Kisspeptin hormone has been recently suggested as a new candidate drug to indirectly stimulate secretion of gonadotropins providing a more physiological approach to the ovarian function, due to concerns about the use of gonadotropins such as the risk of ovarian hyperstimulation syndrome (OHSS). This review describes and compares the results of those clinical trials carried out hitherto with exogenous administration of kisspeptin on humans, with special interest on those focused on healthy women. In addition, the potential use of this hormone on programming and inducing ovulation as an alternative for choriogonadotropin (CG) and GnRH agonists in controlled ovarian stimulation (COS) is also addressed.
Material and methodsMEDLINE/Pubmed (US National Library of Medicine) database was searched for articles published between 2000 and 2014. All searches included the terms ‘kisspeptin’, ‘ovarian stimulation’ and ‘infertility’. Special interest was given to clinical trials.
ResultsAdministration of kisspeptin seems effective in activating the LH surge in women. All trials reported high increases of LH above baseline levels in the preovulatory phase of the cycle. Recent results indicate that a single subcutaneous injection of kisspeptin-54 is enough to induce egg maturation. This strategy might offer the advantage of avoiding OHSS and without the necessity of following a freeze-all strategy as pregnancy rates are maintained after its use. Furthermore, clinical trials show that administration of this hormone is not harmful for women and does not affect their reproductive physiology.
DiscussionIf kisspeptin is able to offer an acceptable ratio of metaphase II oocytes per follicle aspirated with no risk of OHSS and without affecting pregnancy rates in fresh cycles it would be definitely a good option for triggering. Before implementing its routine use, well-designed studies should be carried out in the human model in order to compare IVF results between the different types of triggering proposed to date.
La hormona kisspeptina se ha revelado recientemente como una nueva candidata para la estimulación indirecta de la secreción de gonadotropinas, brindando un efecto más parecido al comportamiento fisiológico del ovario, con el objetivo de evitar riesgos asociados al uso de gonadotropinas, tales como el síndrome de hiperestimulación ovárica (SHO). Esta revisión describe y compara los resultados de los ensayos clínicos efectuados hasta la fecha, con administración exógena de kisspeptina en seres humanos, centrados principalmente en mujeres sanas. Además, se analiza el uso potencial de esta hormona en la práctica clínica, específicamente para la programación y la inducción de la ovulación como alternativa a la gonadotropina coriónica y los agonistas de GnRH en tratamientos de estimulación ovárica.
Materiales y métodosSe realizaron búsquedas en la base de datos MEDLINE/Pubmed (US National Library of Medicine) de artículos publicados entre 2000 y 2014. Todas las búsquedas incluyeron los términos ‘kisspeptina’, ‘estimulación ovárica’ e ‘infertilidad’. Se prestó especial interés a las publicaciones sobre ensayos clínicos.
ResultadosEn mujeres infértiles la kisspeptina parece resultar eficaz, al activar el pico de LH durante la estimulación ovárica. Los resultados recientes indican que basta con una inyección subcutánea de Kisspeptina-54 para inducir la maduración ovocitaria. Esta hormona podría ofrecer la ventaja de evitar el riesgo de SHO sin tener que llevar a cabo una estrategia de “congelar todo”, si se mantienen las tasas de embarazo tras su uso. Además, los ensayos clínicos muestran que la administración de esta hormona no presenta efectos adversos en las pacientes y no afecta a su fisiología reproductiva.
DiscusiónSi la kisspeptina ofreciera un ratio aceptable de ovocitos metafase II por folículo aspirado sin riesgo de SHO, y sin afectar a las tasas de embarazo en ciclos en fresco, constituiría una muy buena opción para programar e inducir la ovulación. Sin embargo, antes de implementar su uso en la práctica clínica habitual, se deben ejecutar más ensayos clínicos aleatorizados para determinar las dosis apropiadas y poder comparar los resultados clínicos con otros inductores de la ovulación propuestos hasta la fecha.
The use of gonadotropins for ovarian stimulation in assisted reproductive treatments (ART) to increase chances of pregnancy insofar as more than a single oocyte is obtained is extensive. Since these drugs were introduced into clinical practice, there is concern about the potential risks derived from their use, such as onset of ovarian hyperstimulation syndrome (OHSS), that is usually mild or moderate, but can be severe or even lethal in some cases (Whelan and Vlahos, 2000). After becoming aware of this problem and attempts having been made to avoid it, in the last decade some strategies have been described to prevent OHSS; e.g., ovulation induction with gonadotropin-releasing hormone (GnRH) agonist in the GnRH antagonist treatment context (Humaidan et al., 2011) to prevent early onset of OHSS; or oocyte and embryo vitrification to prevent late onset of OHSS (Martinez et al., 2013).
Agonist triggering induces LH to peak by simulating the physiological mechanism of final oocyte maturation and ovulation. Its advantage lies in the fact that it avoids the administration of choriogonadotropin (CG) which, in some cases, is the origin of vascular endothelial growth factor (VEGF) release. This, in turn, causes increased capillary permeability and, therefore, the most serious manifestations of OHSS (Soares, 2012).
