The development of antiviral drugs is a very complex process. Currently, around 50 drugs have been approved for human use against viruses such as HSV, HIV-1, the cytomegalo virus, the influenza virus, HBV and HCV. Advancements in this area have been achieved through efforts and technical breakthroughs in different scientific fields. The improvement in the treatment of HCV infection is a good example of what is needed for efficient antiviral therapy. A thorough description of the events that lead to the development of specifically targeted antiviral therapy or HCV (STAT-C) could be useful to further improve research for treating many other viral diseases in the future. Similar to HIV-1 and HBV treatment, combination therapy along with personalized medicine approaches have been necessary to successfully treat HCV patients. This review is focused on what has been done to develop a successful HCV therapy and the drawbacks along the way.
From 1972 to date, more than 50 new viruses have been identified as etiologic agents of human disease.1 These new viral diseases have required more sophisticated therapeutic agents, but the development process of these strategies to this point has been slow and full of hurdles.
Antiviral chemotherapy has advanced at snail-like pace, unlike antibiotics, which in 30 years achieved an advanced therapeutic stage. 34 years elapsed from the description of the antibacterial molecule salvarsan, “the magic bullet”, by Ehlrich in 1910,2 to the discovery of penicillin by Fleming in 1929,3 to Domagk's description of prontosil, the precursor of sulfonamides in 19354 and the isolation of streptomycin, chloramphenicol, erythromycin and tetracycline by Waksman in 1944.5 However, it took almost 60 years for antiviral development to reach its current status of effectiveness. The evolution of the treatment for Hepatitis C is a good example of how complex antiviral development can be and how a combined and specific targeted antiviral therapy has proved to be the best approach to follow for viral disease treatment.
The Hepatitis C virus (HCV) affects over 170 million individuals worldwide, 80% of which are chronically infected.6 This is four times the number of people infected with HIV and about half the number of persons infected with the Hepatitis B virus (HBV). 7 HCV is caused by a hepatotropic virus, which belongs to the Flaviviridae family, genus Hepacivirus. HCV was discovered in 1989 and its viral genome is a 9.6kb-long positive single-stranded RNA. It encodes a single polyprotein precursor of 3010 amino acids and has an internal ribosome entry site at the 5′ untranslated region. This polyprotein precursor is co-translationally processed by cellular and viral proteases into three structural proteins (core, E1 and E2) and seven non-structural proteins (p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B).8 The structural proteins associate with the genomic RNA and a viral particle is assembled inside a lipidic envelope.
Treatment for HCV infection has come a long way. Between 2001 and 2011, a standard of care (SOC) for chronic HCV infection was established worldwide. It consisted of a combination of pegylated interferon (PEG-IFN) and ribavirin (RBV). Nowadays, new specific antiviral agents have been approved. In May 2011, boceprevir and telaprevir, two first-generation NS3/4A protease inhibitors, were authorized for their use in combination with PEG-IFN and RBV for a 24-to-48-week course of treatment in HCV-genotype 1 infections. Two years later (December 2013), Simeprevir (a second-generation NS3/4A protease inhibitor) was approved for use with PEG-IFN and RBV for a 12-week course of treatment in HCV-genotype 1, while sofosbuvir (a NS5B nucleotide polymerase inhibitor) was approved for use with PEG-IFN and/or RBV for a 12/24-week course of treatment in HCV-genotypes 1 to 4. IFN-free regimens have been shown to give better results, because sofosbuvir, combined with simeprevir or an NS5A replication complex inhibitor (ledipasvir or daclatasvir), with or without RBV for a 12-week treatment in genotype 1, resulted in a sustained virological response (SVR) >90%. In addition, ABT-450/r (ritonavir-boosted NS3/4A protease inhibitor)-based regimens, in combination with other direct-acting antiviral agent(s) with or without RBV for 12 weeks in genotype 1, have demonstrated similar results regarding SVR.9
Roadblocks for antiviral drug developmentAs we see in the text above, therapy for HCV infection remained almost the same from 2001 to 2011. After a decade of poorly effective HCV therapy, the development of specific compounds against this virus ramped up HCV treatment on a pace that nearly matched antiretroviral therapy for HIV. “Why did it take such a long time?” is an important question whose answer could help on the approaches towards drug development against untreated diseases. The first complication when studying a virus is the limitations regarding in vitro systems and animal models for experimentation; second, is the low rate of discovery for efficient candidate molecules, and third, the delicate balance between efficacy, toxicity and resistance towards the selected antiviral drug. Additional economical aspects must also be considered. Here we have analysed each of these aspects under the light of the promises and pitfalls related to Hepatitis C research and treatment.
