Chikungunya virus (CHIKV) is a re-emerging mosquito borne alphavirus responsible for the recent outbreak in the Americas. Immunologically naïve population in the Americas favors the spread of epidemics. Chikungunya fever is characterized by an abrupt febrile illness, polyarthralgia and maculopapular rash. In chikungunya fever, shock or severe hemorrhage is very rarely observed; the onset is more acute and the duration of fever is shorter than dengue disease. The pain is much more pronounced and localized to the joints and tendons in chikungunya fever, in comparison to dengue fever. There is no specific and effective antiviral therapy and vaccines are still in trails. The only effective preventive measures consist of individual protection against mosquito bites and vector control. Disease prevention is important due to the economic burden it entails. Clinicians need to distinguish chikungunya fever between dengue fever and other diseases to give a successful treatment and prevent disease spreading.
Chikungunya fever is a viral disease transmitted through the bite of infected Aedes mosquitoes. The disease typically consists of an acute illness with fever, skin rash, and incapacitating arthralgia. The word chikungunya means, “to walk bent over” in the African dialect Makonde, and refers to the effect of the incapacitating arthralgia seen in the affected patients.1 Chikungunya virus (CHIKV) is the etiological agent and a member of the Alphavirus genus in the Togaviridae family.2
Chikungunya cases occur in Africa, Asia and the Indian subcontinent. Human infections in Africa have been at relatively low levels for a number of years. In December 2013, France reported 2 laboratory-confirmed autochthonous cases of in the French part of the Caribbean island of St Martin. Since then, local transmission has been confirmed in over 43 countries and territories in the American region.3 This was the first documented outbreak of CHIKV with autochthonous transmission in the Americas; therefore it is subject of big concern in our continent.
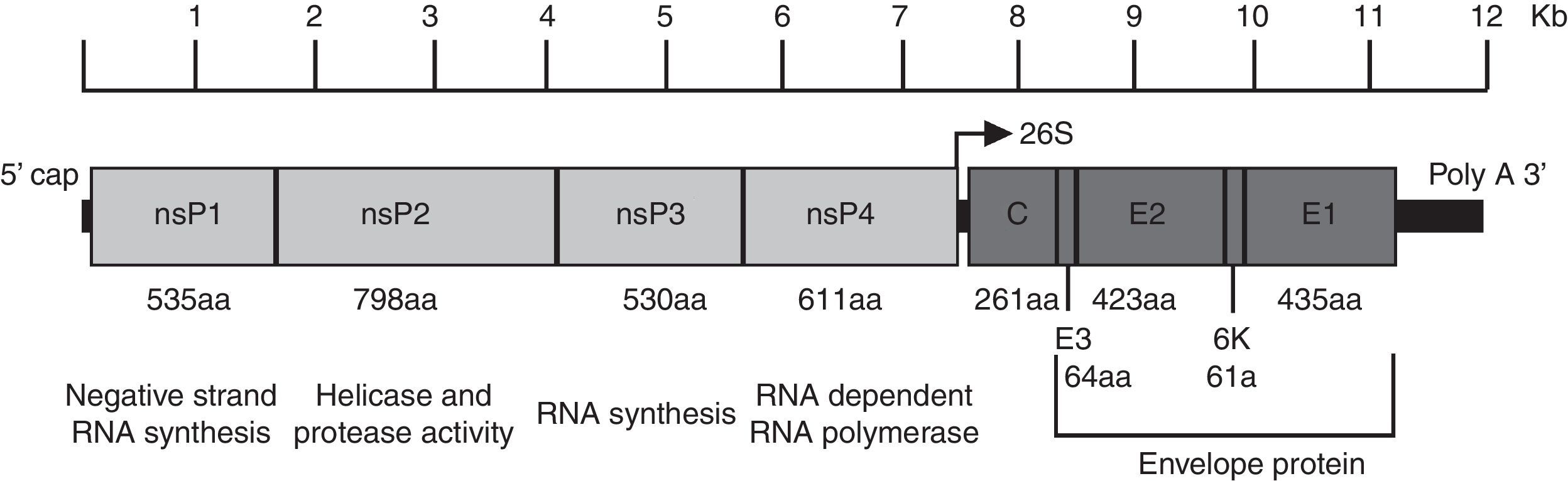
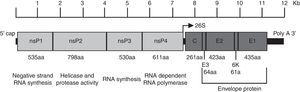
CHIKV genome, structure and replicationChikungunya virus is an enveloped plus-strand RNA virus with icosahedral symmetry. The virion is 70nm in diameter and it is composed of repeating units of the E1 and E2 transmembrane glycoproteins (240 heterodimers of E2/E1 arranged as trimeric spikes on its surface), the capsid (C), a host-derived lipid bilayer, and a single molecule of genome RNA.4 The genome is approximately 12kb in length and encodes the nonstructural proteins (nsPs) at the 5′ end and the structural proteins at the 3′ end. The nsPs are translated from genomic RNA and the structural proteins from a subgenomic RNA (Fig. 1).5
