Introduction
Both, obesity and malnutrition have been considered risk factors for complications and increased relapse and non-relapse mortality in hematopoietic stem cell transplantation (HSCT).1 An inferior outcome after HSCT has been reported in obese adult patients in both allogeneic and autologous HSCT: Overweight individuals seem to develop more complications of graft versus host disease and more infections than its normal counterparts.1 On the other hand, recent data indicate that obesity does not preclude safe and effective myeloablative HSCT.2 To elucidate the impact of pre-transplantation body mass index (BMI) on clinical outcome, we performed a retrospective cohort study with registration data from the Centro de Hematología y Medicina Interna of the Clínica Ruiz in Puebla, Mexico.
Methods
a. Patients: Data were analysed from all patients who underwent autologous HSCT in the Centro de Hematología y Medicina Interna de Puebla of the Clínica Ruiz between May 1993 and December 2010, using the simplified method of autografting avoiding cryopreservation of the hematopoietic stem cells and conducting the procedure fully on an outpatient basis.3-5 Patients were stratified according to pre-transplantation BMI values: low BMI: BMI <18 5 kg m 2, normal BMI: 18.5 kg/m 2 - 25 kg/m 2, and high BMI: >25 kg/m 2.
b. PBSC mobilization and apheresis: The peripheral blood stem cell (PBSC) mobilization schedule was begun at least 30 days after the last dose of chemotherapy. Subcutaneous G-CSF (10 μg/Kg/day/5 days) was given for mobilization of stem cells. Using either a peripheral vein or a Majurkar-type subclavian catheter, the apheresis procedures were performed on days - 3, - 2 and - 1, using a Haemonetics V-50 PLUS machine (Haemonetics Corporation, Braintree MA) or a Baxter C-3000 PLUS machine (Baxter Healthcare, Deerfield IL), and the Spin-Nebraska protocol.6 The apheresis objective was to reach at least 1x106 viable CD34+ cells/Kg.
c. Conditioning and autografting: intravenous melphalan, 200 mg/m2 in a single i.V. dose was used on day -1 in all patients. Ondansetron (8 mg i.v. every 12 h after chemotherapy), ciprofloxacin (250 mg bid) and fluconazole (200 mg bid) were used in all patients. Antibiotics and antimycotics were used until granulocytes were greater than 0.5x109/L. All patients had daily laboratory workup and clinical studies.
d. Apheresis product preservation, studies and infusion: The products of the apheresis and 1 mL aliquots were kept in ACD-A (Baxter Healthcare, Deerfield IL) at 4oC, in 300 ml transfer packs (Baxter Healthcare, Deerfield, IL) composed of gas impermeable, polyvinyl chloride plastic film for up to 72 hours. Enumeration of the total white mononuclear cells (MNC) and CD34 positive cells was done by flow-cytometry7 in an EPiCS Elite ESP apparatus (Coulter Electronics, Hialeah, FL), using for the latter subpopulation the anti-CD34 monoclonal antibody HPCA-2 (Becton Dickinson, San José CA), gating in propidium iodide-excluding CD45(+) MNC population according to forward and 90° angle light scattering. Additional viability studies of the MNC used propidium iodide exclusion and anti-cell antibodies on a flow cytometer. No purging procedures were performed. The apheresis products obtained on days - 3, - 2 and - 1 were re-infused to the patients on days 0, +1 and +2 respectively after keeping them in the conventional blood bank refrigerator.
e. Statistics: The primary objective of the analysis was to assess the survival after the HSCT; accordingly, overall survival (OS) was calculated from the day of HSCT until the day of death or the last follow-up and was estimated according to the Kaplan-Meier method8 using the log-rank chi-square test.