In the last 10 years, the kisspeptin system, composed of the ligand kisspeptin encoded by the Kiss1 gene and its receptor GPR54 or KissR, has generated interest (Pasquier et al., 2014) and kisspeptin has been proposed as a new drug candidate to substitute all these hormones. In humans, kisspeptin is capable of inducing the luteinizing hormone (LH) surge, thus it could be used to trigger ovulation in controlled ovarian stimulation (COS) treatments with the additional advantage of avoiding OHSS and providing a similar behaviour to the ovarian physiology (Thomsen and Humaidan, 2015).
Kisspeptin (Kp or Kiss) is a peptide hormone secreted in the hypothalamus, but it is also produced in other tissues such as the placenta, and in very large amounts (Horikoshi et al., 2003). To date, isoforms Kp-10 and Kp-54 have been widely studied and isolated, and their numbers refer to the amount of the peptide's amino acids. All the isoforms of kisspeptin come from a 145-amino acid precursor encoded by the KiSS1 gene, and a C-terminal RF-amide decapeptide is responsible for both its affinity to the receptor and its biological activity (Kotani et al., 2001).
Scientific and academic interest shown in the therapeutic potential of this hormone increased after the discovery that some patients with Hypogonadotropic Hypogonadism (HH) presented mutations in the gene of protein receptor GPR54, which is found in GnRH secretory fibres and whose ligand is the kisspeptin hormone. Knock-out mice for the GPR54 gene have also been diagnosed with HH and displayed delayed puberty (Seminara et al., 2003; de Roux et al., 2003).
The role of kisspeptin on the Hypothalamic Pituitary Gonadal axis (HPG) has been extensively studied in both human and animal models. Some authors have even suggested that we are currently living in the ‘Kisspeptin Era’ of human reproduction (Kauffman, 2010). There are many reviews about the molecular and neurological control mechanisms of Kisspeptin on the HPG axis (Clarkson et al., 2010; Lehman et al., 2010; Tena-Sempere, 2010; Wahab et al., 2011; Roa et al., 2011; Sonigo and Binart, 2012; Beltramo et al., 2014; Skorupskaite et al., 2014).
After a brief description as to how this hormone controls the HPG axis in animals (and allegedly in humans), this review analyses the results of those clinical trials carried out hitherto with exogenous kisspeptin administration in humans, and shows special interest in those that have focused on healthy women. The potential use of this hormone in clinical practice is also addressed, specifically its potential applications in programming and inducing ovulation as an alternative to CG and GnRH agonists in COS.
MethodsSearches for the articles published between 2000 and 2015 were done in the MEDLINE/Pubmed (US National Library of Medicine) database. All the searches included the keyword ‘kisspeptin’. The keywords ‘ovarian stimulation’ and ‘infertility’ were also used in each search. Articles were classified as review articles or research articles. Another search was done using the keyword ‘kisspeptin’ and the ‘clinical trials’ filter of the database. Those that focussed on clinical trials were analysed separately to extensively analyse their main objectives and protocols.
Given that this review targets such a state-of-the-art topic, searches for authors were also done. The selected authors were those who had been previously mentioned in clinical trial publications as the main authors or as research group leaders of clinical centres that currently carry out trials with the kisspeptin hormone. Only those articles written in English were included in the review. All the found and selected articles can be accessed. Eighty articles were retrieved from the database searches, of which 14 were clinical trials performed in humans, while the rest were review and research articles. Only the review articles about the kisspeptin molecular mechanism of action and the studies carried on animal models that described the same topic were included in this review. All the above-mentioned clinical trials were analysed.
ResultsSignalling mechanism of kisspeptin on the HPG axisIn order to describe the anatomy of secretory nerve fibres of kisspeptin, research groups have mostly used animal models. However, these nerve fibres have already been identified in the infundibular nucleus of the human hypothalamus (Rometo et al., 2007) and in the rostral preoptic area (Hrabovszky et al., 2010). Nevertheless, the murine model is the most extensively studied animal model for kisspeptin signalling pathways and the role that kisspeptin plays in the control of the HPG axis. Using knockout mice models of the kisspeptin receptor GPR54, it has been demonstrated that kisspeptin stimulates secretion of GnRH in the hypothalamus, which occurs through the activation of its receptor in hypothalamic GnRH fibres (Messager et al., 2005; d’Anglemont de Tassigny et al., 2008).
In fact, hypothalamic expression of the genes KISS-1 and GPR54 has been noted to be maximal at puberty and thus necessary to regulate the HPG axis and unleash sexual maturation (Navarro et al., 2005b). Kisspeptin might act through its receptor in GnRH fibres both by modulating neuronal activity enhancing expression of GnRH RNA and by stimulating the release of GnRH to the median eminence (Messager et al., 2005; Novaira et al., 2009). Moreover, it has been also demonstrated that exogenous administration of this hormone indirectly results in the secretion of gonadotropins to the bloodstream (Navarro et al., 2005a,b).
It has also been suggested that sex steroids carry out their feedback loops by means of the Kiss1/GPR54 system. In respect to negative feedback, sex steroids might act directly on expression and secretion of neuropeptides such as neurokinin B and dynorphin, which in turn modulate secretion of kisspeptin. There is strong evidence for the expression of these neuropeptides in kisspeptin fibres (Navarro et al., 2009; Hrabovszky et al., 2010). It has been suggested that steroids may prevent the expression of neurokinin B, but stimulate expression of dynorphins, thus resulting in a lower kisspeptin expression, which in turn down-regulates the HPG axis (Skorupskaite et al., 2014).