Organization of the chikungunya virus genome. The figure shows the nonstructural and structural proteins the way they are organized throughout the genome as well as the non-translatable regions in 5′ and 3′. The function and size in amino acids is showed for each protein. The figure is drawn to scale based on the CHIKV genome with GenBank access number: AM258990. The non-translatable regions sizes were obtained from Hyde et al.92 The figure was adapted from Weaver61 and Solignat.93 nsP, nonstructural protein; C, capsid; E, envelope; AA, amino acid.
Alphaviruses enter target cells by endocytosis. A few receptors (DC-SIGN, L-SIGN, heparin sulphate, laminin and integrins) have been implicated in this process, but their precise roles have not been clearly proven.5 Recently, prohibitin was identified as CHIKV receptor protein.6 Following endocytosis, the acidic environment of the endosome triggers conformational changes in the viral envelope that expose the E1 peptide, which mediates virus–host cell membrane fusion. This allows cytoplasmic delivery of the core and release of the viral genome. Two precursors of non-structural proteins are translated from the viral mRNA, and cleavage of these precursors generates nsP1–nsP4.7 These proteins assemble to form the viral replication complex, which synthesizes a full-length negative-strand RNA intermediate. This serves as the template for the synthesis of both subgenomic (26S) and genomic (49S) RNAs. The subgenomic RNA drives the expression of the C–pE2–6K–E1 polyprotein precursor, which is processed by autoprotolysis. The capsid is released, and further processing generates the pE2 and E1 glycoproteins. PreE2 and E1 associate in the Golgi and are exported to the plasma membrane, where pE2 is cleaved into E2 and E3. Binding of the viral nucleocapsid to the viral RNA and the recruitment of the membrane-associated envelope glycoproteins promote viral assembly. The assembled alphavirus particle, with an icosahedral core, buds at the cell membrane.5,7
Vectors, transmission and reservoirsTwo distinct transmission cycles have been well documented: enzootic and urban. In Africa, an enzootic cycle occurs in forested habitats where arboreal mosquitoes, principally Aedes spp, serve as vectors. Evidence points to nonhuman primates as the principal reservoir and amplification hosts in the enzootic cycle based on their high rates of seroprevalence, documented infection and viremia in nature, and viremia levels in response to experimental infection.8
The enzootic transmission cycle can spill over to infect people who live nearby, and enzootic mosquito vectors may be involved in interhuman transmission during small outbreaks. Epidemics also occur in Africa when CHIKV is introduced into urban areas where the more anthropophilic vectors, Aedes aegypti and Aedes albopictus, can initiate human–mosquito–human transmission.
CHIKV is capable of initiating a sustained, urban transmission cycle that relies only on A. aegypti and/or A. albopictus and human amplification hosts.9 This endemic/epidemic cycle results in high levels of human exposure to mosquito transmission, particularly because these vectors live in close proximity to people.
