (20.6%) | 0.75 |
| 3 | −725C>G, Wt, Wt, Wt, Wt | 3/25 (12%) | 3/29 (10.3%) | 1.0 |
| 4 | Wt, Wt, Wt, del1597C, Wt | 0 | 0 | |
| 5 | Wt, codon 93, Wt, Wt, Wt | 0 | 0 | |
| 6 | Wt, Wt, codon 110, Wt, Wt | 1/25 (4%) | 3/29 (10.3%) | 0.61 |
| 7 | −725C>G, Wt, Wt, Wt, INDEL 14pb | 1/25 (4%) | 2/29 (6.8%) | 1.0 |
| 8 | Wt, Wt, Wt, del1597C, INDEL 14pb | 2/25 (8%) | 1/29 (3.4%) | 0.59 |
| 9 | Wt, codon 93, Wt, Wt, INDEL 14pb | 0 | 4/29 (13.7%) | 0.11 |
| 10 | Wt, Wt, codon 110, Wt, INDEL 14pb | 0 | 0 | |
| 11 | Wt, Wt, codón 110, del1597C, Wt | 0 | 0 | |
| 12 | Wt, codón 93, Wt, del1597C, Wt | 0 | 0 | |
| 13 | −725G>C, Wt, Wt, del1597C, Wt | 0 | 0 | |
| 14 | −725G>C, codon 93, Wt, Wt, Wt | 0 | 0 | |
| 15 | −725G>C, Wt, codon 110, Wt, Wt | 1/25 (4%) | 0 | 0.46 |
| 16 | Wt, Codón 93, codón 110, Wt, Wt | 0 | 0 | |
| 17 | Wt, codón 93, Wt, del1597C, INDEL 14pb | 2/25 (8%) | 0 | 0.21 |
| 18 | Wt, codón 93, codón 110, Wt, INDEL 14pb | 2/25 (8%) | 3/29 (10.3%) | 1.0 |
| 19 | −725G>C, codón 93, Wt, Wt, INDEL 14pb | 0 | 1/29 (3.4%) | 1.0 |
On the one hand we can see the frequency of occurrence of each haplotype in our population. Haplotypes 1 (no presence of polymorphism) and 2 (INDEL 14pb) were the most frequently found in our study population if compared to the rest of haplotypes.
Also, we can see the differences in the frequency of occurrence of a specific haplotype between normal and abnormal embryo karyotypes. No significant differences were observed, in any case, in the frequency of a haplotype specific for HLA-G between the normal and abnormal karyotype samples.
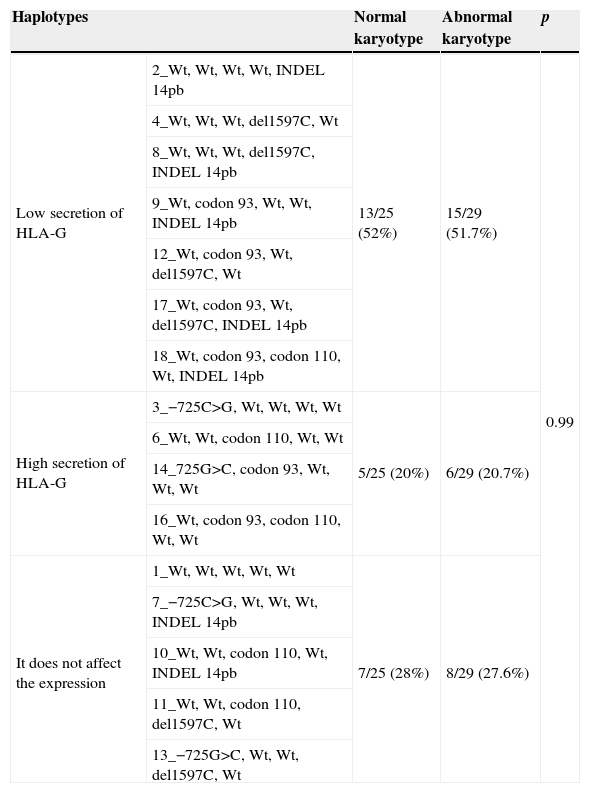
In addition, we wanted to group the different haplotypes generated according to the theoretical HLA-G secretion according to the published data (Table 4). Significant differences did not exist (p=0.99) as for the distribution of the haplotypes secretors, joined by levels of secretion, between abortions of normal and abnormal karyotypes.
