The male factor plays a decisive role in reproduction and, despite being an area that has been extensively studied, there are events that remain unknown, such as sperm DNA fragmentation. This cause of infertility has been the subject of study during the last decades and has been associated with poor semen parameters and low reproductive outcomes. Among the techniques that have emerged to mitigate this problem is the innovative field of microfluidics. Microfluidic-based devices have been developed to be applied as sperm selection methods, reporting promising results in the non-invasive selection of sperm of higher quality, and less DNA fragmentation. The nature of these devices lies in sperm self-selection by simulating an in vivo environment. The present review discusses the mechanisms responsible for sperm DNA fragmentation and its implication in the field of assisted reproduction introducing, in addition, microfluidics as a potential application for human sperm selection.
El factor masculino juega un papel decisivo en la reproducción y, pese a ser un área muy estudiada, hay eventos que siguen siendo desconocidos como es el caso de la fragmentación del ADN espermático. Esta causa de infertilidad ha sido objeto de estudio durante las últimas décadas viéndose asociada con parámetros seminales pobres y bajos resultados reproductivos. De entre las técnicas surgidas para paliar dicho problema se encuentra el novedoso campo de la microfluídica. Los aparatos basados en microfluídica se han desarrollado para ser aplicados como métodos de selección espermática reportando resultados prometedores en la selección no invasiva de espermatozoides de mejor calidad y menor fragmentación en su ADN. La naturaleza de estos aparatos radica en la auto selección espermática mediante la simulación de un ambiente in vivo. La presente revisión desarrolla los mecanismos responsables de la fragmentación del ADN espermático y su implicación en el campo de la reproducción asistida introduciendo, además, la microfluídica como aplicación potencial para la selección humana espermática.
Microfluidics is a relatively recent science and technology based on the use of fluids at scales smaller than micrometers, capable of reproducing the conditions of natural systems. Its multidisciplinary capacity has made it a field of great interest to the scientific community due to its applicability in various areas of life sciences, specifically assisted reproduction (Smith and Takayama, 2017). Within assisted reproduction technology (ART), it has been used both in the in vitro fertilization (IVF) laboratory and in the andrology laboratory. The entry of microfluidics in the IVF laboratory is still too recent to be able to obtain precise conclusions. On the contrary, Andrology laboratory is where its potential has been most exploited, thanks to its great use in the analysis for sperm selection and sample processing (Nosrati et al., 2017).
At present, the evaluation of a semen sample is based on conventional superficial analyses, focused on the movement and morphology of the spermatozoa. These analyses fall short when it comes to making an in-depth diagnosis of the quality of the spermatozoa, specifically at the level of genetic material. As is known, the genetic material from the sperm must reach the oocyte intact, as it is necessary for proper embryonic development (Simon et al., 2017). However, various intrinsic and extrinsic factors are capable of interfering with the correct integrity of sperm chromatin, leading to its fragmentation. Sperm DNA fragmentation (SDF) refers to breaks in the genetic material of the male gamete, distinguishing two types: single strand SDF (ssSDF) and double strand SDF (dsSDF) (Agarwal et al., 2020).
It has been described that 11% of men present semen values within the normal range established by the World Health Organization (WHO) have SDF (Saleh et al., 2002). Therefore, simple observational evaluation under the microscope is not enough to detect this type of abnormality in each individual case.
The trend of publications about SDF has been exponentially increasing since its first study about 40 years ago (Baskaran et al., 2019). Studies have ranged from the impacts that this damage to the paternal genome may have on offspring to possible solutions to prevent it. Among all these alternatives, microfluidic sperm-sorting devices emerged. The nature of these chips is to mimic sperm self-selection as it would in an in vivo environment. This selection is based on progressive sperm motility and/or migration through conditions that simulate the properties of the female reproductive tract (Nosrati et al., 2017). But how can this science improve SDF levels over other conventional methods? The aim of this review is to show an overview of SDF and its effect on assisted reproduction as well as the implication of microfluidics to address this problem.
Mechanisms of SDFAccording to the type of SDF, the origin of these breaks may vary. Although there is no consensus on their exact origin, it is believed that they are mainly caused by maturation defects and abortive apoptosis during spermatogenesis or post-testicular oxidative stress (Sakkas and Alvarez, 2010). During spermatid elongation, chromatin packaging is achieved by the process of protamination. This process involves topoisomerase enzymes that are responsible for making breaks and ligations in the DNA strands. The main function of these breaks is to release the torsional stress involved in the exchange of histones for protamines, which helps to rearrange the chromatin (McPhersson and Longo, 1993). Any impairment or disruption in the repair mechanism of these breaks can result in the release of sperm with SDF in the ejaculate. A malfunction in any of the steps of sperm maturation does not imply direct damage to the genetic material, but it can lead to immature sperm that are susceptible to external factors that can more easily disrupt the structure of their DNA (Sakkas and Alvarez, 2010).
Abortive apoptosis is another process that can produce SDF during spermatogenesis. During the process of apoptosis the defective germ cells are labeled with Fas-type apoptotic markers so that they can be eliminated, preventing the differentiation of these cells into mature spermatozoa. Disruption of this apoptosis may result in defective sperm in the ejaculated sample (Sakkas et al., 1999). The presence of apoptotic markers in the ejaculated sperm has been correlated with detrimental semen parameters and SDF, indicating the importance of apoptosis in maturation pathways (Weng et al., 2002).
Oxidative stress is proposed as the main cause of SDF. In the male reproductive tract, oxidative stress can be generated by deficiencies in antioxidant defense against free radical attack or by a high concentration of reactive oxygen species (ROS) (Bui et al., 2018). These high levels of ROS have a negative impact on chromatin structure, especially if it is poorly compacted, generating oxidized DNA base adducts such as 8-hydroxy-2′-deoxyguanosine (8OHdG) (De Iuliis et al., 2009). Oxidative damage to sperm DNA can be represented as base modifications, chromosomal microdeletions or SDF (Aitken et al., 2016). The main sources of ROS production can be both internal, such as leukocytes, varicocele, or immature germ cells, and external, such as environmental factors, radiation, or centrifugation processes. The latter is the subject of debate due to its implementation in clinical practice in ART laboratories through conventional sperm capacitation methods (Agarwal et al., 2014; Aitken and Clarkson, 1988). Sperm oxidative stress has been negatively associated with fertilization rate (Agarwal et al., 2005) and early embryo development (Burruel et al., 2014).