The behavior and ecology of A. aegypti, in particular, are ideal for epidemic transmission because adult females prefer to feed on humans, often take several partial blood meals during a single gonotrophic cycle, oviposit in artificial containers as their preferred larval sites, and rest inside houses with ready access to human hosts.10
A. albopictus is both zoophilic and anthropophilic, aggressive, silent, active all-day long, and has a longer lifespan than other mosquitoes (up to 8 weeks). In recent decades it has expanded to several areas previously known to be Aedes-free.11,12 It seems that most new introductions of A. albopictus have been caused by vegetative eggs contained in timber and tires exported from Asia throughout the world.13
Although the infectivity of various CHIKV strains varies widely for both A. aegypti and A. albopictus, humans develop high-titer viremias that generally persist during the first 4 days after the onset of symptoms, with the peak estimated on the day of onset at approximately 109 viral RNAcopies/ml14 and infectious titers sometimes exceeding 107PFU/ml.15 These titers generally exceed the oral infectious dose 50% levels for both epidemic vector species, permitting efficient transmission among humans by mosquitoes.16
Human beings serve as the main CHIKV reservoir during epidemic periods. Outside these periods, monkeys, rodents, and birds constitute the virus reservoir sustaining virus circulation in the environment in the absence of human cases.17,18
Geographic distributionCHIKV is typically found being transmitted in Africa and Southeast Asia. Since its discovery in 1952, CHIKV has caused several epidemics in these places.19,20
The last huge outbreak started in Kenya in 2004 and spread through neighbor islands to La Réunion in 2005.21 After that, the virus spread to several islands in the Indian Ocean and India.1,22 From India it spread to Sri Lanka, Thailand, Malaysia and finally to Italy in 2007.23,24 In 2009 CHIKV transmission restarted in La Réunion and lead to CHIKV re-importation to Europe in May 2010.25
During 2011, CHIKV was transmitted in Oceania, Central Africa, Southern and Southeastern Asia, Europe and Western Indian Ocean Islands. In 2012, CHIKV was reported in: Southeastern, Southern and Western Asia; Oceania; Central and Western Africa; and Western Indian Ocean Islands. During 2013, CHIKV was transmitted in Southeastern, Southern and Eastern Asia and Oceania.20
The current outbreak started in, the Caribbean Island, Saint Martin on December 6, 2013.26 During December 2013 and January 2014 it spread to the neighbor islands.27 In February, it continued spreading and reached French Guiana.28 In May, Guiana and almost all the Caribbean Islands reported autochthonous CHIKV infections.29 In June, the first cases of El Salvador were reported.30 By July, autochthonous transmission was reported in Florida, USA, Costa Rica, Panama and Venezuela.31 By September, cases were reported in Guatemala, Colombia and Brazil.32 In October, Nicaragua and Paraguay reported cases for the first time and Guatemalan cases rose.33 By the end of November, Mexico reported its first autochthonous transmission in the southern state of Chiapas. Also by this month, Belize and Honduras reported cases.34
According to the Pan American Health Organization (PAHO), since the current outbreak started, there have been 1,280,953 suspected autochthonous transmission cases and more than 26,300 have been confirmed in America.35 The recent reports obtained from Mexico reveals 405 confirmed autochthonous transmissions.35 Nevertheless, this numbers does not include patients that did not look for medical aid.