HCV study toolsViruses are intracellular organisms which depend on cellular machinery for replication. Therefore, a huge breakthrough in this field was achieved by Enders, Robbins and Weller in 1951, when they developed an in vitro virus propagation system in cell culture.10 Since then, many in vitro and in vivo systems have been implemented for the study of several viruses, such as polio and HIV. Cell assays systems were recently developed for HCV infection and propagation. In the early beginnings of HCV studies, no small animal model existed to study HCV infections, and Chimpanzees, the only animals capable of being infected with HCV, were precluded by both ethical and functional difficulties. The in vitro development for HCV research began with the sub-genomic replicon cell culture system that replicates autonomously in the human hepatoma cell line Huh-7 generated by Bartenschlager et al., in 2001.11,12 This sub-genomic replicon model was further improved by the identification and introduction of adaptive mutations, which enhanced virus replication capacity and lead to the establishment of the full-length replicon system using the highly permissive cell line Huh-7.5.1 in 2003, by Blight and Bartenschlager et al., separately.13–15 These developments allowed the study of HCV infection mechanisms, such as packaging, budding and a more accurate evaluation of potential antiviral molecules. On the other hand, the development of a small animal model that can be infected with HCV became a reality with the T- and B-cell deficient mice with severe combined immunodeficiency (SCID), grafted with human hepatocytes. The first HCV infection studies in this model were performed by Mercer et al. in 2001. In recent years the development of transgenic mice with a chimeric mouse-human liver revolutionized HCV infection research, allowing the assessment of pathological and immunological profiles of the disease.16 Today, scientists rely on a combination of antiviral activity assessment in the HCV replicon cell culture system, cell-based infection systems, and pharmacokinetic profiling in animals as proxy indicators of antiviral drugs’ efficacy, before attempting clinical trials.17
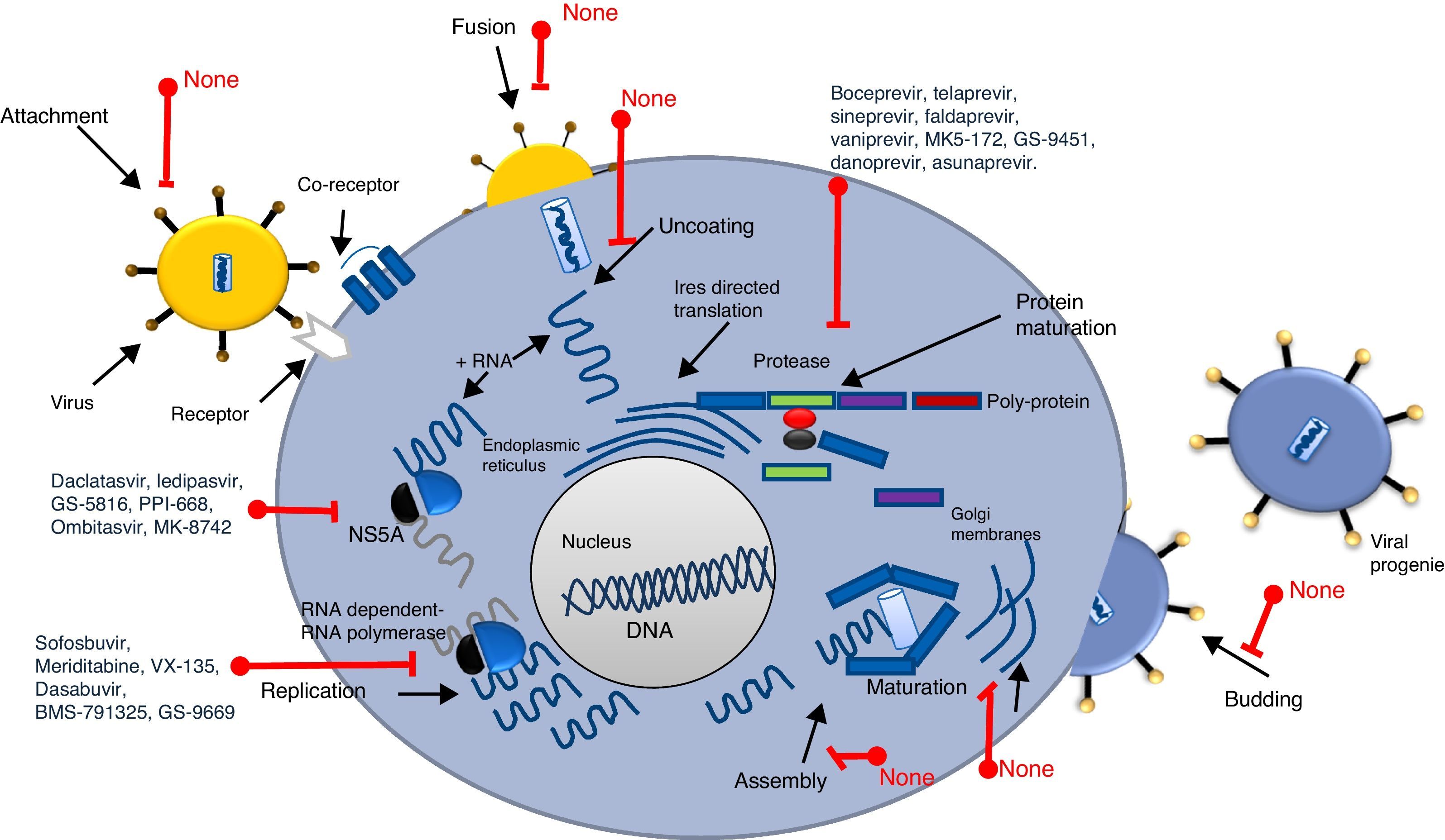
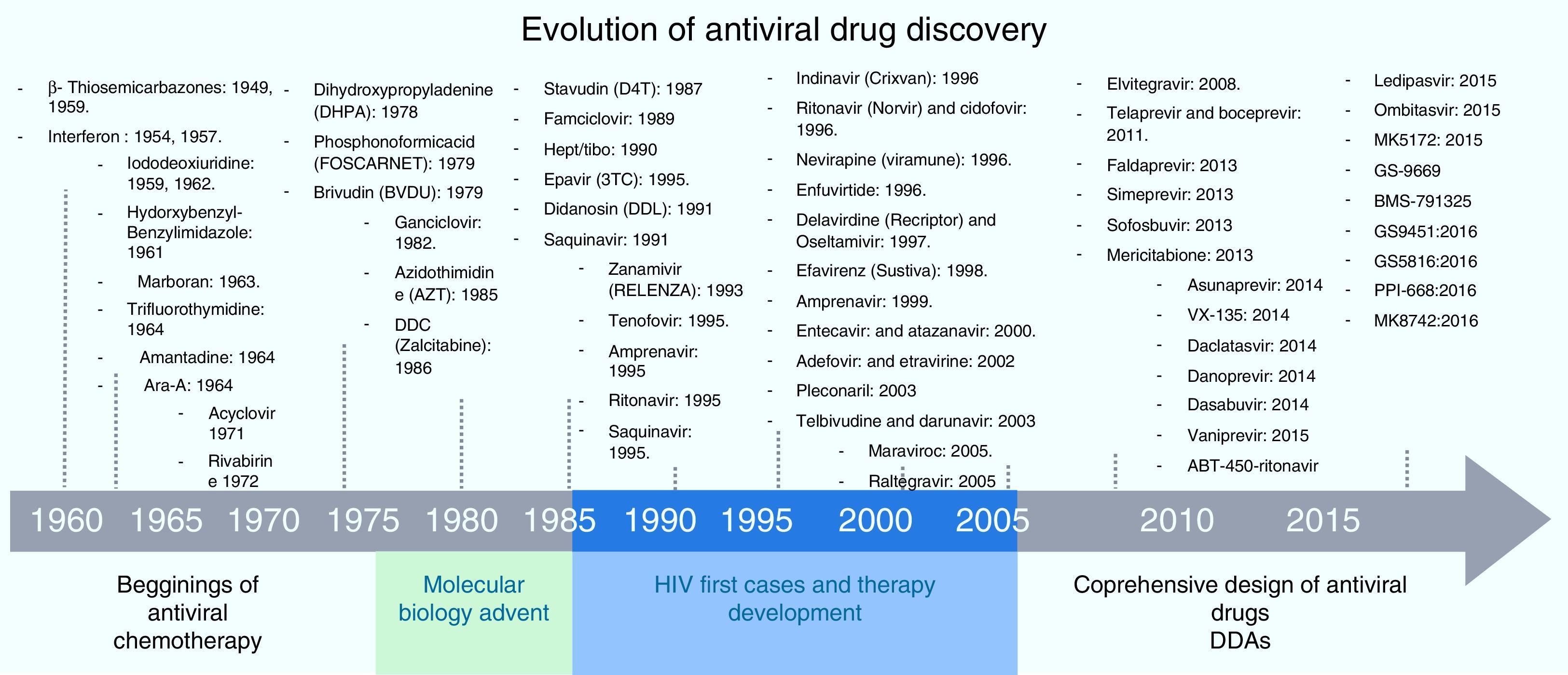
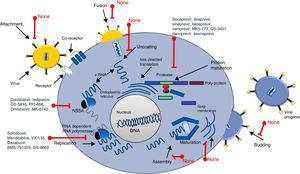
Screening process for antiviral drug discoveryAnother aspect that made antiviral drug discovery a difficult endeavor was the lack of a structured and systematic method for antiviral drug development. Three decades ago most of the first discoveries of antiviral compounds were fortuitous, since molecules originally developed for other purposes were selected as antiviral candidates, based on their success in other medical disciplines. These methods for antiviral discovery were empirical, and most of the time, the biological mechanism behind the observed antiviral effect remained unclear. For instance, the use of thio-semicarbazones against the vaccinia virus, described in 1950 by Hamre et al., and used later as an antibacterial drug against tuberculosis.18 In 1959, the 5-iodo-2-deoxyuridine (IDU), which was originally designed for cancer