Oxidative stress usually generates more ssSDF while maturation defects or abortive apoptosis usually result in higher numbers of sperm with dsSDF. This is somewhat relative since all of these defects can result in DNA breaks regardless of whether the damage occurs on one or both strands. The difference between these 2 types of SDF lies more in the future impact on reproductive outcomes. The dsSDF has been significantly correlated with slower embryo development, worse implantation rates (Casanovas et al., 2019), and a higher risk of recurrent miscarriage (Ribas-Maynou et al., 2012b). In contrast, while no significant impact on embryo development or implantation rates has been observed, ssSDF has been correlated with worse natural pregnancy rates and lack of progressive sperm motility (Ribas-Maynou et al., 2012a).
Factors associated with high SDFAlthough it has already been mentioned that the origin of SDF may be mainly due to defects in its maturation, apoptosis, or oxidative stress, there are multiple factors capable of impairing DNA integrity and favor to elevating the levels of SDF in the seminal sample.
VaricoceleVaricocele is defined as abnormal dilatation of the pampiniform venous plexus in the scrotum. It has a prevalence in men with primary and secondary infertility of about 35% and close to 80%, respectively, being the most common cause of infertility in male population (Jensen et al., 2017). This enlargement of the veins generates venous reflux resulting in scrotal temperature elevation, metabolite reflux, and the formation of a hypoxic environment within the testis. These conditions lead to the production of ROS with corresponding oxidative stress (Jensen et al., 2017). This mechanism may be the explanation for the high levels of SDF reported in patients with varicocele compared to fertile men (Wang et al., 2012). Nevertheless, this pathology can be treated surgically by varicocelectomy, resulting in a reduction of SDF and an improvement in reproductive rates (Lira Neto et al., 2021).
Advanced paternal ageAdvanced paternal age has always been in the spotlight for professionals working in the field of assisted reproduction. Unlike maternal age, there is no clear definition of the specific value to denote advanced paternal age. This particular problem may be responsible for the heterogeneity of the results between men and women present in the current literature. The impact of advanced paternal age on reproductive outcomes is still not fully agreed and an increasing number of studies are emerging about its effect on sperm parameters (Dain et al., 2011). It has been shown that paternal age may increase SDF through mechanisms of defective apoptosis and oxidative stress, affecting the correct chromatin packaging (Gonzalez et al., 2022). The association between advanced paternal age and SDF have been studied for several years. A retrospective study, analyzing 427 infertile men, showed significantly higher levels of SDF in men >40 years compared to younger men (Alshahrani et al., 2014). The same results were observed by Das et al. High SDF levels were significantly correlated with advanced paternal age (>40 years) in normozoospermic patients (Das et al., 2013). A more recent study shows an influence of high paternal age not only on SDF but also on other seminal parameters such as motility or volume (Colasante et al., 2019). On the contrary, other studies reported no significant association between advanced paternal age and SDF (Brahem et al., 2011). However, considering that the average age of couples with gestational desire has been on the rise for the last few decades (Gonzalez et al., 2022), this factor will become more and more important. The effect of age on the offspring will not only focus on the maternal side, but also on the paternal side.
AbstinenceAccording to WHO, the optimal abstinence interval for sample collection ranges from a minimum of 3 days to a maximum of 7 days. The narrowness of this range has been proposed on several occasions, suggesting a maximum of 4 days of abstinence, since it has been seen to have a better effect on seminal parameters (Li et al., 2020). While a positive relationship has been seen between ejaculatory abstinence and seminal volume and concentration (De Jonge et al., 2004), its detrimental effect on motility, morphology and, in particular, SDF cannot be overlooked (Levitas et al., 2005). The accumulation and storage of spermatozoa in the epididymis, with their possible exposure to ROS, can cause damage to sperm DNA (Ramos et al., 2004). For this reason, shorter ejaculatory abstinence has been suggested to improve SDF levels in the seminal sample (Gosálvez et al., 2011; Dahan et al., 2021).
Tobacco/AlcoholCigarette smoking and alcohol consumption are among the most harmful habits for the health of the general population. Smoking potentially causes cancer, chronic diseases, and death (Omolaoye et al., 2021), meanwhile alcohol is linked to an increased risk of liver, brain, and coronary heart disease, among many others (Dawson et al., 2008). Included in all these damages, one area that is also affected by this lifestyle is infertility. This impact is not surprising since more than a quarter of the male population of reproductive age is an official smoker and three quarters are regular drinkers of alcohol (World Health Organization, 2015). This is why studies are increasingly emerging on the impact of smoking and alcohol habit on male gametes. Many of the harmful compounds found in cigarettes reach the seminal sample altering its conditions, such as increasing the concentration of leukocytes, zinc values, or the levels of ROS in the seminal plasma (Taha et al., 2012; Harlev et al., 2015). It has been seen that the levels of SDF or sperm DNA damage increase in male smokers compared to non-smokers (Anifandis et al., 2014). In addition, normozoospermic smokers have not seen alterations in other seminal parameters and their reproductive success, aspects that give rise to debate (Mostafa, 2010). On the other hand, alcohol has been correlated with adverse seminal characteristics and elevated SDF levels (Anifandis et al., 2014). In a meta-analysis, it was concluded that daily alcohol intake negatively affects normal sperm morphology and semen volume, even though moderate consumption showed no detrimental effect (Ricci et al., 2017). Alcohol consumption has been significantly related to testosterone metabolism. Hansen et al. reported modifications in hormonal values, such as an elevation in the estradiol/testosterone ratio, in individuals with high alcohol intakes for 5 days. These results were related to a future effect on seminal quality if this alcohol consumption persisted over time (Hansen et al., 2012). The effect of large alcohol intakes on reproductive outcomes has also been studied. It was observed that male regular drinkers were less likely to achieve pregnancy compared to occasional consumers. Furthermore, those with high alcohol consumption had a higher SDF index (Wdowiak et al., 2016). However, considering the risks that smoking and alcohol have not only for infertility but also for health, it is considered good clinical practice to recommend not having toxic habits.
Environmental conditionsOne of the main external factors causing elevated SDF levels in the semen sample is temperature. As is well known, the process of spermatogenesis is temperature dependent with scrotal temperature being 2–8 degrees below body temperature in order to facilitate this process. Any exposure of the testes to high temperature fluctuations can lead to errors during maturation and abnormalities in future ejaculated spermatozoa (Garolla et al., 2013). In addition, testicular stress induced by scrotal hyperthermia can lead to alterations in chromatin structure (Love and Kenney, 1999). This elevation of scrotal temperature above physiological degrees may be due to multiple factors, but the most commonly found in the literature is the exposure to radio frequency electromagnetic waves. The sources of this type of non-ionizing radiation waves can be both natural (sun or ionosphere) and artificial (telecommunication devices or wireless networks). During the last decades, the exposure to this type of waves has been increasing due to the presence of more compact and easily transportable devices, such as cell phones or laptops (Agarwal et al., 2009). Exposure of the genital area to the use of Wi-Fi through these devices has been correlated with an increase in scrotal temperature and sperm DNA damage induced by oxidative stress (Avendaño et al., 2012). Individuals exposed to occupational ionizing radiation have also been shown to have worse seminal parameters and higher SDF values than unexposed males. Exposure to ionizing radiation generates DNA damage mediated by the production of ROS, affecting not only the functionality of chromatin but also the entire integrity of its structure (Kumar et al., 2014).