PhylogenyThere are four identified CHIKV lineages, each with distinct genotypic and antigenic characteristics. The first phylogenetic study revealed that CHIKV spread from Africa where two major lineages circulated: West African and East/Central/South African (ECSA). Posteriorly the ECSA lineage spread to Asia and originated the Asian lineage.36,37 Until 2004, these were the only identified CHIKV lineages that were circulating. The source of the 2005 Indian Ocean epidemic was traced to the ECSA lineage.38 When the Indian Ocean epidemic started in Kenya 2004, the first CHIKV isolates from La Réunion epidemic exhibited an alanine at E1 envelope protein residue 226, but later isolates showed an A226V substitution. This and additional substitutions gave rise to the fourth lineage, Indian Ocean lineage.38,39
PathogenesisThe pathogenesis of CHIKV infection in humans is still poorly understood, but recent outbreaks have helped providing insights into the cells and organs involved in viral replication. Following intradermal inoculation by infected mosquitoes, CHIKV directly enters the subcutaneous capillaries and infects susceptible cells in the skin: macrophages, fibroblasts and endothelial cells, where limited replication occurs.40 Locally produced viruses are transported to secondary lymphoid organs, where it infects migratory cells and release viruses to the lymph circulation and proceed to blood.20 Once in the blood, the virus has access to various parts of the body, including the liver, muscle, joints and brain.41 In these tissues, the infection is associated with a marked infiltration of mononuclear cells. The mononuclear cell infiltration and viral replication in muscles and joints are associated with pain.42,43
CHIKV infection elicits strong systemic innate responses, principally involving the production of antiviral IFN-α as well as many pro-inflammatory cytokines, chemokines, and growth factors.44 This is followed by the activation of the adaptive immunity through activation and proliferation of CD8+ T cells in the early stages of the disease. A classical switch to CD4+ T-cell response and the production of anti-inflammatory proteins IL-1RA and IL-2RA are characteristics of later stages of the acute phase.44
CHIKV infection induces a strong inflammatory response that is possibly orchestrated by the production of IL-16, IL-17, monocyte chemoattractant protein 1 (MCP-1), IP-10, and MIP-1α. The end of the acute phase is characterized by the production of proinflammatory MIF, MIP-1β, SDF-1α, and IL-6 and IL-8. CCL5 (RANTES) levels were also high in all patients during the first week after symptom onset.44 CCL5, MCP-1, IP-10, MIP-1β, and IL-8 are produced by activated macrophages that are susceptible to CHIKV infection.40 These chemokines play a major role in leukocyte recruit to sites of infection, coordinating the deployment of efficient antiviral defenses.
CHIKV infection also induces strong cellular immune response. High plasma levels of IFN-γ, IL-4, IL-7, and IL-12p40, cytokines that promote the adaptive immunity, suggested the involvement of cellular responses.44A key role for natural killer cells in the clearance of infected cells and in the development of CHIKV arthralgia has also been suggested.45 The B cell-promoting cytokines IL-4 and in some cases IL-10, were also up regulated in the first few days after symptom onset probably initiating the production of CHIKV-specific IgG. Additionally, CD4+ T lymphocytes, which are also involved in the promotion of humoral responses, were strongly activated toward the end of the acute phase.44
IgG antibodies are detected in the first week after infection, indicating rapid seroconversion and high levels of antibody responses among CHIKV-infected individuals.46 Specific IgM lasts for 3–4 months from the onset of the disease, and that IgG lasts more than 6 months.47 However, their role in chronic arthralgia is not very well understood.