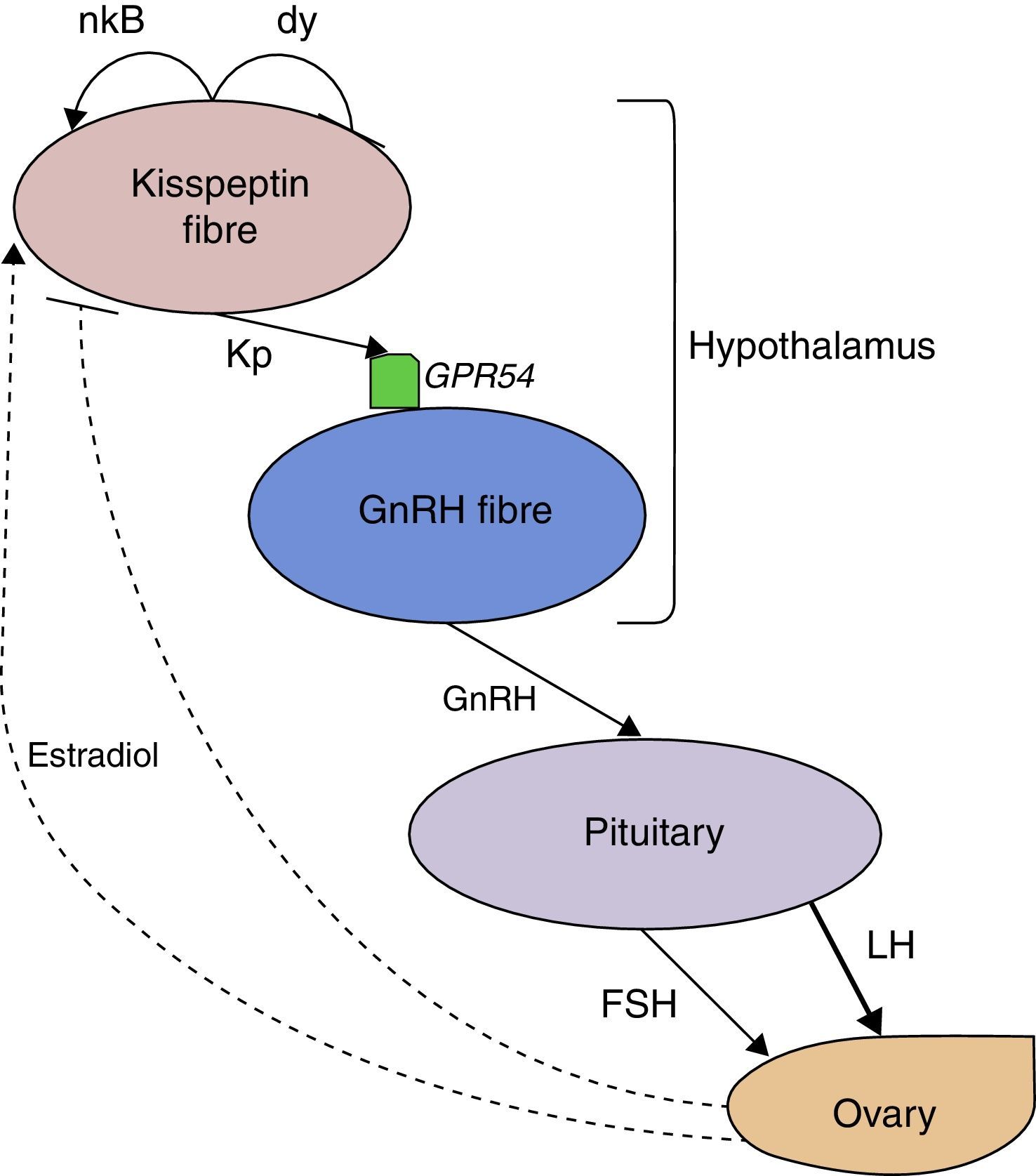
On the other hand, positive feedback of sex steroids leading to the LH surge appears to be also mediated by kisspeptin fibres. In the preovulatory phase of reproductive cycles of mice, oestrogens produced by gonads activate oestrogen alpha receptors (ERα), which are located in kisspeptin secretory fibres and are absent in GnRH fibres, to result in the secretion of GnRH and, consequently, of the luteinising hormone (LH) surge that induces ovulation (Clarkson et al., 2008). This suggests that kisspeptin performs the upstream regulation of the HPG axis. Other studies in mammals indicate that administration of GnRH antagonists can prevent the LH surge from occurring after kisspeptin (Gottsch et al., 2004), which, in turn, implies that kisspeptin acts directly on GnRH fibres and not at a pituitary level. This stresses the pivotal role of the Kiss1/GPR54 system on the HPG axis signalling pathways. Fig. 1 schematises the most accepted model of kisspeptin control on the HPG axis based on the murine model and accepted in mammals.