treatment, proved to exhibit antiviral activity against the Herpes Virus, but due to its high cytotoxicity, its use was limited to topical application. IDU boosted antiviral development, and from its discovery many antiviral molecules were proposed for the treatment of various viral diseases.19 In Fig. 1, a time line of the milestones in the development of antiviral agents shows the early years of this discipline and how it evolved to become a structured and methodic science.20 At the time of IDU discovery, only a handful of viruses were known to cause diseases in humans. The first antiviral drugs were directed to treat herpes, polio, smallpox and influenza, as they were the most relevant viral diseases of that time. Some of them that we can mention are the following: triflouro-thymidine (TFT), a nucleoside analogue used to treat herpes; adenine arabinoside (Ara-A) a nucleoside analogue against the herpes simplex virus21; 2-(α-hydroxybenzyl) benzimidazole for the treatment of poliomyelitis; Marboran for the treatment of smallpox and amantadine and rimantadine to treat influenza, which were identified by traditional biological screening assays in the early 1960s and was shown to be inhibitory for influenza A viruses in cell culture and animal models. In the last two decades, medicinal chemistry has developed into a recognized discipline, in which a lead compound was usually identified by screening a large collection of molecules. This method was improved with the introduction of combinatorial chemistry and high-throughput screening.22 Today, more structured rationales are implemented when looking for new antiviral drugs; simple screening, blind screening and programmed screening have become more sophisticated, as the tools to analyze structure, protein interaction and viral behavior have evolved. For HCV therapy development, many attempts to treat the infection were implemented, with rather poor results.23 Due to the lack of a serological test, systematic treatment protocols could not be performed, and so several “informal” studies were reported, evaluating many kinds of molecules. But it was not until 1986 that Hoofnagle reported the beneficial effect of Interferon Alpha in a pilot study to treat Non-A/Non-B hepatitis.24 This report primed a boom in HCV therapeutics and many randomized controlled clinical trials were performed to improve HCV treatment. In 1990 Ribavirin was first proposed to treat HCV infection and the first clinical trial for the assessment of its efficacy began in 1991.25,26 After the efficacy of the combined antiviral therapy of Pegylated Interferon-α (PEG-IFN-α) and ribavirin against HCV infection was proven, it became the standard of care (SOC) for this disease, and despite its shortcomings (50% response rate and 50% relapse rate on patients infected with genotype 1b, and unwanted side effects), it remained as such for more than 15 years.27,28 During this time, using blind screening approaches, some molecules were found to reduce HCV-RNA levels in vitro, but none of them were significant enough to be implemented clinically. It was not until May, 2011 that the improved understanding of the HCV life cycle led to the discovery, assessment and FDA approval of the HCV protease inhibitors Telaprevir and Boceprevir, that effectively reduce viral load on chronic HCV infected patients, in treatment of naïve patients and in prior relapsers and non-responders. 