Clinical manifestationsChikungunya fever is characterized by an abrupt febrile illness, polyarthralgia and maculopapular rash. The incubation period lasts 2–4 days (rage 1–12 days) and asymptomatic infections occur in 5–15% of the cases.48
Studies conducted in infected patients during La Réunion outbreak indicated that arthralgia was bilateral and symmetrical in 78.4% of the patients. It affected mostly ankles, knees, hands, wrists, feet, shoulders, and elbows.49–52 Rash was present in 54% of the patients, predominantly on the trunk and arms. Periarticular edema was reported in 45% of the patients, affecting the ankles in a greater proportion. Myalgia and headache were present in 72 and 63% of the patients, respectively. Hemorrhagic signs such as gingivorrhagia and epistaxis were only present in 10.6% of the patients.49–52
Radiological findings are normal and biological markers of inflammation like erythrocyte sedimentation rate and C-reactive protein are moderately elevated.49,50,52–54 Iridocyclitis and retinitis are the most common ocular manifestations associated with CHIKV infection and have a benign course with complete resolution and preservation of vision.55
The acute signs and symptoms usually resolve in less than 2 weeks, but arthralgia may last for weeks, months or even years;56,57 this is a clinical symptom that may distinguish CHIKV from dengue virus infection. Also in a univariate analysis, CHIKV infected patients displayed fewer gastrointestinal symptoms compared to dengue-infected patients, and a higher proportion of myalgia and arthralgia.58
Chikungunya is not generally considered life threatening; nevertheless severe forms can also be present. Patients with severe chikungunya fever requiring hospitalization tend to be older and have comorbidities such as cardiovascular, neurologic, and respiratory disorders or diabetes, which are independent risk factors for severe disease.49,59 Severe chikungunya can manifest as encephalopathy and encephalitis, myocarditis, hepatitis, and multiorgan failure. These rare forms can be fatal and typically arise in patients with underlying medical conditions.60,61 Neonates are also at risk for severe infection associated with neurologic signs. The rate of infection of neonates born to viremic mothers and exposed to the virus during birth can reach 50%, leading to severe disease and encephalopathy, resulting in long-term neurological sequelae and poor outcome.62