Group of the haplotypes depending on his supposed HLA-G protein secretion.
| Haplotypes | Normal karyotype | Abnormal karyotype | p | |
|---|---|---|---|---|
| Low secretion of HLA-G | 2_Wt, Wt, Wt, Wt, INDEL 14pb | 13/25 (52%) | 15/29 (51.7%) | 0.99 |
| 4_Wt, Wt, Wt, del1597C, Wt | ||||
| 8_Wt, Wt, Wt, del1597C, INDEL 14pb | ||||
| 9_Wt, codon 93, Wt, Wt, INDEL 14pb | ||||
| 12_Wt, codon 93, Wt, del1597C, Wt | ||||
| 17_Wt, codon 93, Wt, del1597C, INDEL 14pb | ||||
| 18_Wt, codon 93, codon 110, Wt, INDEL 14pb | ||||
| High secretion of HLA-G | 3_−725C>G, Wt, Wt, Wt, Wt | 5/25 (20%) | 6/29 (20.7%) | |
| 6_Wt, Wt, codon 110, Wt, Wt | ||||
| 14_725G>C, codon 93, Wt, Wt, Wt | ||||
| 16_Wt, codon 93, codon 110, Wt, Wt | ||||
| It does not affect the expression | 1_Wt, Wt, Wt, Wt, Wt | 7/25 (28%) | 8/29 (27.6%) | |
| 7_−725C>G, Wt, Wt, Wt, INDEL 14pb | ||||
| 10_Wt, Wt, codon 110, Wt, INDEL 14pb | ||||
| 11_Wt, Wt, codon 110, del1597C, Wt | ||||
| 13_−725G>C, Wt, Wt, del1597C, Wt | ||||
Immunostaining was performed on 25 patients. Of the 27 trophoblast samples, 13 showed an abnormal karyotype and 14 had a normal karyotype.
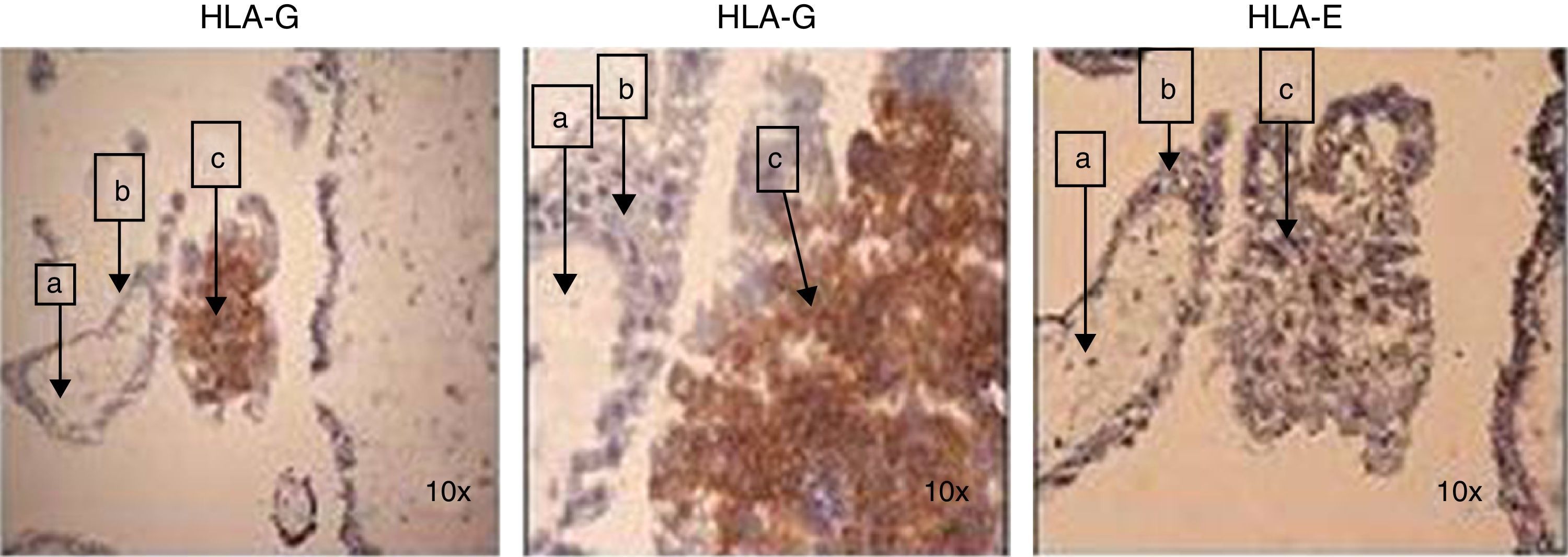
All the samples (100%) were positive for the HLA-G expression, while the HLA-E expression was weakly positive in only 14 of the 27 samples (51.8%) (Fig. 1).