29,30 Telaprevir and boceprevir were the first direct-acting antiviral agents (DAAs) that selectively target HCV. However, new DDAs have been recently added to this list: simeprevir (protease inhibitor), sofosbuvir (NS5b polymerase inhibitor), daclatasvir (NS5A protein inhibitor), and faldaprevir (second-wave NS3/4A protease inhibitor), all of them showing very promising results and some have even been proposed as the treatment backbone for Interferon-free HCV therapies.31,32 With these selective HCV protease inhibitors, the establishment of STAT-C therapy became a reality. Today, several DAAs (including HCV protease inhibitors, polymerase inhibitors, and NS5A inhibitors) are in various stages of clinical development. Current research is attempting to improve the pharmacokinetics and tolerability of these agents, define the best regimens, and determine treatment strategies that produce the best outcomes. Some of these DAAs will reach the market simultaneously, and resources will be needed to guide the use of these drugs. It is also worth mentioning that different lines of research are currently evaluating other ways to improve HCV chemotherapy. For example, taribavirin, a prodrug for the long-known nucleoside analogue ribavirin, is at 3rd phase clinical trials and has shown promising results.33 This new antiviral would further boost HCV therapy in the coming years. Fig. 2 shows the major HCV potential targets for antiviral chemotherapy.
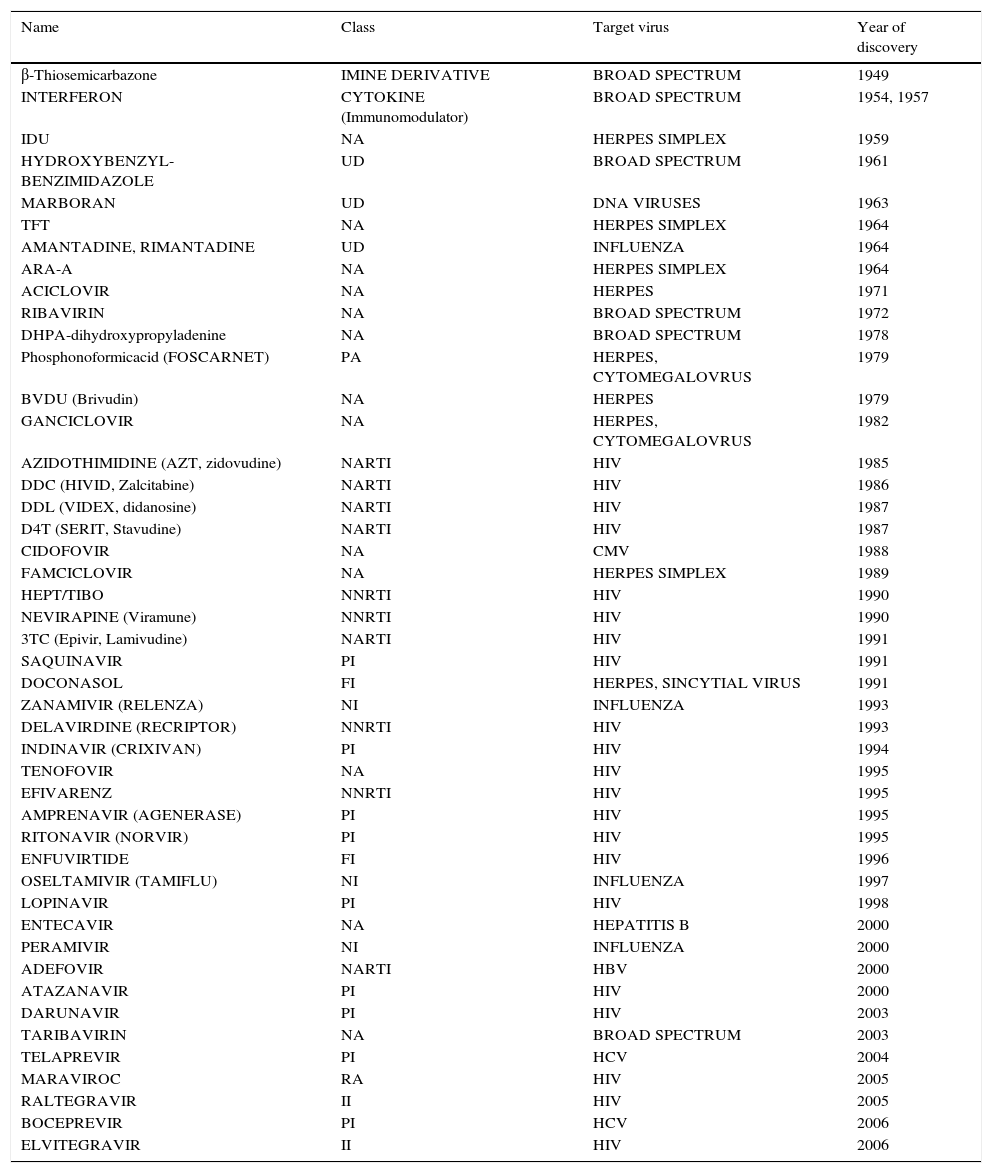
Since the discovery of IDU 50 years ago, only a few molecules have proven to be effective and safe when used for selective antiviral therapy. A huge breakthrough that came from the better understanding of virus-host interaction was the inception of 9-(2-hydroxyethoxymethyl) guanine (Acyclovir). It was the first highly selective antiviral drug, being a substrate for the Herpes Simplex Virus-encoded thymidine-kinase. It displayed a direct inhibitory effect against viral replication and practically no adverse effects on the host. The achievement of selective viral toxicity by Acyclovir and other similar molecules were thought of as the beginning of a new therapeutic age for a well-established, effective and safe antiviral therapy. Acyclovir is a pro-drug, which means it has to be further metabolized in vivo before entering the infected cell wherein further metabolism may or may not be required to yield the active inhibitor. The key to Acyclovir's specificity is the selective phosphorylation of the acyclic guanosine nucleoside by the Herpes virus-encoded pyrimidine deoxynucleoside kinase, which means it would only be active on Herpes-infected cells.34 After Acyclovir's discovery and study, several nucleoside analog pro-drugs have been developed, all of them with relatively high specificity (Table 1 shows a list of the most important antiviral drugs, including their mode of action). Sadly, new challenges