In general, CHIKV infection has a good prognosis. Nevertheless, older patients (<45 years old) are more likely to evolve toward relapsing and lingering chronic rheumatic musculoskeletal pain.63 A study showed that subjects who experienced severe initial rheumatic involvement (six or more painful sites with at least four other symptoms) at the acute stage of infection were more likely to exhibit chronic rheumatic musculoskeletal pain on follow up.63 Moreover, a positive association between high titers of CHIKV-specific IgG in the plateau phase and long-lasting arthralgia has been observed contemporaneously from a pilot study and in the Italian cohort.63,64 A possible mechanistic hypothesis may be that an imbalance toward B cell expansion and differentiation, in response to IL-6 secretion following the progression of both immunosenescence65 and chikungunya is triggered by viral persistence in host sanctuaries.66,67
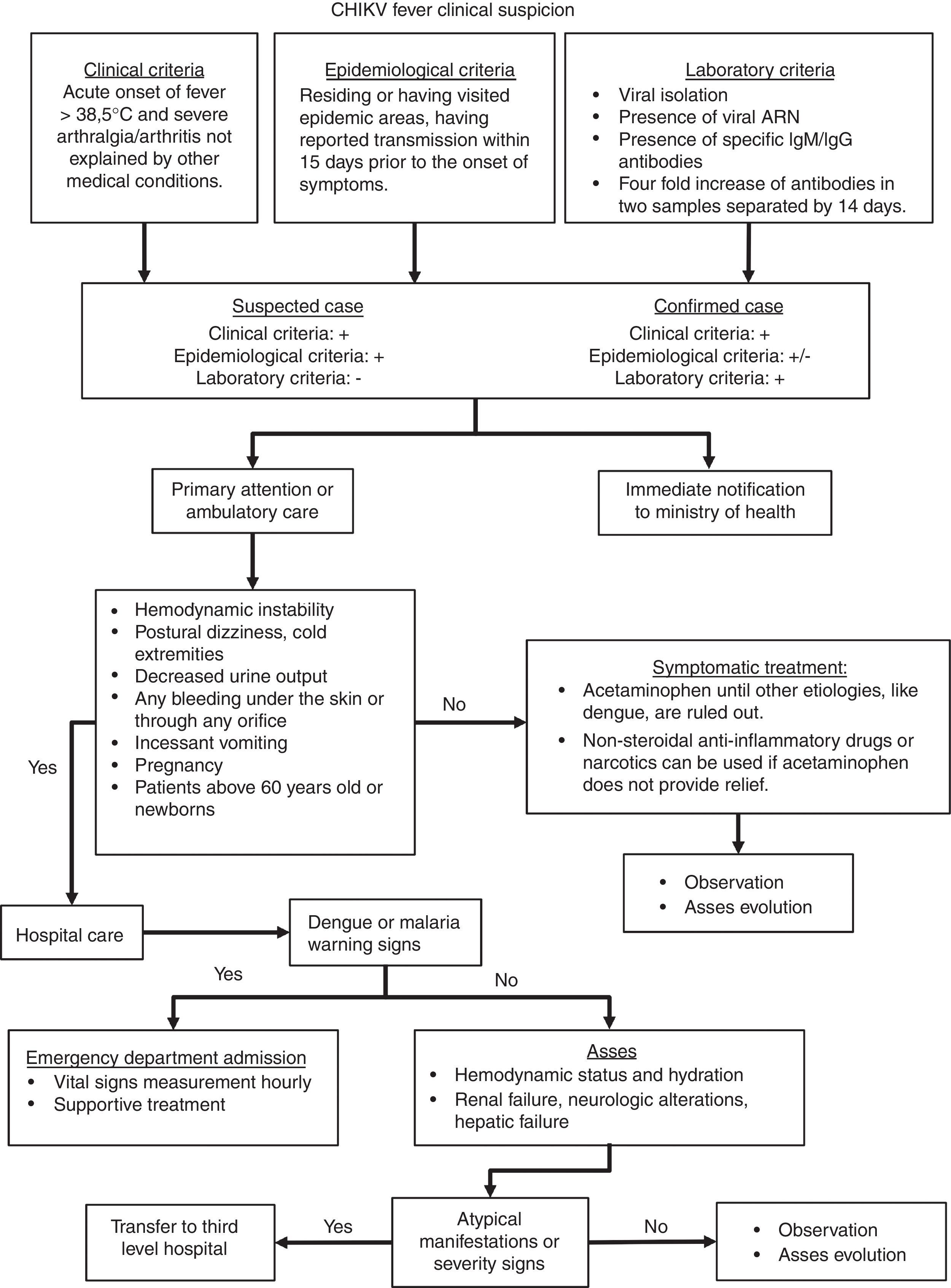
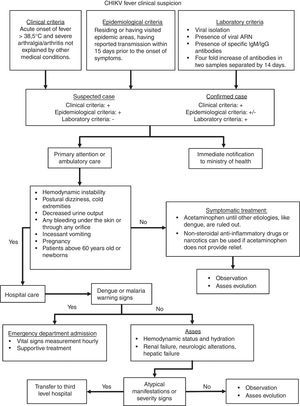
Taking in consideration the clinical features mentioned above, the algorithm presented in Fig. 2 would guide the clinician throughout case definition, and management. This algorithm is an adaptation from Palacios-Martinez.68
CHIKV infection diagnostic and therapeutic algorithm.
Chikungunya infection is diagnosed on the basis of clinical, epidemiological and laboratory criteria. An acute onset of fever and severe arthralgia or arthritis that is not explained by other medical disorders is considered a possible CHIKV case.20Three main types of laboratory tests are used for diagnosing CHIKV infection: virus isolation, reverse transcriptase-polymerase chain reaction (RT-PCR), and serology.
Virus isolation can be performed on field collected mosquitoes or acute serum specimens (≤8 days). Serum obtained from whole blood collected during the first week of illness can be inoculated into a susceptible cell line or suckling mouse at a reference laboratory. This can be achieved if the sample is transported cold (between 2°C and 8°C or dry ice) and as soon as possible (within 48h).69
Several RT-PCR assays for the detection of CHIKV RNA have been published. Real-time, closed system assays should be used, due to their increased sensitivity and lower risk of contamination. Taking into account the sensitivity, PAHO recommends the use of the CHIKV RT-PCR protocols from the Centers for Disease Control and Prevention and the Institute Pasteur.14,70 Serum from whole blood is used for PCR testing as well as virus isolation.