Results
a. Patients: One hundred and six cases were included in the study. The Table 1 depicts some of the salient features of the patients. There were 39 patients with multiple myeloma, 31 with acute leukemia, 13 with Hodgkin´s lymphoma and 7 with non-Hodgkin´s lymphoma. The median age was 47 years (range 8 to 78 years); there were 56 males and 50 females. Patients were stratified according to pre-transplantation BMI values (vide supra): 4 patients had low BMI, 45 had normal BMI and 57 patients had high BMI.
b. PBSC mobilization and apheresis: A median of three apheresis sessions were needed to collect a minimum of 1x106 CD34 viable cells/kg of the recipient; the range was 2 to 4 sessions to obtain enough CD34+ cells.
c. Conditioning and autografting: All patients were conditioned with intravenous melphalan. In cases in which more than three apheresis sessions were needed to obtain a minimum of 1x106 CD34 viable cells/Kg of the recipient, the dose of melphalan was adjusted to 180 mg/m2 (90% of the planned dose).9,10
d. Apheresis product studies: The median number of transplanted CD34 viable cells was 3.2x106 CD34 viable cells/kg of the recipient; the range was 0.8 to 9.6. In all cases the viability of the CD34 cells was above 85% prior to being re-infused to the patients.
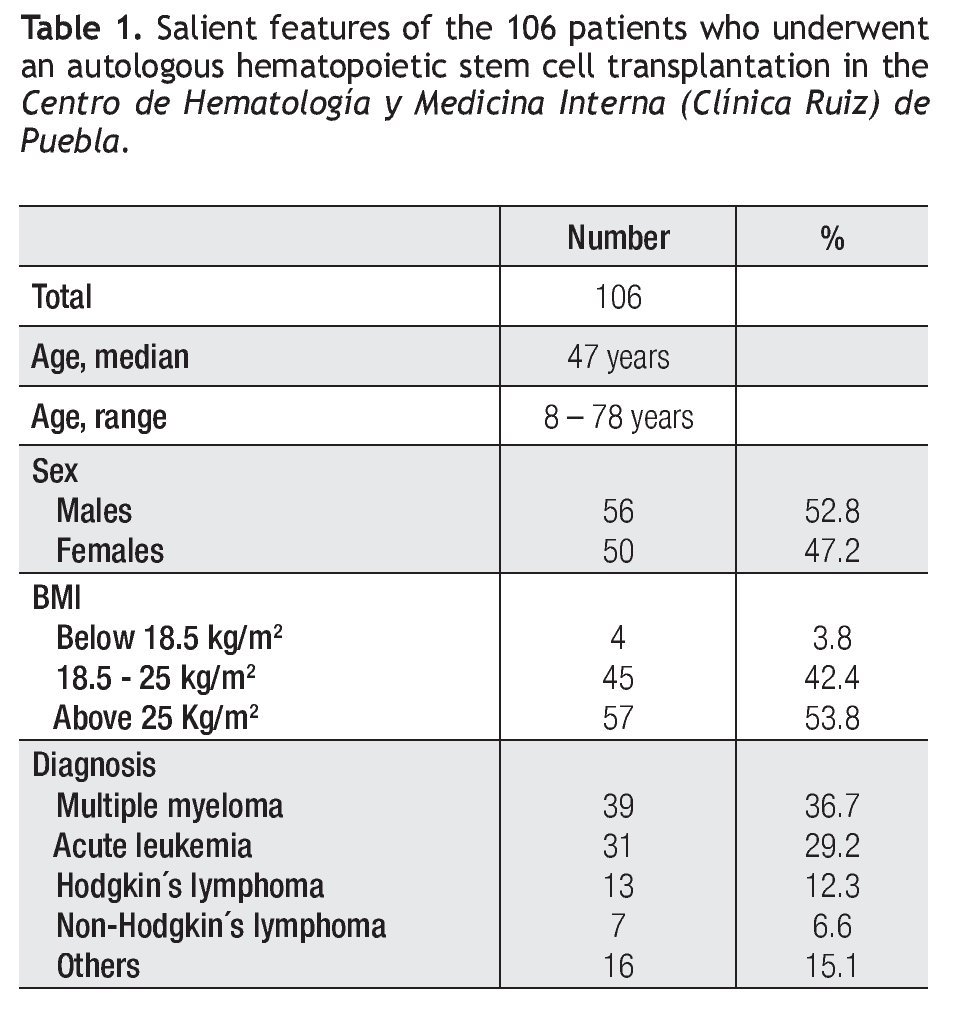
e. Overall survival (OS): The OS of the 106 patients was 55% at 190 months, with a median OS which has not been reached, being above 190 months (Figure 1). The OS was different in the three subsets of individuals according to the BMI: Patients with an increased BMI had an 80-month OS of 65%, whereas those with a BMI between 18.5 kg/m2 and 25 kg/m2, had an OS of 47% at 190 months (p NS) (Figure 2). The small subset of 4 patients with a low BMI (below 18.5 kg/m2) had an OS of 100% at 152 months. There were no significant differences in the subsets of patients according to sex or to the diagnosis of the entity which indicated the autograft.