arose for antiviral treatment. Several resistant mutants have been identified, making it more difficult to achieve a complete viral eradication and therefore demands for a successful antiviral therapy became more complex, involving many aspects that were previously not considered. One undeniable fact is that most of our current knowledge on viral and antiviral science comes from the study of HIV. The science of antiviral research was well established when HIV/AIDS appeared as a major viral disease in early 1980s. An increase of antiviral therapy studies with no equal took place when the first cases of HIV were reported. Azidothymidine (AZT), among other antiviral molecules already in existence, proved to have selective toxicity against HIV. However, it was during the treatment of HIV that medicine confronted new obstacles. The concept of resistant strains was long known in the microbiological world, but for the young and developing antiviral terrene, it was an issue of little importance until then. HIV was one of the first chronic viral diseases discovered to have a considerable impact on public health. Although antiviral research and development were ignited by the HIV threat, many HIV patients were not responsive to the treatment. The discovery of AZT was followed by several other dideoxynucleoside (ddN) analogues (ddI, ddC, d4T, 3TC, ABC, FTC) (Fig. 2). All these NRTIs act in a similar fashion; after their phosphorylation to triphosphates, they interact as ‘chain terminators’ of the HIV-reverse transcriptase, thus preventing the formation of the proviral DNA. Even though they had great success, drug resistance forced HIV treatment to evolve. Today, it is known that two inevitable and important consequences of antiviral therapy have to be taken into account when planning a treatment strategy for viral chronic diseases. The first is that, given its nature, long-term antiviral therapy automatically selects resistant mutants that will survive and become dominant strains. Resistant mutants are even more frequent in viral than in bacterial infection, and this becomes more evident when treating chronic viral infections such as HIV and HCV.35–37 For viral infections, any attempt to attack the virus’ metabolism could have an effect on host cells. It is evident then, that modifications of these two aspects of antiviral therapy, could improve the results of treatment for chronic patients. This barrier was overcome in part through the use of combinatorial therapy. In addition to that, the concept of a broad spectrum or at least a “pangenotypic” antiviral molecule that could be effective on a wide range of viral pathogens is paradoxically self-defeating if we think that specificity is required to avoid cell toxicity and the opposite is needed to broaden the spectrum of a given antiviral molecule. With our current knowledge on viral metabolism and host interaction, three aspects of viral infection can be targeted for antiviral treatment: inhibition of viral genes and proteins, blocking of host genes and enzymes that interact with viral counterparts, and modulation of host metabolic pathways involved in the virus life cycle.