For serological diagnosis, serum obtained from whole blood is used in enzyme-linked immunosorbent assay (ELISA). The serum (or blood) specimen should be transported at 2–8°C and should not be frozen. Serologic diagnosis can be made by demonstration of IgM antibodies specific for CHIKV or by a four-fold rise in IgG titer in acute and convalescent specimens.69
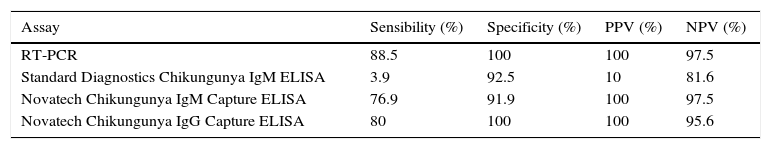
The determination of IgM can be made by different commercially available techniques. However, it should be taken into account that the best sensitivity is from techniques that use the complete virus as antigen compared to those that use recombinant proteins. Since the first commercially available kits had poor results, it is recommended that in house techniques for IgM/IgG ELISA be implemented using the purified viral antigen and following the CDC protocols.60 Recent ELISA assays have improved sensibility and specificity as shown in Table 1.71 The use of rapid tests is not recommended. The second sample for serological determination should be taken between 1 and 2 weeks after the first sample. Seroconversion can also be detected as an increase in IgG by a factor of 4 or more between acute-phase and convalescent-phase serum samples.69
Accuracies and sensitivities of different chikungunya fever diagnostic assays.
| Assay | Sensibility (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| RT-PCR | 88.5 | 100 | 100 | 97.5 |
| Standard Diagnostics Chikungunya IgM ELISA | 3.9 | 92.5 | 10 | 81.6 |
| Novatech Chikungunya IgM Capture ELISA | 76.9 | 91.9 | 100 | 97.5 |
| Novatech Chikungunya IgG Capture ELISA | 80 | 100 | 100 | 95.6 |
PPV, positive predictive value; NPV, negative predictive value; RT-PCR, reverse transcriptase-polymerase reaction; IgM, immunoglobulin M; IgG, Immunoglobulin G; ELISA, enzyme-linked immunosorbent assay.
There is no specific antiviral drug treatment for CHIKV infection. Symptomatic treatment is recommended after excluding more serious conditions like malaria, dengue, and bacterial infections.20,21
In acute infection, treatment is symptomatic and supportive, comprised of rest and the use of acetaminophen to relieve fever (<4g/day). The use of ibuprofen, naproxen, or another non-steroidal anti-inflammatory agent (NSAID) to relieve the arthritic component of the disease can be used when dengue infection is discarded.60 In patients with severe joint pains that are not relieved by NSAID, tramadol or narcotics (e.g., morphine) can be advised.60,61 Patients should be advised to drink plenty of fluids to replenish fluid lost from sweating, vomiting, and other insensible losses.60,72
While recovery from CHIK is the expected outcome, convalescence can be prolonged and persistent joint pain may require pain management, including long-term anti-inflammatory therapy. Specific treatment for diffuse post-CHIKV polyarthralgia in chronic stage consists of oral or topic NSAIDs plus a short course of oral corticotherapy or corticoid injection in affected joint. Neuropathic pain can treated with tricyclic antidepressants, antiepileptic drugs or tramadol.73
Although an older study suggested that hydroxychloroquine phosphate offered some benefit in arthralgia,74 posterior studies failed to confirm its efficacy.73,75,76
In patients with refractory joint symptoms, alternative therapies such as methotrexate (MTX) can be evaluated. In a retrospective study made in La Réunion Island, 54 out of 72 patients had a positive clinical response when treated with MTX.73,77 When MTX is contraindicated or ineffective, immune-modulating biologic agents as etanercept, rituximab, or tocilizumab can be used.73
It has been shown that patients with rheumatoid arthritis have low vitamin D levels and a negative correlation with disease activity.78 Therefore, reposition of vitamin D could improve disease severity. In a study made in India, patients with chronic CHIKV-related arthritis were treated with vitamin D and calcium for five months which improved joint pain and fatigue.79
In addition to pharmacotherapy, cases with arthralgia and joint stiffness may benefit from a program of graduated physiotherapy in acute and chronic stages of the disease. Movement and mild exercise tend to improve morning stiffness and pain, but heavy exercise may exacerbate symptoms.