No significant differences (p=0.75) were found in the mean HLA-G intensity in both the normal and abnormal karyotype samples (2.92 vs. 2.65, respectively). Neither the normal nor the abnormal types had significant differences (p=0.76) at the HLA-E expression level (1.17 vs. 1.44, respectively). Finally, there were no significant differences between observers (quadratic Kappa index >70%).
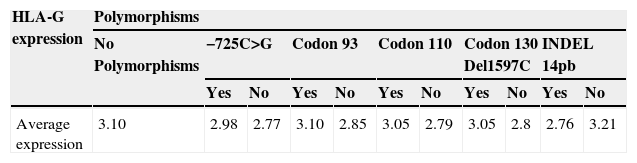
Association between polymorphisms and haplotypes with the protein expression of HLA-GNo association was observed between the presence of specific polymorphisms and HLA-G secretion at the cytotrophoblast level (Table 5). The presence of a particular polymorphism does not alter the expression of HLA-G (p=0.79).
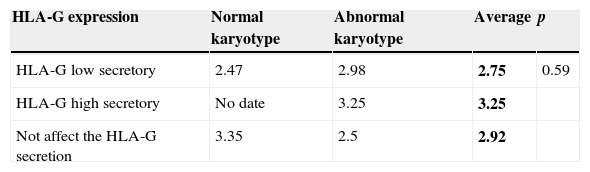
In addition, as mentioned above, the different generated haplotypes were grouped based on the theoretical HLA-G secretion in accordance with the literature. There is a correlation between the theoretical grouping of haplotypes and the real secretion of HLA-G produced by these haplotypes (Table 6). However, no significant differences were found in the average of HLA-G expression by cytotrophoblast cells between the three types of secretory haplotypes (2.75 vs. 3.25 vs. 2.92) (p=0.59) (Table 6).
DiscussionMany causes for first trimester spontaneous abortions have been proposed. Although the aetiology of the majority of spontaneous abortions lies in chromosomal anomalies, their pathology is associated at the end with the immune system responses (Vassiliadou et al., 1999; Olivares et al., 2002).
In this study, we decide to study the molecules of the HLA-G and HLA-E since they are good candidates to protect the embryo against the maternal immune system using several functions. It has been observed that the presence of some polymorphisms in the HLA-G gene is related with an alteration in the levels of HLA-G expression (Mendes-Junior et al., 2010; Rebmann et al., 1999, 2001; Ober et al., 1996; Chen et al., 2008; Gonzalez et al., 2010). Diminished or insufficient HLA-G expression can be associated with certain complications in pregnancy, such as pregnancy loss (Pfeiffer et al., 2000, 2001; Athanassakis et al., 1999; Alegre et al., 2007; Emmer et al., 2002).
In this study we aimed to determine the role of HLA-G by analyzing some genetic polymorphisms that might be relevant during pregnancy and which have been related with an altered HLA-G protein expression. Besides, we decided to evaluate the presence and quantity of the expression of this molecule, and also of the HLA-E expression, in the embryo cytotrophoblast of spontaneous abortion samples by comparing pregnancies which were probably arrested by a chromosomal vs. a non-chromosomal cause. However, neither the HLA-G genotype nor the HLA-G and HLA-E protein expression were different in chromosomally abnormal miscarriages compared to the normal ones. Furthermore no association was found between the HLA-G gen polymorphisms and local HLA expression in the trophoblast cells in any type of abortion.
The novel aspect of this work lies in using embryo trophoblast samples from early-stage abortions, using the technique known as hystero-embryoscopy, with which, almost certainly, is not affected by maternal contamination and obtain high reliability of the embryonic karyotype diagnosed (Ferro et al., 2003).