Major antiviral compounds developed and approved for use in humans.
| Name | Class | Target virus | Year of discovery |
|---|---|---|---|
| β-Thiosemicarbazone | IMINE DERIVATIVE | BROAD SPECTRUM | 1949 |
| INTERFERON | CYTOKINE (Immunomodulator) | BROAD SPECTRUM | 1954, 1957 |
| IDU | NA | HERPES SIMPLEX | 1959 |
| HYDROXYBENZYL-BENZIMIDAZOLE | UD | BROAD SPECTRUM | 1961 |
| MARBORAN | UD | DNA VIRUSES | 1963 |
| TFT | NA | HERPES SIMPLEX | 1964 |
| AMANTADINE, RIMANTADINE | UD | INFLUENZA | 1964 |
| ARA-A | NA | HERPES SIMPLEX | 1964 |
| ACICLOVIR | NA | HERPES | 1971 |
| RIBAVIRIN | NA | BROAD SPECTRUM | 1972 |
| DHPA-dihydroxypropyladenine | NA | BROAD SPECTRUM | 1978 |
| Phosphonoformicacid (FOSCARNET) | PA | HERPES, CYTOMEGALOVRUS | 1979 |
| BVDU (Brivudin) | NA | HERPES | 1979 |
| GANCICLOVIR | NA | HERPES, CYTOMEGALOVRUS | 1982 |
| AZIDOTHIMIDINE (AZT, zidovudine) | NARTI | HIV | 1985 |
| DDC (HIVID, Zalcitabine) | NARTI | HIV | 1986 |
| DDL (VIDEX, didanosine) | NARTI | HIV | 1987 |
| D4T (SERIT, Stavudine) | NARTI | HIV | 1987 |
| CIDOFOVIR | NA | CMV | 1988 |
| FAMCICLOVIR | NA | HERPES SIMPLEX | 1989 |
| HEPT/TIBO | NNRTI | HIV | 1990 |
| NEVIRAPINE (Viramune) | NNRTI | HIV | 1990 |
| 3TC (Epivir, Lamivudine) | NARTI | HIV | 1991 |
| SAQUINAVIR | PI | HIV | 1991 |
| DOCONASOL | FI | HERPES, SINCYTIAL VIRUS | 1991 |
| ZANAMIVIR (RELENZA) | NI | INFLUENZA | 1993 |
| DELAVIRDINE (RECRIPTOR) | NNRTI | HIV | 1993 |
| INDINAVIR (CRIXIVAN) | PI | HIV | 1994 |
| TENOFOVIR | NA | HIV | 1995 |
| EFIVARENZ | NNRTI | HIV | 1995 |
| AMPRENAVIR (AGENERASE) | PI | HIV | 1995 |
| RITONAVIR (NORVIR) | PI | HIV | 1995 |
| ENFUVIRTIDE | FI | HIV | 1996 |
| OSELTAMIVIR (TAMIFLU) | NI | INFLUENZA | 1997 |
| LOPINAVIR | PI | HIV | 1998 |
| ENTECAVIR | NA | HEPATITIS B | 2000 |
| PERAMIVIR | NI | INFLUENZA | 2000 |
| ADEFOVIR | NARTI | HBV | 2000 |
| ATAZANAVIR | PI | HIV | 2000 |
| DARUNAVIR | PI | HIV | 2003 |
| TARIBAVIRIN | NA | BROAD SPECTRUM | 2003 |
| TELAPREVIR | PI | HCV | 2004 |
| MARAVIROC | RA | HIV | 2005 |
| RALTEGRAVIR | II | HIV | 2005 |
| BOCEPREVIR | PI | HCV | 2006 |
| ELVITEGRAVIR | II | HIV | 2006 |
NA: nucleoside analogue; NARTI: nucleoside analogue-reverse trancriptase inhibitor; UD: undetermined; PA: pyrophosphate analogue; NNRTI: non-nucleoside analogue-reverse trancriptase inhibitor; NARTI: nucleoside analogue-reverse trancriptase inhibitor; PI: protease inhibitor; FI: fusion inhibitor; NI: neuraminidase inhibitor; RA: receptor antagonist; II: integrase inhibitor.