Additionally, it has been reported that cellular exposure to environmental toxins favors the formation of breaks in the sperm genome. Some of these toxic substances studied are pesticides, including organophosphates, carbamates, and pyrethroids (Miranda-Contreras et al., 2013; Bian et al., 2004), perfluorinated compounds (Governini et al., 2015), phthalates (Al-Saleh et al., 2019), cadmium (Pant et al., 2014), nitric oxide (Santiso et al., 2012), styrene (Migliore et al., 2002), and pollutants (Pizzol et al., 2021).
OthersAdditionally, other factors such as genital tract infections (Gallegos et al., 2008), inflammatory reactions (Fraczek et al., 2013), chemotherapy treatments (Delbes et al., 2007), diabetes mellitus (La Vignera et al., 2012), obesity (Dupont et al., 2013), and anxiety (Vellani et al., 2013) have been shown to impair chromatin integrity and favor male infertility.
Techniques to measure SDFSDF can be measured using four different techniques: sperm chromatin structure assay (SCSA), sperm chromatin dispersion (SCD), terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay, and Comet assay (See Table 1).
Summary of the main aspects of SDF measurement techniques.
| Technique | Measurement and principle | Pros | Cons |
|---|---|---|---|
| SCSA | DNA integrity assessment by acridine orange staining after a partial denaturation | Standardized protocols availableLarger amount of data | Do not distinguish between ssSDF and dsSDFComplex equipment and skilled personnel requiredPoor predictive power |
| SCD | Determination of the dispersion of a halo around the head formed by DNA loops characteristic of sperm without SDF | No complex equipment or skilled personnel neededEasy and accessible for standardizationShort time periods | Do not distinguish between ssSDF and dsSDFPoor predictive powerInter-observer subjectivity |
| Tunel assay | Fluorochrome signaling of DNA breaks and their 3’-OH ends by TdT enzyme | Large number of cells analyzedHigh predictive powerNo inter-observer subjectivity | Do not distinguish between ssSDF and dsSDFComplex equipment and skilled personnel requiredPoor standardization |
| Comet assay | Migration of DNA fragments in an electrophoresis gel forming a comet-like tailCell lysis is performed under neutral or alkaline pH conditions. | Able to distinguish between ssSDF and dsSDFHigh predictive power | Long time, complex equipment and skilled personnel requiredPoor standardization |
SCSA, first described by Evenson et al., was the first reported technique for the measurement of fragmented DNA in spermatozoa. It is based on the partial denaturation of DNA using an acidic solution. This solution maintains the structural configuration of the DNA double helix in those spermatozoa that maintain the integrity of their genome intact, while breaking the double strand of spermatozoa that have fragmented DNA. The presence of denatured or undenatured DNA can be detected by acridine orange staining using flow cytometer. This type of staining emits a red fluorescence when interacting with single-stranded DNA structures and a green fluorescence when interacting with double-stranded DNA structures (Evenson et al., 1999). As the first technique to be patented, it is the one with the largest amount of data capable of correlating a patient's risk of infertility with his SDF percentage (Evenson, 2016). Nevertheless, the mandatory use of the flow cytometer makes it not very accessible to daily laboratories as they cannot provide this instrument in their workspaces.
SCDFollowing this methodology, a variation of the technique called SCD emerged. It is based on a controlled process of DNA denaturation, to facilitate the removal of proteins and membranes contained in each spermatozoon. For this purpose, the seminal sample is introduced into an agarose matrix and subsequently treated with acid solutions and lysis buffer. In this way, a halo is dispersed around the head of the spermatozoon formed by DNA loops. After this procedure, when there is no DNA fragmentation, a large halo can be observed around the sperm head. On the contrary, in spermatozoa with fragmented DNA the halo dispersion is absent or minimal. Based on this principle, the sample can be observed, analyzed, and visually categorized under a bright-field microscope by eosine and thiazine staining or by fluorescence microscopy using DNA-specific fluorescent markers such as 6-diamino-2-phenylindole (DAPI) (Fernández et al., 2003). SCD proved to be as effective in detecting SDF as other techniques such as SCSA or TUNEL (Ribas-Maynou et al., 2013). Its mechanism gives it an advantage over the rest of the techniques on the market because it is simple to use, does not require expensive instrumentation and is easily reproducible in any assisted reproduction laboratory (Fernández et al., 2003). However, when interpreting the halos under the microscope, inter-observer variability is present. Although this subjectivity has not been proven to greatly affect the results, it is the main limitation of this technique (Agarwal et al., 2016).
TUNEL assayThe TUNEL assay is a widely known technique used in various areas of biology. Its mechanism consists of an enzyme called terminal deoxynucleotidyl transferase (TdT) capable of detecting DNA breaks and signaling their 3’-OH end in both single- and double-stranded DNA. This marking occurs through an enzymatic reaction by the enzyme in which it adds fluorochrome-labeled deoxyribonucleotides to these free terminals so that they can be detected and analyzed by flow cytometry or fluorescence microscopy. It measures the percentage of spermatozoa with fragmented DNA (Gorczyca et al., 1993). TUNEL has been considered among all the techniques to have the highest predictive value for clinical pregnancy, miscarriage rate, and live birth rate (Sharma et al., 2021). But this robust technique also has its limitations. As in the case of SCSA, TUNEL requires complex equipment for proper assessment, such as flow cytometer or confocal microscope. Equipment which is not very accessible for routine laboratories of both andrology and IVF.