Despite other studies have employed placenta tissue from the first pregnancy after VPI (Hiby et al., 1999; Hviid et al., 2003) or abortion (Moreau et al., 2008), but they did not specify the method followed to obtain samples, so it is assumed that chromosome determination was done by conventional curettage. Some of the mentioned studies have looked for associations between certain genotypes with RNAm levels, but they did not analyze the actual protein HLA-G presence on the cytotrophoblast cells.
Polymorphisms of the HLA-G gene and their distribution between normal and abnormal karyotype miscarriagesAccording to our results, no differences were found in the frequency of the studied polymorphisms between trophoblast samples with normal and abnormal karyotypes. The frequency of the polymorphisms found in our study were within the range of other published data in women suffering of recurrent abortion (Hviid et al., 2002; Aruna et al., 2010; Sipak-Szmigiel et al., 2007).
Nowadays, there are contradictory results among the various genetic studies conducted on HLA-G. Some authors found no association between some polymorphisms and abortion, while others believe that a deletion or mutation in the HLA-G gene can affect the generation of the final protein, and can even lead to pregnancy loss. The variations between studies might be due to study design, small sample size, use of a coding or non-coding region, or even the analysed exon (Hviid et al., 2002). Besides, the results may vary depending on whether the polymorphisms in the HLA-G gene have been analysed in either the peripheral blood taken from the mother only, from either parents, or the foetal tissue itself that is unable to successfully achieve a term pregnancy. In our study the analysis we carried out in abortive tissue.
Generated haplotypesAfter grouping the polymorphisms in haplotypes, trophoblast samples that contain no polymorphisms in the HLA-G gene, as are haplotype 1 (wt) and INDEL 14 pb polymorphisms (haplotype 2), were significantly (p<0.05) the most frequent in both chromosomally normal and abnormal samples. To some extent, this corroborates the low polymorphism of the HLA-G gene as compared with other classic HLAs.
Despite no differences being observed in the distribution of a given polymorphism between normal and abnormal karyotype trophoblast samples, we wanted to evaluate if the haplotypes could show differing frequencies between our two study populations. However, no significant differences were found in the distribution of these haplotypes generated between normal and abnormal karyotype samples. In other words, samples with a normal and abnormal karyotype present a similar incidence of frequency in appearing in a given haplotype (see Table 3).
The same occurred when we grouped the haplotypes obtained according to their theoretical protein-secreting HLA-G. No significant differences were observed for the frequency of secreting HLA-G haplotypes, which were grouped according to secretion levels, between trophoblast samples with normal and abnormal karyotypes (see Table 4).
Although many studies have analysed the frequency of a certain haplotype in a group of individuals, even today, there is no study which compares the frequency with which a haplotype appears in spontaneous abortion patients, and none has compared chromosomally normal and abnormal abortions.
Expression of HLA-G and HLA-EVariations the HLA-G gene can entail alterations in the immune response by either affecting the functionality and superficial expression of the HLA-G molecule (Van der Ven et al., 1998) or by affecting the sequence of its leader peptide. This sequence is indispensable for the superficial HLA-E expression, which would imply in the embryo lacking protection against the mother (Hviid et al., 1999; O’Callaghan and Bell, 1998).
According to the literature, the polymorphisms studied in this project (−725C>G, codon 93, codon 110, codon 130 and INDEL 14bp) do not relate with changes in the tertiary HLA-G structure. In principle, the interaction functions of this molecule with others should not be affected; for example, the inhibition of NK cells by the interaction with their receptors. Nonetheless, all these polymorphisms have been related with changes in the superficial HLA-G expression level, so, when the quantity of HLA-G increases excessively and when it also lowers, some of its functions might be affected.
After detecting the presence of some HLA-G polymorphisms in our study samples, we decided to evaluate the impact they have on the protein secretion of the HLA-G molecule in the embryonic trophoblast, and its effect on the HLA-E expression.