Alleged signalling pathway of Kisspeptin on the HPG axis. Kisspeptin secreted in the hypothalamus binds to its receptor GPR54 and stimulates the secretion of GnRH, which results in gonadotropin production by the pituitary. The effect on LH production is greater than FSH's. Dotted arrows show the putative role in the positive and negative feedback of oestradiol through the Kiss1/GPR54 system. The co-expression and secretion of neuropeptides neurokinin B and dynorphin, which probably mediate feedback responses, are also represented. Kp: kisspeptin, nk B: neurokinin B, dy: dynorphin, LH: luteinising hormone, FSH: follicle-stimulating hormone.
The numbers 10 and 54 refer to the quantity of the amino acids of each isoform of kisspeptin after processing the 145-amino acid precursor encoded by the KiSS1 gene. Even though the C-terminal decapeptide of the isoforms suffices to activate the GPR54 receptor (Kotani et al., 2001), some differences in their effects have been observed after administrating both isoforms to humans. For instance, in all the studies conducted with Kp-54, gonadotropin secretion has been stimulated (Dhillo et al., 2005, 2007; Jayasena et al., 2009, 2010, 2013a, 2014a,b).
However, results with intravenous (IV) boluses of Kp-10 appear to be somewhat inconsistent given that in some studies Kp-10 failed to do so in the follicular phase of healthy women (Jayasena et al., 2011), but has increased gonadotropin release above baseline in others (George et al., 2012; Chan et al., 2012). These differences in the effects of both isoforms might be due to their different times of action in the bloodstream after its administration.
The group of Jayasena et al. (2011) estimated the half-life of Kp-10 in the human bloodstream to be approximately 4min. At the same time, they detected Kp-54 in the bloodstream 4h after IV injection. This might, therefore, be the reason why Kp-54 appears more effective to elicit a response from the hypothalamus and pituitary. Nonetheless, detection assays of kisspeptin in the bloodstream are conducted mostly with immunoreactivity assays, which can also detect the products of the metabolism of this hormone, thus, small biologically inactive peptides may have interfered with the aforementioned estimations (Chan, 2013).
Another peculiarity of the isoform 54 is that its administration to women with hypothalamic amenorrhoea resulted in tachyphylaxis after two daily subcutaneous injections for 2 weeks. Moreover, a partial desensitisation to this isoform has been reported after administrating two doses per week for 2 weeks (Jayasena et al., 2009, 2010). Desensitization has not been reported when administering the isoform Kp-10, again perhaps due to its shorter half-life.
Despite the differences in effects of both isoforms, it is clear that Kp-54 administration achieves the release of gonadotropins in more cases than isoform Kp-10. However, Kp-10 does not lack biological activity. Studies administering kisspeptin to humans based their decision of which isoform to use on previous experiences with an isoform and its properties. For instance, the effects of Kp-10 were tested for the first time on men (George et al., 2011) and women (Jayasena et al., 2011) because there was at the moment no data on humans and also because Kp-10 seemed to posses a stronger pharmacological potential than Kp-54 given its cheaper and easier manufacture.
Later on, due to the fact that Kp-10 resulted in a lower magnitude of LH secretion compared to Kp-54 (Jayasena et al., 2011), some further studies focused their research on the long isoform because they considered that Kp-54 had more potential to be used therapeutically and because it previously proved to be able to stimulate LH secretion after a subcutaneous single bolus in the follicular phase (Dhillo et al., 2007; Jayasena et al., 2011). It is clear, however, that more research is required to characterise each isoform and to determine which is the most ideal to be used in ARTs.
Ways of exogenous kisspeptin administrationLack of uniformity among clinical trials as to how to administer kisspeptin to women makes it more difficult to compare results from trial to trial and to distinguish the most advantageous protocol. Some studies have claimed that subcutaneous (SC) injections exert more efficient effects because of good absorption to the bloodstream (Dhillo et al., 2007). Nevertheless, the same research group has stated that Kisspeptin may face degradation in subcutaneous tissue; therefore, IV boluses guarantee a higher level of the peptide in blood, which would result in more sustained effects (Jayasena et al., 2011).
Both SC and IV injections have resulted in gonadotropin release, but there is still no evidence to prove that one way of administration is better than the other. Nevertheless, single bolus injections (either SC or IV) are preferable to infusions or multiple boluses because of higher feasibility. A single injection is surely less disturbing to patients and can be employed in a protocol more easily. Intravenous administration has resulted in shorter periods of elevated circulating kp-10 levels compared to subcutaneous administration, and IV is clearly more time-consuming (Jayasena et al., 2011).
Clinical trials with kisspeptinAs mentioned earlier, most of the knowledge available about kisspeptin and its biological functions stems from the experimentation done with animal models. However, this section of the review describes the main outcomes of the clinical trials conducted in humans and the effects of kisspeptin on gonadotropin production by the pituitary, with a special emphasis on healthy women.
Study subjectsKnowing the sex of study subjects is extremely important to accurately interpret the results of trials because it has been evidenced that the effects of kisspeptin administration present a strong sexual dimorphism. For instance, the doses that generate very high serum LH levels in men have no effect in the follicular phase of healthy women (Dhillo et al., 2005; George et al., 2011; Jayasena et al., 2011; Chan et al., 2011) (Table 1).