Comet assayComet assay, also known as single cell gel electrophoresis assay, is another technique to measure DNA damage. Its principle lies in the migration of DNA fragments, resulting from sperm cell lysis, through an agarose gel while electrophoresis is applied. The key point of this assay is that the DNA fragments that were previously damaged are displaced forming a comet-like tail, hence the name. Those undamaged DNA fragments will remain immobilized in the head of the comet forming a more rounded shape. By using fluorescent dyes that bind to DNA, each strand of the genetic material can be observed and evaluated by fluorescence microscopy (Singh et al., 1988). The SDF index is positively correlated with the displacement of each DNA fragment from the head of the comet to the end of its tail. There are 2 types of comet assay depending on the conditions under which the denaturation of DNA breaks is performed, neutral or alkaline (pH < 13) (Ribas-Maynou et al., 2012a). The main difference between these 2 techniques lies in the ability to detect the type of SDF breakage. The neutral comet assay is unable to measure ssSDF, however, the alkaline comet assay is capable of detecting both types of SDF and alkali-labile DNA sites. Therefore, by performing 2 combined electrophoresis, one under neutral pH conditions and the other under alkaline conditions, we can obtain the proportion of dsSDF and ssSDF, respectively present in the seminal sample (Enciso et al., 2009). Although it has limitations such as the long time required, the use of skilled personnel, or the need for complex equipment, it is the only technique on the market available to distinguishing between both types of breaks. This fact gives it a great advantage over its competitors, being the strongest predictor of male infertility (Ribas-Maynou et al., 2013).
Reproductive outcomes affected by SDFThere is discrepancy about the negative impact of SDF as multiple studies have reported no relationship between elevation of this variable and clinical outcomes (Green et al., 2020). However, there is also emerging evidence that has proven a detrimental effect on the fate of the ART cycle. The reproductive variables affected by elevated SDF values present in the semen sample are shown in Table 2. Results vary between studies due to the technique used to measure SDF and its cut-off value. As there is no definitive consensus on the threshold value at which a sample can be not considered normal, the percentage chosen usually ranges from 4% to 60%. Many of these values are selected arbitrarily from the literature, through statistical tests, or based on the target reproductive outcome (Agarwal et al., 2020). In addition, this cut-off percentage may fluctuate depending on the measurement technique selected, making this discrepancy in cut-off values more attenuated between studies.
Reproductive outcomes affected by sperm DNA fragmentation in ICSI and IVF cycles.
| Outcome measure | Evidence | Study design (n) | Treatment | SDF assay | Main result (p > .05) | Cut-off value |
|---|---|---|---|---|---|---|
| Fertilization rate | (Sun et al., 1997) | Prospective (298 couples) | IVF | TUNEL | ↑SDF ↓fertilization rate | >4% |
| (Høst et al., 2000) | Prospective (50 couples) | IVF | TUNEL | ↑SDF ↓fertilization rate | ≥4% | |
| (Benchaib et al., 2003) | Prospective (104 cycles) | IVF/ICSI | TUNEL | ↑SDF ↓fertilization rate | >10% | |
| (Huang et al., 2005) | Retrospective (303 couples) | IVF/ICSI | TUNEL | ↑SDF ↓fertilization rate | >10% | |
| (Tavalaee et al., 2009) | Prospective (66 couples) | ICSI | TUNEL | ↑SDF ↓fertilization rate | NA | |
| (Simon et al., 2010) | Prospective (230 couples) | IVF | Alkaline Comet assay | ↑SDF ↓fertilization rate | >60% | |
| (Simon et al., 2011a) | Prospective (73 couples) | IVF | Alkaline Comet assay | ↑SDF ↓fertilization rate | >40% | |
| (Pregl Breznik et al., 2013) | Prospective (883 oocytes) | IVF | SCD | ↑SDF ↓fertilization rate | NA | |
| (Xue et al., 2016) | Retrospective (135 couples) | ICSI | SCSA | ↑SDF ↓fertilization rate | >22.3% | |
| (Oleszczuk et al., 2016) | Retrospective (1633 IVF/ICSI cycles) | IVF | SCSA | ↑SDF ↓fertilization rate | >10% | |
| Embryo development | (Sun et al., 1997) | Prospective (298 couples) | IVF | TUNEL | ↑SDF ↓embryo cleavage rate | >4% |
| (Morris et al., 2002) | Prospective (40 couples) | ICSI | Neutral Comet assay | ↑SDF ↑impairment of embryo cleavage | NA | |
| (Seli et al., 2004) | Prospective (49 couples) | IVF | TUNEL | ↑SDF ↓blastocyst development | ≥20% | |
| (Virro et al., 2004) | Prospective (249 couples) | IVF/ICSI | SCSA | ↑SDF ↑risk for low blastocyst rate | ≥30% | |
| (Borini et al., 2006) | Prospective (50 cycles) | ICSI | TUNEL | ↑SDF ↓post-implantation development | ≥10% | |
| (Pregl Breznik et al., 2013) | Prospective (883 oocytes) | IVF | SCD | ↑SDF ↓embryo development | NA | |
| (Wdowiak et al., 2015) | Prospective (165 couples) | ICSI | SCD | ↓SDF ↑faster embryos reaching blastocyst stage | NA | |
| (Sedó et al., 2017) | Prospective (82 oocytes from donation cycles) | ICSI | TUNEL | ↑SDF ↓blastulation rate | ≥15% | |
| (Esbert et al., 2018) | Retrospective (971 embryos from 135 cycles) | ICSI | TUNEL | ↑SDF ↑delayed cleavage divisions | >20.15% | |
| (Casanovas et al., 2019) | Prospective (196 embryos from 43 couples) | ICSI | Alkaline Comet assay | ↑dsSDF ↑delay in embryo development and implantation | NA | |