As we mentioned before, a new era of therapeutics is currently emerging for Hepatitis C treatment, since several other direct-acting HCV antiviral drugs are being developed (Protease inhibitors: faldaprevir, asunaprevir, danoprevir, vaniprevir, ABT-450-ritonavir, MK5172, GS-9451; NS5A inhibitors: ledipasvir, ombitasvir, GS-5816, PPI-668, MK-8742 and daclatasvir; NS5b inhibitors: mericitabine, VX-135, dasabuvir, BMS-791325, GS-9669), which have been shown to reduce viral RNA levels, reaching SVR in up to 95% of the treated patients.38,39 However, there are several challenges to be addressed to combat HCV using new drugs. DAA's directly attack the Hepatitis C virus and, similar to some of the drugs used to treat HIV, these new molecules target the enzymes needed for viral protein processing; the virus should counterpart this effect (Fig. 2). Based on that, HCV genetic variability and drug resistance are the bigger obstacles that DAAs must overcome. HCV has a high rate of replication, with 1012 virions produced daily, along with an equally high mutation rate, meaning that, for any given drug, there are already resistant mutants present on the infected subject that would ultimately render single drugs useless. However, Hepatitis C resistance may be delayed or prevented by using combinations of potent antiviral drugs without cross-resistance profiles and optimizing patient adherence to therapy.38 On the other hand, accessibility to the new and approved HCV therapies is a challenge in combating the Hepatitis C, mainly because of the high cost of the combined treatments (between 100,000 and 250,000 USD). Availability and accessibility of new protease inhibitors (PI), telaprevir, boceprevir, simeprevir, and the recently approved RNA polymerase inhibitor (RPI) sofosbuvir depends on the region where patients are located and their access to governmental health programs. In most countries, accessibility to these drugs is possible only for those patients who can afford treatment for themselves, as public health systems do not yet have policies for application of the new HCV therapy to the general population through insurance systems.40 This will likely require concerted public and political mobilization to pressure originator companies to reduce prices and stimulate generic competition. In addition, lower prices could make widespread access to HCV treatment possible in low and middle income countries.
Where we stand todayAfter almost 20 years since HCV's discovery, today we account for a solid-yet-not-completely effective treatment landscape to fight hepatitis infection. First, modern biomolecular diagnostic tools are used to determine genotype and viral load as a base to design an accurate therapeutic regimen; second, viral load dynamics is monitored in order to determine drug resistance, and third the liver's state and the presence of infection are assessed in patients who have completed the therapy. In an effort to provide a condensed set of treatment guidelines, the American Association of Liver Disease (AASL), Infectious Disease Society (IDSA) and the International Antiviral Society (IAS-USA) generated the Guidelines for HCV infection treatment which are based on patient's previous exposure to treatment, HCV genotype, relapsing profile and hepatic status.41 In Table 2 we show a compendium of the recent treatment guidelines for HCV infection therapy. It is important for physicians to evaluate patient clinical history (naïve or not), HCV genotype, treatment effectiveness and HIV co-infection in order to avoid unwanted drug interactions.
Summary of the recent treatment guidelines for HCV infection therapy [described by the American Association for the Study of Liver Diseases (AASLD), Infectious Disease Society of America (IDSA) and the International Antiviral Society (IASUSA)].
| HCV genotype | Naïve patientsb | Non-responders to traditional IFN-RBV therapy | Resistant to Sofosbuvir | Resistant to traditional therapy and 1st generation protease inhibitors | Patients with Cirrhosisb |
|---|---|---|---|---|---|
| 1a | Combination of Ledispavir 90mg/sofosbuvir 400mg for 12 wks | Combination of Ledispavir 90mg/sofosbuvir 400mg for 12 wks | Combination of Ledispavir 90mg/sofosbuvir 400mg for 12 wks | Extend treatment for 24 wks | |
| Paritaprevir 150mg/ritonavir 100mg/ombitasvir 25mg/twice daily dose of dasabuvir 250mg and RBVa for 12 wks | Paritaprevir 150mg/ritonavir 100mg/ombitasvir 25mg/twice daily dose of dasabuvir 250mg and RBVa for 12 wks | Combination Of Ledispavir 90mg/sofosbuvir 400mg plus RBVa for 12 wks. | Extend treatment for 24 wks | ||
| Sofosbuvir 400mg/Simeprevir 150mg/RBVa for 12 wks | Sofosbuvir 400mg/Simeprevir 150mg/RBVa for 12 wks | Extend treatment for 24 wks | |||
| 1b | Ledipasvir 90mg/sofosbuvir 400mg for 12 wks | Ledipasvir 90mg/sofosbuvir 400mg for 12 wks | Ledispavir 90mg/sofosbuvir 400mg plus RBVa | Combination Of Ledispavir 90mg/sofosbuvir 400mg for 12 wks | Extend treatment to 24 wks |
| Paritaprevir 150mg/ritonavir 100mg/ombitasvir 25mg/twice daily dose of dasabuvir 250mg for 12 wks | Paritaprevir 150mg/ritonavir 100mg/ombitasvir 25mg/twice daily dose of dasabuvir 250mg for 12 wks | Combination Of Ledispavir 90mg/sofosbuvir 400mg plus RBVa for 12 wks. | Plus RBV | ||