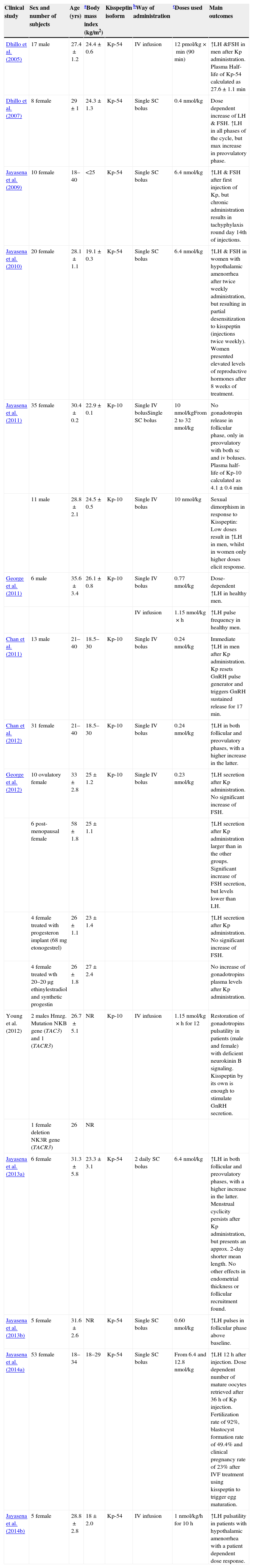
Characterisation of study subjects of 14 clinical trials using kisspeptin in humans.
| Clinical study | Sex and number of subjects | Age (yrs) | aBody mass index (kg/m2) | Kisspeptin isoform | bWay of administration | cDoses used | Main outcomes |
|---|---|---|---|---|---|---|---|
| Dhillo et al. (2005) | 17 male | 27.4±1.2 | 24.4±0.6 | Kp-54 | IV infusion | 12pmol/kg×min (90min) | ↑LH &FSH in men after Kp administration. Plasma Half-life of Kp-54 calculated as 27.6±1.1min |
| Dhillo et al. (2007) | 8 female | 29±1 | 24.3±1.3 | Kp-54 | Single SC bolus | 0.4nmol/kg | Dose dependent increase of LH & FSH. ↑LH in all phases of the cycle, but max increase in preovulatory phase. |
| Jayasena et al. (2009) | 10 female | 18–40 | <25 | Kp-54 | Single SC bolus | 6.4nmol/kg | ↑LH & FSH after first injection of Kp, but chronic administration results in tachyphylaxis round day 14th of injections. |
| Jayasena et al. (2010) | 20 female | 28.1±1.1 | 19.1±0.3 | Kp-54 | Single SC bolus | 6.4nmol/kg | ↑LH & FSH in women with hypothalamic amenorrhea after twice weekly administration, but resulting in partial desensitization to kisspeptin (injections twice weekly). Women presented elevated levels of reproductive hormones after 8 weeks of treatment. |
| Jayasena et al. (2011) | 35 female | 30.4±0.2 | 22.9±0.1 | Kp-10 | Single IV bolusSingle SC bolus | 10nmol/kgFrom 2 to 32nmol/kg | No gonadotropin release in follicular phase, only in preovulatory with both sc and iv boluses. Plasma half-life of Kp-10 calculated as 4.1±0.4min |
| 11 male | 28.8±2.1 | 24.5±0.5 | Kp-10 | Single IV bolus | 10nmol/kg | Sexual dimorphism in response to Kisspeptin: Low doses result in ↑LH in men, whilst in women only higher doses elicit response. | |
| George et al. (2011) | 6 male | 35.6±3.4 | 26.1±0.8 | Kp-10 | Single IV bolus | 0.77nmol/kg | Dose-dependent ↑LH in healthy men. |
| IV infusion | 1.15nmol/kg×h | ↑LH pulse frequency in healthy men. | |||||
| Chan et al. (2011) | 13 male | 21–40 | 18.5–30 | Kp-10 | Single IV bolus | 0.24nmol/kg | Immediate ↑LH in men after Kp administration. Kp resets GnRH pulse generator and triggers GnRH sustained release for 17min. |
| Chan et al. (2012) | 31 female | 21–40 | 18.5–30 | Kp-10 | Single IV bolus | 0.24nmol/kg | ↑LH in both follicular and preovulatory phases, with a higher increase in the latter. |
| George et al. (2012) | 10 ovulatory female | 33±2.8 | 25±1.2 | Kp-10 | Single IV bolus | 0.23nmol/kg | ↑LH secretion after Kp administration. No significant increase of FSH. |
| 6 post-menopausal female | 58±1.8 | 25±1.1 | ↑LH secretion after Kp administration larger than in the other groups. Significant increase of FSH secretion, but levels lower than LH. | ||||
| 4 female treated with progesteron implant (68mg etonogestrel) | 26±1.1 | 23±1.4 | ↑LH secretion after Kp administration. No significant increase of FSH. | ||||
| 4 female treated wth 20–20μg ethinylestradiol and synthetic progestin | 26±1.8 | 27±2.4 | No increase of gonadotropins plasma levels after Kp administration. | ||||
| Young et al. (2012) | 2 males Hmzg. Mutation NKB gene (TAC3) and 1 (TACR3) | 26.7±5.1 | NR | Kp-10 | IV infusion | 1.15nmol/kg×h for 12 | Restoration of gonadotropins pulsatility in patients (male and female) with deficient neurokinin B signaling. Kisspeptin by its own is enough to stimulate GnRH secretion. |
| 1 female deletion NK3R gene (TACR3) | 26 | NR | |||||
| Jayasena et al. (2013a) | 6 female | 31.3±5.8 | 23.3±3.1 | Kp-54 | 2 daily SC bolus | 6.4nmol/kg | ↑LH in both follicular and preovulatory phases, with a higher increase in the latter. Menstrual cyclicity persists after Kp administration, but presents an approx. 2-day shorter mean length. No other effects in endometrial thickness or follicular recruitment found. |
| Jayasena et al. (2013b) | 5 female | 31.6±2.6 | NR | Kp-54 | Single SC bolus | 0.60nmol/kg | ↑LH pulses in follicular phase above baseline. |