| (Setti et al., 2021a) | Retrospective (118 couples) | ICSI | SCD | ↑SDF ↑delay in cell divisions and blastulation | ≥30% | |
| (Setti et al., 2021b) | Retrospective (540 couples) | ICSI | SCD | ↑SDF ↓blastocyst development and implantation rates in women >40 years | ≥30% | |
| Embryo quality | (Velez de la Calle et al., 2008) | Prospective (622 couples) | IVF/ICSI | SCSA | ↑SDF ↓grade I embryos | >18% |
| (Avendaño et al., 2010) | Prospective (36 couples) | ICSI | TUNEL | ↑SDF ↓embryo quality | NA | |
| (Simon et al., 2010) | Prospective (230 couples) | IVF | Alkaline Comet assay | ↑SDF ↓total embryo cumulative score | >60% | |
| (Simon et al., 2011a) | Prospective (73 couples) | IVF | Alkaline Comet assay | ↑SDF ↓good quality embryos | >40% | |
| (Simon et al., 2014) | Cross-sectional (115 couples) | ICSI | Alkaline Comet assay | ↑SDF ↓good quality embryos | >71% | |
| (Oleszczuk et al., 2016) | Retrospective (1633 IVF/ICSI cycles) | IVF | SCSA | ↑SDF ↓good quality embryo per retrieved oocytes | >20% | |
| Pregnancy rate | (Larson et al., 2000) | Prospective (24 couples) | IVF/ICSI | SCSA | ↑SDF ↓pregnancy rate | ≥27% |
| (Spanò et al., 2000) | Retrospective (1301 cycles) | Natural conception | SCSA | ↑SDF ↓pregnancy rate | >40% | |
| (Henkel et al., 2003) | Prospective (65 couples) | IVF/ICSI | TUNEL | ↑SDF ↓pregnancy rate | >36.5% (IVF) >24.3% (ICSI) | |
| (Larson-Cook et al., 2003) | Retrospective (89 couples) | IVF/ICSI | SCSA | ↑SDF ↓ clinical pregnancy | >27% | |
| (Virro et al., 2004) | Prospective (249 couples) | IVF/ICSI | SCSA | ↑SDF ↓ongoing pregnancy rate | ≥30% | |
| (Frydman et al., 2008) | Prospective (117 couples) | IVF | TUNEL | ↑SDF ↓clinical and ongoing pregnancy rates per retrieved oocyte | >35% | |
| (Velez de la Calle et al., 2008) | Prospective (622 couples) | IVF/ICSI | SCSA | ↑SDF ↓pregnancy rate | >18% | |
| (Tarozzi et al., 2009) | Prospective (50 cycles) | ICSI | TUNEL | ↑SDF ↓pregnancy rate | NA | |
| (Avendaño et al., 2010) | Prospective (36 couples) | ICSI | TUNEL | ↑SDF ↓pregnancy potencial | >17.6% | |
| (Speyer et al., 2010) | Prospective (155 cycles) | ICSI | SCSA | ↑SDF ↓ continuing pregnancy rate | ≥19% | |
| (Meseguer et al., 2011) | Prospective (210 couples) | IVF/ICSI | SCD | ↑SDF ↓pregnancy rate using own oocytes | NA | |
| (Simon et al., 2011b) | Prospective (75 couples) | IVF | Alkaline Comet assay | ↑SDF ↑ risk of failure to achieve pregnancy | >52% | |
| (Sedó et al., 2017) | Prospective (82 oocytes from donation cycles) | ICSI | TUNEL | ↑SDF ↓pregnancy rate | ≥15% | |
| (Setti et al., 2021b) | Retrospective (540 couples) | ICSI | SCD | ↑SDF ↓pregnancy rates in women >40 years | ≥30% | |
| Miscarriage rate | (Benchaib et al., 2007) | Prospective (322 cycles) | IVF/ICSI | TUNEL | ↑SDF ↑risk of miscarriage | >15% |
| (Ribas-Maynou et al., 2012b) | Prospective (45 couples) | NA | Neutral Comet assay | ↑dsSDF ↑unexplained recurrent miscarriage | >77.5% | |
| (Setti et al., 2021b) | Retrospective (540 couples) | ICSI | SCD | ↑SDF ↑miscarriage rate | ≥30% | |
| Live birth rate | (Frydman et al., 2008) | Prospective (117 couples) | IVF | TUNEL | ↑SDF ↓live birth rate per retrieved oocyte | >35% |
| (Simon et al., 2013) | Prospective (203 couples) | IVF | Alkaline Comet assay | ↑SDF ↓live birth rate | >50% | |
| (Oleszczuk et al., 2016) | Retrospective (1633 IVF/ICSI cycles) | IVF | SCSA | ↑SDF ↓live birth rate per retrieved oocyte | >20% |
NA: Not available.
Conventional sperm selection methods have proven to be insufficient to select spermatozoa with low SDF, as they integrate the centrifugation process in their protocols. As mention previously, centrifugation has been associated with increased levels of ROS (Aitken and Clarkson, 1988). Based on this problem and with the aim of avoiding centrifugation in the protocols, several microfluidic-based chips capable of avoiding ROS formation and selecting sperm with low SDF have appeared on the market. These microfluidic devices focus on selecting spermatozoa according to different sperm conditions, such as progressive motility/migration or sperm interactions. The mechanism of each can be considered active, such as laminar flow, chemical gradients, or electrophoresis, or passive, such as filtration of the seminal sample through porous membranes (Nosrati et al., 2017). Any of these systems has been tested in one way or another to select while respecting the integrity of the sperm DNA structure.
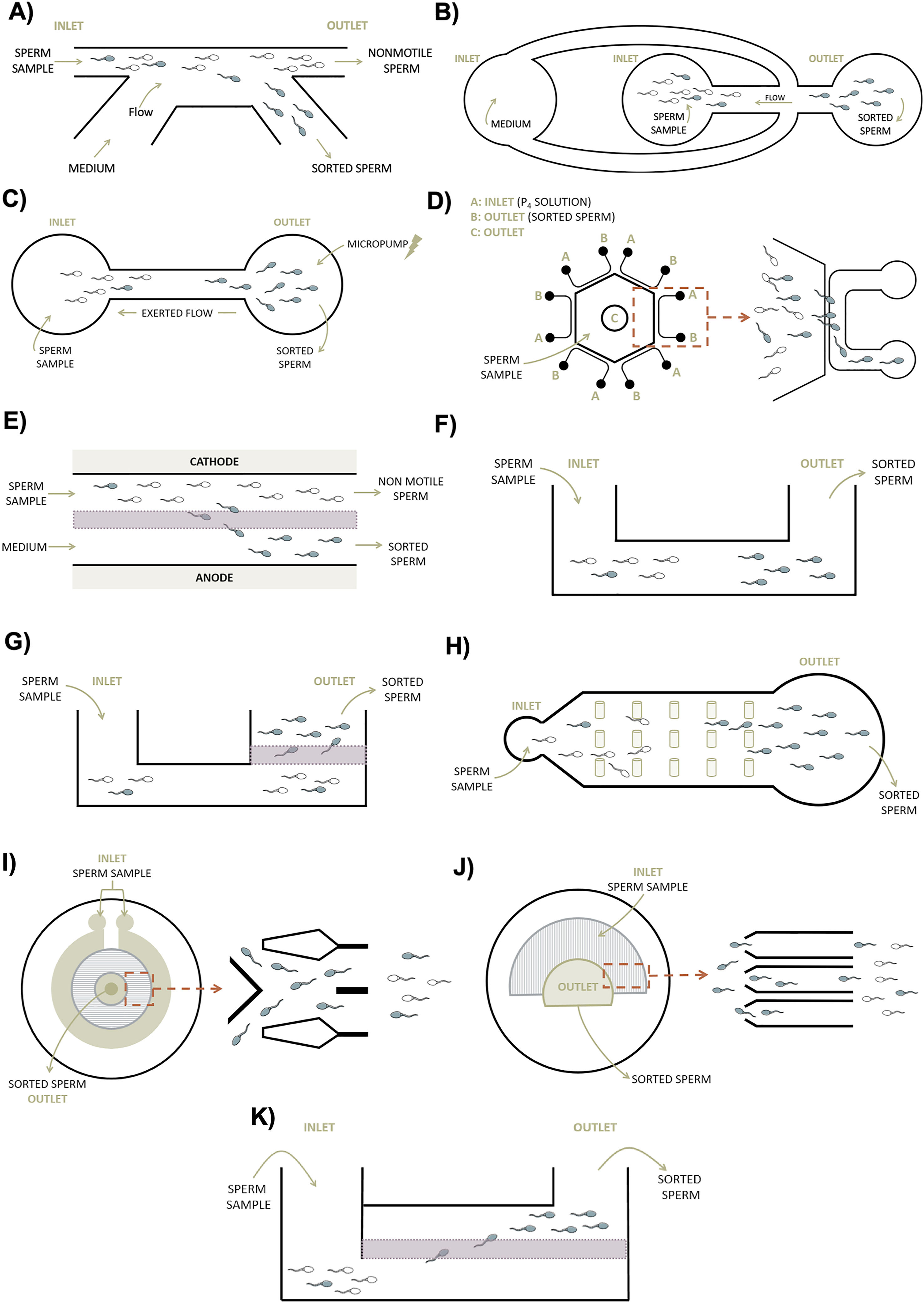