According to our data, the immunohistochemistry technique did not reveal significant differences in the HLA-G expression between trophoblast samples from normal and abnormal karyotype abortions. Abnormal ones were employed as reference samples where the cause of abortion was almost certainly the chromosomal anomaly. Thus, we cannot state that in those cases of cause of abortion not known or chromosomally normal abortion(s) the cause of arrested pregnancy is an absent or diminished HLA-G expression in the foetal cytotrophoblast. Besides, our data coincide with those reported by other authors (Bhalla et al., 2006; Patel et al., 2003), where HLA-G does not appear to be a molecule that determines pregnancy maintenance. Furthermore, we wanted to see the distribution and intensity of the HLA-E protein expression in the trophoblast samples and whether its expression is somehow conditioned by HLA-G. It is well-known that for the HLA-E expression to appear on the cell surface, the presence of a leader peptide from other class I HLA molecules is required. As mentioned earlier, there is only one HLA-G expression in the cytotrophoblast, so the HLA-E expression will be subjected to the leader peptide of HLA-G and, therefore, indirectly to the HLA-G expression. After observing that some samples express HLA-G in the embryo cytotrophoblast, but they cannot express HLA-E, we can specify that the HLA-G expression is not determinant enough to lead to the HLA-E expression. These data contradict findings reported by other authors (Ishitani et al., 2003; Llano et al., 1998) who believe that wherever a soluble HLA-G expression, or one joined to the membrane, is found, there must be a HLA-E expression. Hence, we assume that many other factors must influence this surface expression mechanism.
We observed a considerably and generally reduced HLA-E expression in our studied samples as compared to the HLA-G expression. Besides, and coinciding with the fact that no correlation was found with HLA-G, we observed no significant differences in the HLA-E expression between the trophoblast samples from normal and abnormal karyotype abortions. Therefore, we can only assume that either their diminished expression or the fact that HLA-E was absent in some cases in the embryo cytotrophoblast is not responsible for pregnancy loss. Our results coincide with those of similar study by another author (Bhalla et al., 2006).
Association between the studied polymorphisms, and their corresponding haplotypes, and the HLA-G protein expressionIn our study, no significant differences were observed in the HLA-G expression for each polymorphism between normal and abnormal karyotype samples. After grouping samples irrespectively of their embryonic karyotype, and having taken the mean HLA-G expression, no significant differences (p=0.79) were obtained in the mean HLA-G expression between the trophoblast samples containing no polymorphism (a value of 3.1) and those that did (values of 2.7–3.1). Therefore, we deduce that the presence of a specific polymorphism does not influence the HLA-G expression levels in the trophoblast samples (see Table 5).
Despite each polymorphism not presenting differences in the HLA-G expression, we wished to see if there were any differences in the HLA-G expression when grouping polymorphisms and generating different haplotypes. Haplotypes were grouped in function of their theoretical secretion of HLA-G described in the literature. The HLA-G expression in the three generated secreting haplotypes corresponded to the theoretical secreting haplotype kind (2.75 for the low-secreting ones, 3.25 for the high-secreting ones and 2.92 for the haplotypes that did not affect expression) (see Table 6). However, these differences in expression of HLA-G among the three generated secretory haplotypes were not statistically significant (p=0.59). Besides, no haplotype was found which presented a significantly higher HLA-G expression than another haplotype. Also, no significant differences were found in the HLA-G expression of the secreting haplotypes in accordance with a normal or abnormal karyotype kind.
ConclusionBased on the data obtained in the present study it seems that, HLA-G production in the foetal-maternal interphase is carried out constitutively, and no significant differences are noted in the various polymorphic variants or among the protein expression data among arrested pregnancies due to chromosomal and unknown causes.
Given the immunocompetent machinery in the human uterus that facilitates pregnancy being accepted, we are inclined to believe that pregnancy loss must depend on other regulation defects that are not necessarily triggered by the HLA-G molecule, such as genetic anomalies in NK cells or their inhibitory receptors, to such an extent that they do not properly perform their beneficial functions to maintain pregnancy or a specific maternal immunological situation at this specific gestational time. Therefore, we consider that a study in the deciduas using our study model could contribute new data in future studies to clarify causes of pregnancy loss.
FundingThis project was supported by R+D programme from the Centre for Industrial Technological Development (CDTI, ID 2007003), a public corporation of the Spanish Ministry of Economy and Competitiveness and the IMPIVA R+D (IM IDTF/2007/166) programme of the Generalitat Valenciana (Regional Valencian Government).
Conflict of interestNone of the authors declared a conflict of interest.
Thanks to the general operating room of the IVI Valencia for the withdrawal of samples, to IVIOMICS laboratory for his help in the analysis of the samples.