| Jayasena et al. (2014a) | 53 female | 18–34 | 18–29 | Kp-54 | Single SC bolus | From 6.4 and 12.8nmol/kg | ↑LH 12h after injection. Dose dependent number of mature oocytes retrieved after 36h of Kp injection. Fertilization rate of 92%, blastocyst formation rate of 49.4% and clinical pregnancy rate of 23% after IVF treatment using kisspeptin to trigger egg maturation. |
| Jayasena et al. (2014b) | 5 female | 28.8±2.8 | 18±2.0 | Kp-54 | IV infusion | 1nmol/kg/h for 10h | ↑LH pulsatility in patients with hypothalamic amenorrhea with a patient dependent dose response. |
Most of the studies performed on humans have taken into account only healthy individuals, except for three which included women with hypothalamic amenorrhoea (Jayasena et al., 2009, 2010, 2014b) and one whose study subjects were men presenting deficiencies in the signalling pathway of neurokinin B in the hypothalamus caused by mutations in ligand neurokinin B and its receptor (Young et al., 2013). Table 1 shows the main clinical trials related to the administration of kisspeptin performed both in males and females.
The reason why studying subjects with hormonal disorders has been included in some works depends on their research objectives. For example, those studies that assessed the effects of kisspeptin on women with hypothalamic amenorrhoea intended to observe how kisspeptin can trigger secretion of sex hormones and to, thus, assess its potential to restore menstrual cyclicity in these patients. Although gonadotropins secretion was observed after administering kisspeptin, the effect did not suffice to eliminate amenorrhoea in these patients (Jayasena et al., 2009, 2010, 2014b).
In addition, kisspeptin administration in patients with a neurokinin-pathway deficiency resulted in increased serum levels of gonadotropins which, in turn, confirmed the upstream role of neurokinin B on the HPG axis (Young et al., 2013). Kisspeptin has, therefore, been suggested to be a potential treatment for these patients.
Kisspeptin administration in healthy womenIn order to obtain a more accurate view of the potential of kisspeptin to trigger egg maturation, and hence ovulation, it is necessary to specially focus on those studies performed on healthy women. Seven clinical trials performed in humans of the 14 analysed included only healthy women.
All women in the studies presented spontaneous natural cycles (Table 2) except for one study which included six postmenopausal women (as a low sex steroid and a high gonadotropin condition) and eight other women using either contraceptive pill or progestogen contraceptive implants, so as to observe responses of kisspeptin administration in a high sex steroid and a low gonadotropin condition (George et al., 2012).
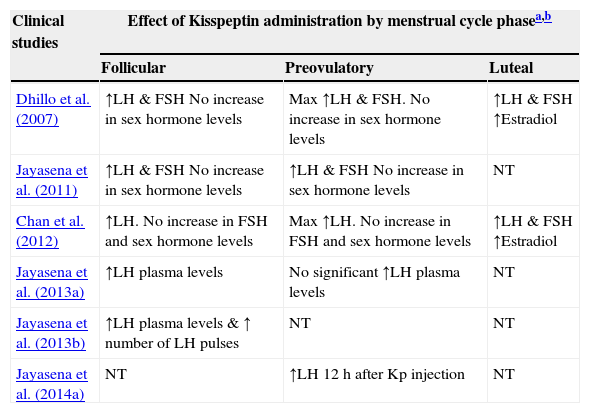
Effects of Kisspeptin administration to healthy women.
| Clinical studies | Effect of Kisspeptin administration by menstrual cycle phasea,b | ||
|---|---|---|---|
| Follicular | Preovulatory | Luteal | |
| Dhillo et al. (2007) | ↑LH & FSH No increase in sex hormone levels | Max ↑LH & FSH. No increase in sex hormone levels | ↑LH & FSH ↑Estradiol |
| Jayasena et al. (2011) | ↑LH & FSH No increase in sex hormone levels | ↑LH & FSH No increase in sex hormone levels | NT |
| Chan et al. (2012) | ↑LH. No increase in FSH and sex hormone levels | Max ↑LH. No increase in FSH and sex hormone levels | ↑LH & FSH ↑Estradiol |
| Jayasena et al. (2013a) | ↑LH plasma levels | No significant ↑LH plasma levels | NT |
| Jayasena et al. (2013b) | ↑LH plasma levels & ↑ number of LH pulses | NT | NT |
| Jayasena et al. (2014a) | NT | ↑LH 12h after Kp injection | NT |
Two isoforms of exogenous kisspeptin were administered to women: Kp-54 and Kp-10. The choice of which isoform to employ in each study depended on the properties of each above-mentioned isoform. Both the infusion and single bolus injections were used in the trials; however, only the effects of single bolus injections of kisspeptin were further analysed since this way of administration seems to offer more clinical potential than a long-lasting infusion of the hormone.