Active mechanism devicesCho et al. were the first reported evidence of microfluidic devices to sperm sorting based on the motility of the spermatozoon itself (Fig. 1A). These early devices consisted of 2-inlet and 2-outlet linked by channels composed of polydimethylsiloxane (PDMS). The sample is deposited in one of the inlets and a constant flow of medium is added to the other. The fluid with the spermatozoa flows while an active sorting system is produced. Non-motile spermatozoa and other cell types that have maintained their initial flow are discharged from one of the outlets, while motile spermatozoa deviate in parallel from this initial streamline and end up in another outlet for later collection. This bifurcation occurs thanks to the properties of parallel flow in these small channels avoiding the turbulent mixing between streams and the rapid velocity of motile spermatozoa (Cho et al., 2003). The use of this PDMS-chip based on laminar flow has been studied for the entrapped of sperm with low SDF. A commercial version called Sperm Sorter Qualis® (Menicon Co., Japan), was developed and tested against the conventional swim-up method. The SDF index, measured by SCD assay, was significantly reduced from the initial semen sample showing a percentage of 27.7–5.9% after using the microfluidic sperm sorter Qualis® (Kishi et al., 2015). Testing the efficacy of the same commercial product, Shirota et al. were able to isolate spermatozoa with less than 1% SDF (assessed with SCSA) compared to swim-up (Shirota et al., 2016). Recently, Gai et al. also noted an improvement in the percentage of spermatozoa with high DNA integrity by applying surface acoustic waves to this laminar flow (Gai et al., 2020).
Microfluidic-based devices for sperm selection. A| First device based on sperm motility. It is consisted of 2-inlet and 2-outlet linked by channels. The exerted flow runs parallel to the passive sample current, which allows sorting of motile sperm in a separate chamber. B| Device based on sperm rheotaxis with 3 reservoirs and 4 channels. A flow is generated by hydrostatic pressure allowing the selection of sperm that swims against it. C| Microfluidic sorting device composed of two chambers connected to a micropump which generates a flow to select sperm according to rheotaxis behavior. D| Device consisting of a pool surrounded by U-shape channels. Different progesterone concentrations are added at inlet A while motile sperm is sorted at outlet B. E| Represents the CS-10 designed with an electric field which allows the transport of the motile sperm across a membrane. F| System that selects the motile sperm swimming fastest through a passive flow. G| Passive mechanism composed of a porous membrane capable of filtering sperm with better motility and fragmentation. H| The hydrodynamic behavior and swimming direction of the sperm is simulated in the SPARTAN for selection. It is made of 2 chambers connected by a channel composed of spaced micropillars. I| Based on boundary-following behavior, the selection in this device occurred by tracking sperm in a circular space of 500 microchannels. J| In this device, a patterned layer is placed forming a semi-circular structure while a covering layer is superimposed to establish inlet and outlet chambers to collect the highest quality sperm. K| The commercially available microchip is composed of 2 wells separated by a membrane with the capacity to filter sperm with better motility and fragmentation during a short incubation period. White sperm: non-motile. Blue sperm: motile. Green arrows indicate the direction of flow and red ones magnify the selected section. Adapted from A) Cho et al., 2003 B) Seo et al., 2007 C) Zhang et al., 2015a D) Zhang et al., 2015b E) Ainsworth et al., 2005 F) Zhang et al., 2011 G) Asghar et al., 2014 H) Chinnasamy et al., 2018 I) Nosrati et al., 2014 J) Xiao et al., 2021 K) Meseguer et al., 2021. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Traditionally, the movement and direction of spermatozoa within the female reproductive tract was attributed to the movement of these cells in response to the concentration of chemical agents present in the environment, a phenomenon known as chemotaxis (Eisenbach, 1999). However, over the years it was realized that this phenomenon is not the only one involved and that some others intervene, such as thermotaxis or rheotaxis. The latter, defined as the ability of an organism in a fluid to move in the same or opposite direction to the current, has been considered the most robust system used by sperm to reach the oocyte in the process of natural fertilization (Zhang et al., 2016). These types of taxis have been studied in microfluidics in order to create devices that take advantage of these biological dynamics. Seo et al. developed a microfluidic-based device to select motile spermatozoa on the basis of rheotaxis (Fig. 1B). They generated a device made of PDMS with 3 reservoirs and 4 microchannels to which they applied a flow generated by hydrostatic pressure. Motile sperm have the tendency to orient themselves and swim against the current, so this self-motion allows their separation by the fabrication chip (Seo et al., 2007). Rheotaxis has been employed in other devices with the aim of obtaining better sperm DNA integrity. Zhang et al. developed another device that was connected through the outlet to a micropump generating a reverse flow (Fig. 1C). A total of 33 infertile patients were studied, showing better results in DNA integrity (measured by the comet assay) when the semen sample was processed by the chip rather than by conventional methods (Zhang et al., 2015a). Furthermore, Rappa et al. concluded that sperm rheotaxis can be used to select a population with better semen parameters when compared to unprocessed samples (Rappa et al., 2018). Recent groups have studied this same mechanism through the generation of flows by different pump-driven systems. They compared the microfluidic device with samples to which no additional flow was applied, obtaining improved SDF values and embryo developmental rates (Romero-Aguirregomezcorta et al., 2021).