Discussion
Obesity has become a pandemic, affecting both children and adults; in México this situation is even worse;11 our study indicates that the prevalence of overweight and obesity in the population of patients undergoing autologous HSCT in this single institution is rather high: 57/106 persons (53.8%) in this analysis had an increased BMI: this figure compares with that informed in other publications: 39% in North-American children receiving allogeneic cord blood grafts,12 43% in Mexican patients receiving an allograft,13 57% in North-American lymphoma patients receiving an autograft,14 16% in Japanese patients undergoing allogeneic HSCT,1 46% in North-American patients with acute myelogenous leukemia receiving a HSCT.2 The prevalence of an increased BMI seems to be higher in persons undergoing autologous than allogeneic HSCT and may have also a relation with cultural and social factors.1,2,11-14
Both obesity and malnutrition have been considered as adverse prognostic factors in patients undergoing HSCT.1,2,12-16 Obesity is associated with an increased risk of hyperglycemia, which can lead to an inferior outcome after allogeneic HSCT.13,14 On the other hand, malnutrition has been reported to be associated with an increased risk of early death after allogeneic HSCT.14,15 There are, however, other studies which indicate that an increased BMI does not preclude safe and effective allogeneic HSCT, employing either myeloablative2 or non-myeloablative conditioning regimens.13
In this study, the overall survival (OS) of all the patients was 55% at 190 months, with a median OS which has not been reached being above 190 months (Figure 1). Interestingly, an increased BMI (>25 kg/m2) was not associated with a worse outcome after the autograft; by the contrary, an increased BMI was associated with a better long-term post-autograft outcome, even though the differences were not statistically significant: Patients with an increased BMI had an 80-month overall survival (OS) of 65%, whereas those with a BMI below 25 kg/m2, had an OS of 47% at 190 months (Figure 2), this information being consonant with the recent observation which indicates that obesity does not preclude safe and effective HSCT.2
Figure 1. Overall survival of the 106 patients which were autografted in the Centro de Hematología y Medicina Interna (Clínica Ruiz) de Puebla.
Figure 2. Overall survival of the patients which were autografted, classified according to the body mass index: Above (n = 57) or below (n = 49) 25 kg/m2.
The low number of patients with a low BMI (four cases), despite showing an improved OS militates against further statistical analysis.
Our findings demonstrate a high prevalence of both overweight and obesity in Mexican patients undergoing an autologous HSCT and a correlation between pretransplantation BMI and post-transplantation survival. Although BMI depends strongly on multiple factors, the effect of and increased BMI on clinical outcome should be evaluated in a prospective study. There is currently no agreement regarding a suitable target range of pretransplantation BMI for clinical management.
Conclusion
These results should provide insight into how to better manage nutritional support for patients undergoing HSCT.
Correspondence: Dr. Guillermo J. Ruiz Argüelles.
8-B Sur 3710 CP 72530. Puebla, México.
E-mail: gruiz1@clinicaruiz.com
Recibido: Mayo 2011.
Aceptado: Julio 2011