Response variability during the menstrual cycleIn the studies, kisspeptin was administered in a specific cycle phase, whether it was the follicular, preovulatory or luteal phase depended on the objectives of the study in order to describe the responses in each phase. Kisspeptin administration in the follicular phase of all healthy women led to LH to subtly increase above the baseline levels (Dhillo et al., 2007; Jayasena et al., 2011; Chan et al., 2012; George et al., 2012; Jayasena et al., 2013a,b). The increase in LH above the baseline levels was even slighter in the patients who used combined oral contraceptive pill or progestogen implants (George et al., 2012). Only one study showed that IV boluses as high as 10nmol/kg of isoform Kp-10 resulted in a non-significant increase above the baseline level compared to the saline control (Jayasena et al., 2011).
Administration of both Kp-54 and Kp-10 in the preovulatory phase (15–16 days before their next predictive period) gave rise to sharp increases in LH above the baseline levels in all the trials, with serum levels peaking up to 61.3±16.7IU/L after 40min of an IV single bolus of Kp-10 at 0.24nmol/kg (Chan et al., 2012). So since the highest LH levels were obtained immediately after kisspeptin administration in the preovulatory phase of cycles, such increases were considered to be the LH surge, like the one which occurs naturally, in order to restore oocyte maturation and to induce ovulation. Table 1 summarises the ways of kisspeptin administration, whose isoform was used, as were the dose used in the studies that resulted in the highest LH levels in the preovulatory phase and the time when these peaked.
With respect to the luteal phase, only two studies have addressed the effect of Kisspeptin in this phase of the menstrual cycle. In both studies, administration of this hormone elicits an immediate response increasing LH and FSH plasma levels, as it also happens in the preovulatory phase (Dhillo et al., 2007; Chan et al., 2012). However, the magnitude of this increases are considerably lower than in the preovulatory phase, but at the same time, always higher than in the follicular phase. The fact that higher magnitudes of gonadotropin secretion are reported in the luteal phase in contrast to the follicular phase might be due to a change in pituitary sensitivity during the menstrual cycle (Chan et al., 2012).
All the studies that measured FSH levels after kisspeptin administration reported very low or no increases above the baseline levels, unlike what happens with LH, which is apparently due to the different pulsatility patterns of GnRH when its secretion is stimulated by exogenous doses of kisspeptin which, in turn, favours LH secretion (George et al., 2012). For serum oestrogen, no significant increases in the baseline levels were observed in any cycle phases after kisspeptin administration.
Kisspeptin as a novel therapeutic optionClinical trials using kisspeptin have shown that administration of this hormone is not harmful for women and does not affect their reproductive physiology. Regarding its use in IVF cycles, a proof-of-concept study has been recently published by the group of Jayasena, trying to determine the effects of Kp-54 on egg maturation. In this study, infertile women were stimulated with recombinant FSH under a GnRH antagonist protocol. Oocyte retrieval was scheduled 36h after the single SC injection of different doses of Kp-54 (1.6, 3.2, 6.4 and 12.8nmol/kg). They proved that the two highest doses of kisspeptin elicited the LH surge in about 4–6h and resulted in acceptable rates of egg maturation, ranging between 75 and 85% of metaphase II oocytes per follicle aspirated greater than 14mm of diameter. Ninety-two percent of patients had at least one fertilized oocyte and similar outcomes were observed as regards the blastocyst formation rate (49.4%) if compared to the protocols that employed conventional triggers of oocyte maturation (51.2%). Overall, clinical pregnancy rate was 23% and live birth rate was 19% and finally, 12 healthy children were born without any abnormalities (Jayasena et al., 2014a).
This interesting study demonstrates that there is real potential for this hormone to be employed in IVF treatments given the undeniable evidence for successful human pregnancy and live births after its employment. Nonetheless, major issues remain to be addressed and clarified, such as which isoform is used, what dose provides the best results, and which way of administration is the most efficient.
It may seem hasty to start discussing the perfect Kisspeptin protocol because there is still a myriad of research to be conducted before this hormone is clinically employed in ART cycles; however, some considerations can be pointed out. As this hormone elicits the secretion of gonadotropins in the bloodstream, it is also tempting to suggest kisspeptin as a drug to be employed in ovarian stimulation protocols to stimulate oocyte recruitment and to increase the amount of preovulatory follicles. However, continuous kisspeptin administration results in tachyphylaxis (Jayasena et al., 2009), which hinders its use in this part of COS. Likewise, the levels of gonadotropins resulting from kisspeptin administration are similar to these physiological serum levels, which renders this hormone useless to hyperstimulate the ovary.
Moreover, in order to define the ideal kisspeptin dose for triggering, more trials that focus on egg maturation (e.g., percentage of metaphase II oocytes per oocyte aspirated) and fertilization rates are required. So far only one research work has addressed this issue after attempting single SC injections of up to 12.8nmol/kg of Kp-54 (Jayasena et al., 2014a). The results it reports hint at how kisspeptin should be used in COS cycles; e.g., oocyte retrieval was carried out 36h after Kisspeptin administration, exactly as in COS cycles after CG administration.