As mentioned above, chemotaxis is also a very important process in sperm navigation routes. As a result, many microfluidic devices have sought to integrate this biological property into their mechanism for sperm selection. Zhang et al. developed a diffusion-based microfluidic chip that was composed by a 4 mm hexagonal pool surrounded by 6 U-shape channels with inlet and outlet chambers (Fig. 1D). Gradient profiles were calibrated and sperm chemotaxis behavior was observed under 2 different concentrations of progesterone (100 pM and 1 mM). They described a significant recruitment of a greater amount of motile spermatozoa in comparison with the control group (Zhang et al., 2015b). Also using progesterone as a chemoattractant, the property of sperm chemotaxis was integrated into the mechanism of a device to obtain good quality spermatozoa in normal and subfertile patients. After device capacitation, the level of SDF (measured by Comet Assay) and sperm oxidative stress decreased significantly (Gatica et al., 2013). Apart from progesterone, other chemoattractants have been tested to study the behavior of sperm chemotaxis, but most of them are still in the experimental phase and results have only been confirmed in animal models (Bhagwat et al., 2021). The effect of this biological response has also been explored by adding thermotaxis to its focus of study. Thermotaxis is a type of taxis by which an organism moves in one direction or another depending on a temperature gradient (Pérez-Cerezales et al., 2018). Yan et al. developed a device in order to study sperm response to thermotaxis and chemotaxis. Interestingly, they argued that in response to temperature fluctuations, sperm changed speed and direction, while, under certain chemoattractant conditions, sperm modulated their movement, swimming in different trajectories or shapes (Yan et al., 2021). These various forms of expression may be of interest for integration into the fabrication of microfluidic devices intended for sperm sorting. The limitation of using conditions based on these types of taxis to improves SDF values routinely is that they have not yet been tested on clinical human samples (Pérez-Cerezales et al., 2018). Further studies are needed before they can be implemented in future manufactured chips.
During sperm maturation, the membrane is exposed to various chemical changes in its composition in order to provide negative charge to the spermatozoon and allow its communication with the extracellular medium. This endowment and changes are essential for the subsequent processes of interaction with the oocyte necessary for fertilization to occur (Simon et al., 2015). In recent decades, science has taken advantage of this negative charge that covers the sperm head to isolate mature individuals with intact chromatin structure for ART cycles. Ainsworth et al. was the first to combine electrophoresis technology together with flow chambers in a device they named Cell Sorter-10 or CS-10. CS-10 consisted of a design of 2-inlet and 2-outlet chambers constrained by two 5 μm porous polyacrylamide membranes (Fig. 1E). Once the semen sample and pure buffer were deposited in the inlet chambers, an electric field was applied to transport the best quality spermatozoa to one of the collecting outlet chambers. Membranes had a specific pore size to allow the passage of spermatozoa based on sperm size and charged, but leaving behind any other excludable cells present in the seminal sample. Electrophoretic separation of the device collected a sperm population with better SDF values (measured by TUNEL) and other seminal parameters compared to processing by density gradients (Ainsworth et al., 2005). A few years later, a prospective controlled trial was performed using this same device and mechanism. Various sperm variables of samples processed either with the device or with density gradients were analyzed. SDF index showed a slightly, but not significantly, higher value in semen samples after density gradient processing compared to CS-10 (Fleming et al., 2008). The integration of dielectrophoresis in microfluidic platforms has also been proposed and tested for the manipulation and analysis of sperm samples (Ohta et al., 2010). Living, motile sperm with high DNA integrity are attracted in these devices to positively charged regions once polarized through electric fields. In contrast, dead, immotile, and DNA-damaged sperm repel or respond in a neutral manner to these electrical forces. However, these advances are being studied mainly in animal models or in other scientific areas without taking into consideration the clinical side of ART. The cost and complexity of these techniques are the major reasons why their use in routine clinical practice has not yet been validated.
Passive mechanism devicesOver the years, other chips have emerged with different structures but trying to achieve the same results. In 2011, a new space-constrained microfluidic sorting (SCMS) chip was designed on the basis of passive sorting (Fig. 1F). Its principle is based on different wells, 1-inlet and 1-outlet, connected by constrained 50 μm microchannels (Zhang et al., 2011). The seminal sample is placed in the inlet chamber and the sperm swim through microchannels until they reach the outlet chamber. Only the fastest sperm capable of swimming and reaching the outlet chamber first during the given incubation time will be collected. Sperm migration is constrained by the dimensions and characteristics of the microchannels between the 2 chambers. This mechanism, apart from avoiding centrifugation, allows a fast and simple selection since no experience or laborious processes are required in the protocol (Zhang et al., 2011).
Later, the structure of this chip was modified while maintaining the same properties. It also consisted of 2 chambers, but this time separated by a porous polycarbonate membrane (Fig. 1G). The seminal sample is deposited in the initial chamber and the sperm swim through microchannels. From this point on, self-selection occurs by sperm migration, in which sperm with the ability to pass through the pores of the membrane and reach the outlet chamber are selected. Once the incubation time has elapsed, all dead, immotile sperm, or other cell bodies will be left behind enabling the selection of motile sperm (Asghar et al., 2014). This device structure has been tested to improve sperm quality in terms of motility, viability, and DNA integrity, being the latter our focus of interest. Quinn et al. tested this commercially available version of the chip (ZyMōt®Fertility; DxNow Inc., USA) for the SDF index in infertile patients. Samples processed with the chip showed undetectable SDF values compared to processing with density gradients or directly unprocessed samples (Quinn et al., 2018). Moreover, Parrella et al. demonstrated a significant decrease in SDF from 20.7% present in the fresh ejaculate to 1.8% after using the microfluidic chip (Parrella et al., 2019). Recently, in a study enrolling patients with >60% dsSDF, a decrease up to 46% in this value was observed after using this chip compared to either raw sample or after processing with swim-up (Pujol et al., 2021). The enhancement of other variables such as clinical pregnancy, embryo quality or live birth rate has been also studied with this chip (Yetkinel et al., 2019).
In parallel, Chinnasamy et al., designed a sorting device named SPARTAN, which simulates the hydrodynamic behavior and direction of sperm traversing a periodic pillar array (Fig. 1H). It consisted of 2 chambers joined by a channel in which micropillars were arranged with different spacings between them. Depending on the spacing of the pillars and other features in their dimensions, immotile or abnormal spermatozoa were not able to pass through this array increasing the spatial distance with respect to the progressive motile ones. Therefore, after an effective 10-min incubation, the SPARTAN is able to separate motile and morphologically normal spermatozoa from the rest of the semen sample, with a significant decrease in the SDF index compared to other conventional methods or unprocessed samples (Chinnasamy et al., 2018).