PreventionPending vaccine development, the only effective preventive measures consist of individual protection against mosquito bites and vector control. Control of both adult and larval mosquito populations uses the same model as for dengue and has been relatively effective in many countries and settings.80 Mosquito control is the best available method for preventing CHIKV infection. Breeding sites must be removed, destroyed, frequently emptied, and cleaned or treated with insecticides.3
For protection, clothing which minimizes skin exposure to the day-biting vectors is advised. Repellents can be applied to exposed skin or to clothing in strict accordance with product label instructions. Repellents should contain DEET (N,N-diethyl-3-methylbenzamide), IR3535 (3-[N-acetyl-N-butyl]-aminopropionic acid ethyl ester) or icaridin (1-piperidinecarboxylic acid, 2-(2-hydroxyethyl)-1-methylpropylester). Mosquito coils or other insecticide vaporizers may also reduce indoor biting.3
VaccinesThere is currently no commercial vaccine for CHIKV, although some candidate vaccines have been tested in human beings.81,82
Several technologies have been used to develop CHIK vaccines, including inactivated viral vaccines, live-attenuated viruses, alphavirus chimeras, recombinant viral vaccines, consensus-based DNA vaccines, recombinant subunit vaccines and more recently, a virus-like particle (VLP) vaccine.
Two vaccine candidates have finished phase I trials: a live recombinant measles-virus-based chikungunya vaccine and the VRC-CHKVLP059-00-VP, VLP vaccine. The live recombinant measles-virus-based chikungunya vaccine had good immunogenicity, even in the presence of measles immunity, was safe, and had a generally acceptable tolerability profile.83 The VLP vaccine, VRC-CHKVLP059-00-VP was also immunogenic, safe, and well tolerated.84
Economic burdenIn India, the chikungunya epidemic in 2006 imposed heavy epidemiological burden and productivity loss to the community. National burden of chikungunya was estimated to be 25,588 DALYs lost during 2006 epidemic. Persistent arthralgia was found to impose heavy burden, accounting for 69% of the total DALYs. The productivity loss in terms of income foregone was estimated to be a minimum of 6 million USD.85 Other studies made in India reported that the burden for Andhra Pradesh was 6600 DALYs (cost: US$12,400,000). While the burden was moderate, costs were high and mostly out of pocket.86
A study made with military policemen at La Réunion in June 2006 reported that most symptomatic patients (93.7%) complained of a chronic stage of the disease, which is characterized by pains in joints or bones, or both, although the inquiry was made 6 months after the epidemic peak. Most working adults are disabled with loss of mobility, hand disability, and depressive reaction, which can each last for weeks to months and has negative consequences in health, social organization, and economy in epidemic areas.87,88
With these antecedents, if the outbreak spreads throughout Mexico, the infected working adults will be incapacitated, and will increase the economic burden.
ConclusionThe arrival of CHIKV to America will be a challenge to the public health system and a significant economic burden. The probability of autochthonous transmission in the rest of Mexico and USA is high due to the vector ubiquity. Economic development does not protect countries from vector-borne diseases; modern lifestyles may amplify an epidemic through travel, population aging, and production of solid waste that can shelter Aedes mosquitoes.
FundingNo financial support was provided.
Conflict of interestThe authors have no conflicts of interest to declare.