| Sofosbuvir 400mg/Simeprevir 150mg for 12 wks | Sofosbuvir 400mg/Simeprevir 150mg plus RBVa for 12 wks | Extend treatment for 24 wks | |||
| 2 | Sofosbuvir 400mg and RBVa for 12 wks | Sofosbuvir 400mg and RBVa for 12 wks | Extend treatment for 16 wks | ||
| Sofosbuvir 400mg and RBVa plus weekly PEG-IFN for 12 wks | |||||
| 3 | Sofosbuvir 400mg/RBVa | Sofosbuvir 400mg and RBVa for 12 wks | |||
| Sofosbuvir 400mg/RBVa plus Weekly PEG-IFN for 12 wks | Sofosbuvir 400mg and RBVa plus weekly PEG-IFN for 12 wks | ||||
| 4 | Ledipasvir 90mg/Sofosbuvir 400mg for 12 wks | Ledipasvir 90mg/Sofosbuvir 400mg for 12 wks | |||
| Paritaprevir 150mg/ritonavir 100mg/ombitasvir 25mg/and RBVa for 12 wks | Paritaprevir 150mg/ritonavir 100mg/ombitasvir 25mg/and RBVa for 12 wks | ||||
| Sofosbuvir 400mg/RBVa for 24 wks | Sofosbuvir 400mg plus RBVa plus Weekly PEG-IFN for 12 wks | ||||
| Sofosbuvir 400mg plus RBVa plus Weekly PEG-IFN for 12 wks | Sofosbuvir 400mg plus RBVa for 24 wks | ||||
| 5 | Sofosbuvir 400mg plus RBVa plus Weekly PEG-IFN for 12 wks | Sofosbuvir 400mg plus RBVa plus Weekly PEG-IFN for 12 wks | |||
| Weekly PEG-IFN plus RBVa for 48 wks | Weekly PEG-IFN plus RBVa for 48 wks | ||||
| 6 | Ledispavir 90mg/Sofosbuvir 400mg for 12 wks | Ledispavir 90mg/Sofosbuvir 400mg for 12 wks | |||
| Sofosbuvir 400mg plus RBVa plus weekly PEG-IFN for 12 wks | Sofosbuvir 400mg plus RBVa plus weekly PEG-IFN for 12 wks | ||||
RBV (Ribavirin) dosage is weight based (1000mg [<75kg] and 1200mg [>75kg]).
All indications refer to daily doses unless is otherwise clarified in the text.
Definitions for treatment criteria.42,43
(Treatment) Naïve patient: A person who has never undergone any HCV therapy.
– Rapid Virologic Response (RVR): It is defined as an undetectable HCV RNA at week 4 of treatment.
– Sustained Virological Response (SVR): It is defined as undetectable HCV RNA 12 weeks (SVR12) or 24 weeks (SVR24) after treatment completion.
– Non-response: Refers to a patient who do not achieve undetectable HCV RNA during the first 24 weeks of treatment. There are two forms of non-responders: Partial responders and null responders.
– Partial response: It is a sub-category of non-response and describes a decrease in HCV RNA levels by at least 2 Log10 at week 12 of treatment but detectable levels at week 24.
– Null response: Is a sub-category of non-response and refers to the situation when a patient does not suppress their HCV RNA levels by at least 2 Log10 by week 12 of treatment.
– Drug resistant: A patient who is Partial or Null responder to a specific treatment for which a HCV “resistant” mutant remains immune making necessary to change the therapeutical approach.
– Liver Cirrhosis: Liver disease severity should be assessed prior to therapy. Identifying patients with cirrhosis is of particular importance as their prognosis is altered and their treatment regimen may be adapted. Liver biopsy remains the reference method for grading the activity and histological progression (staging) of the disease (fibrosis and cirrhosis). Some non-invasive methods can also be used: Assessment for Liver stiffness, muscle atrophy, patient skin, sclera and mucous; skin turgor, jaundice, spider angiomas and palmar erythema along with elevation of liver enzymes (AST, ALT and LDH). Patients with liver cirrhosis must also be assessed for Hepatocellular Carcinoma.
Antiviral therapy is a well-established discipline with a promising future. Based on economic, scientific and medical interest, and a continuous need for new drugs to avoid resistance, it is most likely that the development of antiviral drugs over the next 20 years will be focused on HIV and HCV. Today, well-established diagnostic and study systems are available for HCV and other viruses. New targets against HCV, such as inhibitors for the scavenger receptor type B1 (SR-B1) and CD81, neutralizing antibodies against the viral glycoproteins and the NS5B polymerase, as well as the NS2/3 auto-protease, the NS3 helicase, and non-enzymatic targets such as NS4B and NS5A proteins are in development (Fig. 1). Other potential drugs targeting HCV replication include compounds active against the IRES element and antisense inhibition. As mentioned before, virus factors are not the only potential targets for inhibition, but host targets are as well, including microRNAs, cellular receptors, adhesion molecules and cyclophilins. For the near future, a combination of host and viral inhibitors will provide a variety of drug regimes appropriate for different patients that could lead to interferon-free therapies that can consistently clear the infection.
A new era of HCV treatment and the increasing knowledge about viruses and their mechanisms of infection, combined with the rapid discovery of novel antiviral strategies and techniques, will speed up the development of novel antiviral drugs.
FundingFinancial support was provided by the CONACYT, grant number CB-2011-1-58781 to A.M.R.E.
Conflict of interestThe authors have no conflicts of interest.
We thank Sergio Lozano-Rodriguez, M.D. for his assistance in reviewing the manuscript.