Nosrati et al., aiming to select spermatozoa with low SDF, also developed a high-throughput microfluidic chip (Fig. 1I). The selection occurred by tracking spermatozoa in a circular space of 500 microchannels with dimensions of 100 μm × 75 μm, based on boundary-following behavior like previous devices. To simulate the routes taken by sperm through the female reproductive system, the microchannels were pre-filled with a viscoelastic medium. The results showed a significant improvement of >80% in DNA integrity in normozoospermic human samples (Nosrati et al., 2014). With the same device, a significant improvement in chromatin compaction and SDF index has also been reported from other studies (Eamer et al., 2016).
Recently, Xiao et al. developed a chip capable of recovering a higher percentage of sperm for ICSI than the raw sample. The platform, referred to as FertDish, had a Petri dish on which a 2-layer film was placed, one patterned and the other as a covering layer. The patterned layer was composed of 60 microchannels of 10 μm × 50 μm in width and 10 mm in length forming a semi-circular structure (Fig. 1J). The covering layer was superimposed with the patterned one to establish inlet chambers for the seminal sample and outlet to recover the final result. Its use allowed an improvement of 91% in the SDF value and 75% in high quality spermatozoa (Xiao et al., 2021). In addition, other commercially available microchips are being tested to improve SDF indices, such as the SwimCount™ Harvester (MotilityCount ApS, Denmark) studied by Meseguer et al (Fig. 1K). In this initial study, they demonstrated lower sperm chromatin fragmentation values after using the Harvester compared to density gradients, another capacitation method (Meseguer et al., 2021). These early advances indicate the upcoming implementation of all these microfluidic devices in our assisted reproduction laboratories, bringing with them promising results.
Discusion and future perspectivesCurrent evaluation of sperm quality is performed based on parameters such as motility, morphology, or vitality. These analyses do not explore the sperm in depth and are merely visual, providing sometimes unreliable diagnoses. One of the parameters that is not included in the daily clinical evaluation is the SDF. This value has been related in multiple occasions with slow embryo development (Sedó et al., 2017), risk of pregnancy loss (Zini et al., 2008), and lower live birth rates (Osman et al., 2015).
One of the main sources causing a high percentage of sperm with SDF in the semen sample is centrifugation mediated by the production of ROS. Conventional sperm selection methods such as swim-up or density gradients, as they have centrifugation in their protocol, expose sperm to free oxygen radicals increasing the SDF levels.
The use of antioxidants has been used as a strategy to decrease the level of SDF present in the sample. However, there are discrepancies in the role of these antioxidants since recent evidence has reported adverse effects of some of them. An example may be the case of vitamin C, which has been shown to increase sperm chromatin decondensation, interfering with subsequent preimplantational development (Ménézo et al., 2007).
The field of microfluidics emerged as a method to study fluids at different scales and mimic processes as they would be done in in-vivo environments. Among all its applications, microfluidic-based sperm sorting has recently become very relevant. Over the last decades, multiple microfluidic-based devices have been developed capable of selecting spermatozoa based on different sperm conditions such as motility or morphology. By skipping the centrifugation step, they have been used for the selection of sperm with low levels of SDF with favorable results. Many of them select on the basis of better progressive motility, a condition significantly correlated with SDF. Some of the prototype microfluidic chips reported in the literature are already being commercialized as is the case of the ZyMōt®Fertility or Qualis® and many of them try to outdo each other by incorporating efficient structures into their designs. This is the case of Raman microscopy, which seeks to combine microfluidics with optical technology to non-invasively locate nuclear DNA damage and distinguish it from spermatozoa with better quality (Mallidis et al., 2014). This area is innovating over the years, and it is not unusual to expect to find devices that select spermatozoa not only focusing on their motility or morphology, but also on other intrinsic conditions of the spermatozoa.
However, the impact of SDF on reproductive outcomes remains unclear, so the implementation of measurement techniques for clinical diagnosis has not yet been established in the laboratory. Additionally, the SDF test presents some limitations. To begin with, as it can be analyzed with different techniques, each of them has its own weaknesses, not allowing reproducibility of the results in different laboratories with different resources. On the other hand, there is a lack of a specific cut-off value to establish when a sperm has high or low SDF. In general, a normal spermatozoon presents fragmentation in its DNA since these are processes that occur at the molecular level for the correct packaging of chromatin and the functioning of the spermatozoon. But, as we have commented above, an error in these processes can produce high percentages of SDF with a detrimental impact on reproductive cycles. Currently, there is no consensus on the cut-off value to determine whether these reproductive outcomes will be affected. Multiple values have been proposed to act as a cut-off value, with the general range being between 15 and 50%. In a meta-analysis, Santi et al. compared the 4 techniques for measuring SDF and identified 20% as the ideal predictive value for differentiating between fertile and infertile patients with a sensitivity and specificity of 79% and 86%, respectively (Santi et al., 2018).
At the same time, there is a debate about the entry of microfluidics into assisted reproduction laboratories, as it would imply some disadvantages. On the one hand, the prices of the materials used for their manufacture are still too high and large-scale production would not be possible. On the other hand, the use of microfluidic devices would lead to the automation of multiple processes, which for many people is a major drawback. However, as mentioned by Casciani et al. they can offer many improvements. By working with small volumes, they would reduce culture media costs and allow for more optimal conditions for individual sperm handling. The structures of these chips are usually compact, saving space, and complexity of use. In addition, by simulating in vivo conditions, it improves throughput and achieves standardization by reducing viability (Casciani et al., 2021).
In conclusion, the area of microfluidics is here to stay. Microfluidic-based devices allow for non-invasive selection of spermatozoa with low SDF. This system would help to improve the clinical reproductive outcomes of patients such as blastocyst formation or pregnancy rates. Although the implementation of microfluidic-based chips in ART laboratories is not widely consolidated, the good results obtained after their use are irrefutable. Advances in microfluidics are helping us to better understand the behaviors of gametes in order to be able to apply them to clinical practice. The study of this field is increasing and more and more innovations are emerging to adapt to new generations. Its regular use in the clinic will allow us to optimize laboratories and reproductive cycles, giving us a new era.
FundingThe present investigation has not received specific aid from public sector agencies, commercial sector or non-profit organizations.
Conflict of interestThe authors have no conflicts of interest to disclose.