Notwithstanding, some other relevant details must be taken into account when working with kisspeptin. This hormone indirectly stimulates gonadotropin release through the HPG axis, thus only GnRH antagonists can be used in the COS protocol if kisspeptin is going to be used as a triggering option, paying special attention to when to administer the GnRH antagonists so that it does not affect kisspeptin response. Furthermore, protocols using drugs other than kisspeptin to substitute CG in COS cycles require the support of the luteal phase due to the low progesterone levels released (Kol and Humaidan, 2013); however, progesterone after kisspeptin administration in healthy women reached levels above 10nmol/L and a normal corpus luteum was observed (Jayasena et al., 2013a).
Safety of kisspeptin administration in humansAs with any other drug of therapeutic potential in humans, concerns are voiced as to whether or not its administration is safe enough for patients. One of the main reasons for such concerns is the fact that the expression of receptor GPR54 has been detected in animal models in not only the hypothalamus, but also in the liver, the placenta, pancreas and gonads (Ohtaki et al., 2001). There is also some evidence to suggest that some isoforms of kisspeptin are potential vasoconstrictors in humans given their paracrine activity which regulates muscular tone (Mead et al., 2007).
In all the clinical trials with exogenous kisspeptin administration to humans published to date and referred to in Table 1, no side effects have been reported, such as changes in blood pressure, heart rate or renal failure. Likewise, exogenous kisspeptin neither influences endometrial thickness nor affects the number of follicles recruited in a natural cycle, although a slight increase in the diameter of dominant follicles has been reported (Jayasena et al., 2013a). The same study also observed that although exogenous kisspeptin does not alter menstrual cyclicity, all the cycle phases (follicular, periovulatory and lutheal) appeared 2 days in advance when compared with the cycles of healthy women who, as a control group, received only saline injections.
Nonetheless, new concerns have been voiced about the use of kisspeptin in IVF treatments. For instance, after using a single bolus of Kp-54 to trigger egg maturation in an ovarian hyperstimulation protocol, three of the 53 patients presented tubal pregnancies. Although ectopic pregnancies occur in IVF cycles, especially when women suffer tubal infertility, it is still not absolutely certain that kisspeptin administration had nothing to do with this result (Jayasena et al., 2014a). Nevertheless, sample size was too small to draw any conclusions about this finding. The same study also pointed out that concerns about alterations to epigenetic markers after using kisspeptin have also flourished. Unfortunately, there are currently no published data about this alleged event.
Finally, another argument that reinforces the innocuousness of kisspeptin is the high serum level of this hormone during pregnancy. During the third trimester, concentrations of up to 9000pmol/L have been detected, unlike those levels in non-pregnant women of 1.3pmol/L (Horikoshi et al., 2003).
ConclusionsKisspeptin is a promising alternative to the drugs commonly used to induce and programme ovulation in ovarian hyperstimulation (e.g., choriogonadotropin (CG) or GnRH agonist) as it stimulates the HPG axis in a more physiological way, which undoubtedly makes it more appealing to be used in ARTs. In infertile women, kisspeptin seems effective to activate the LH surge during a COS cycle, and offers the advantage of avoiding OHSS without having to follow the freeze-all strategy as pregnancy rates are apparently maintained after its use.
The advantage of GnRH agonist triggering over CG triggering is that OHSS syndrome is avoided in the majority of cases. Its disadvantage lies in the low pregnancy rates observed due to corpus luteum insufficiency after its use. To cope with this, some strategies with satisfactory results have been developed; e.g., freeze-all the embryos and transfer them in an ulterior thawed cycle, or even the use of drugs in the luteal phase to maintain pregnancy rates in the fresh cycle, such as oestrogens, progesterone, or even the use of small amounts of CG to maintain the corpus luteum function (Humaidan et al., 2013; Fatemi et al., 2013). For this reason, if kisspeptin is able to offer an acceptable ratio of metaphase II oocytes per follicle aspirated, and if it can ensure that OHSS does not occur without affecting pregnancy rates in fresh cycles, it may seem a very good option for triggering. On the other hand, it is also important to mention that the high costs of the kisspeptin peptide manufacture compared to other triggering options such as GnRH analogs makes kisspeptin, to date, an impractical alternative in routine clinical practice.
Nonetheless, there is still a long way to go before its routine use can be implemented. Firstly, future research should focus on deciphering how kisspeptin should be administered (IV vs. SC; bolus vs. infusion) and the exact dose needed to obtain the best results. Recent results have suggested that a single subcutaneous injection of Kisspeptin-54 suffices to induce egg maturation with a minimum dose of 6.4nmol/kg (Jayasena et al., 2014a). Secondly, randomised controlled trials are mandatory to compare IVF results between different types of triggering, including that with kisspeptin. Finally, the clinical use of kisspeptin goes beyond ovarian stimulation because it is a potential drug that can be used to cope with other infertility disorders, such as Hypothalamic Amenorrhea and Hypogonadotropic Hypogonadism (Jayasena et al., 2009; Young et al., 2013). This reinforces the need to conduct further research in order to meticulously describe and understand the effects and mechanism of action of this hormone in the human body.
Conflict of interestsThe authors declare no conflict of interest.