Donor sites of split-thickness skin grafts (STSGs) are painful and limit patient rehabilitation. We conducted this study to assess the efficacy of a non-adherent polyurethane dressing in reducing pain and its effect on the epithelialization rate of donor sites of STSGs.
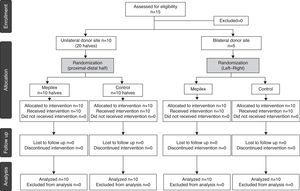
MethodsFifteen patients requiring an STSG were included. In 10 patients the donor sites were randomly divided into two halves and covered with either a non-adherent polyurethane dressing or a standard non-adherent gauze. In five patients with bilateral donor sites, one side was covered with the non-adherent polyurethane dressing and the other with non-adherent gauze. The pain was assessed with a visual analog scale and epithelialization was also assessed, calculating non-epithelialized areas with image software by a blinded surgeon. Epithelialization of the wounds covered with the non-adherent polyurethane dressing was assessed at day 8 and 10 and those with non-adherent gauze at day 10.
ResultsPostoperative pain significantly decreased with the non-adherent polyurethane dressing during the length of the study (6.07±1.46 vs. 1.72±1.6) and at each time point (p<0.001). Epithelialization was not affected with the polyurethane dressing, compared to the standard method.
ConclusionsNon-adherent polyurethane dressing achieves a significant reduction of pain in the skin-grafted donor sites without affecting epithelialization.
Split thickness skin grafts (STSGs) are routinely used to cover a variety of wounds caused by burns, trauma or tumor excisions, etc. Due to their reliability and the relatively high availability of donor sites, STSGs represent one of the first options in reconstruction.1–3 However, harvesting STSGs invariably produces a new open wound in the donor site, which can be painful and requires fast and effective re-epithelialization.
Typically, the donor site is covered with non-adherent fine-meshed gauze impregnated with different ointments.4,5 Unfortunately, this technique is usually painful and is one of its main drawbacks.6 In fact, pain related to donor sites is the most important patient complaint within the first ten days after graft harvest.7 This is particularly important in those cases where prompt rehabilitation is required, e.g., severely burned patients.
Recent technological advances have made the creation of new dressings designed to cause less discomfort in donor site wounds possible.8,9 As newer options are seen on an almost daily basis, the current trend in donor-site management is oriented to reduce pain as well as promote rapid and effective re-epithelialization.10,11
Among the dressings that have been used in donor site wounds are hydrocolloids (Duoderm®) that typically forms a scab over the wound and an exudate with an unpleasant odor macerating the surrounding skin, and Biobrane®, a biocomposite porcine type I collagen attached to a flexible synthetic membrane that has been effective in reducing pain.12 One of the main issues with Biobrane® is that fluid accumulates underneath if not properly used, making the area prone to infection.12
Mepilex® (Mölnlycke Health Care, US, LLC, Norcross, GA) is a non-adherent polyurethane dressing consisting of a polyurethane absorbing sponge, adaptable with Safetac Technology®. According to the manufacturers, this technology permits the dressing to adhere to the surrounding skin, but not to the moist wound bed, potentially reducing pain, preventing maceration and minimizing the drag of epithelial cells at removal.13,14 Furthermore, it seals the wound to prevent leakage of exudate and isolates the wound from the environment, minimizing skin infections.14
Due to these characteristics, it is potentially beneficial for STSGs. We conducted a prospective and randomized study to assess the efficacy of Mepilex® in reducing pain of STSG donor sites and on epithelialization compared with our traditional management (non-adherent dressing).
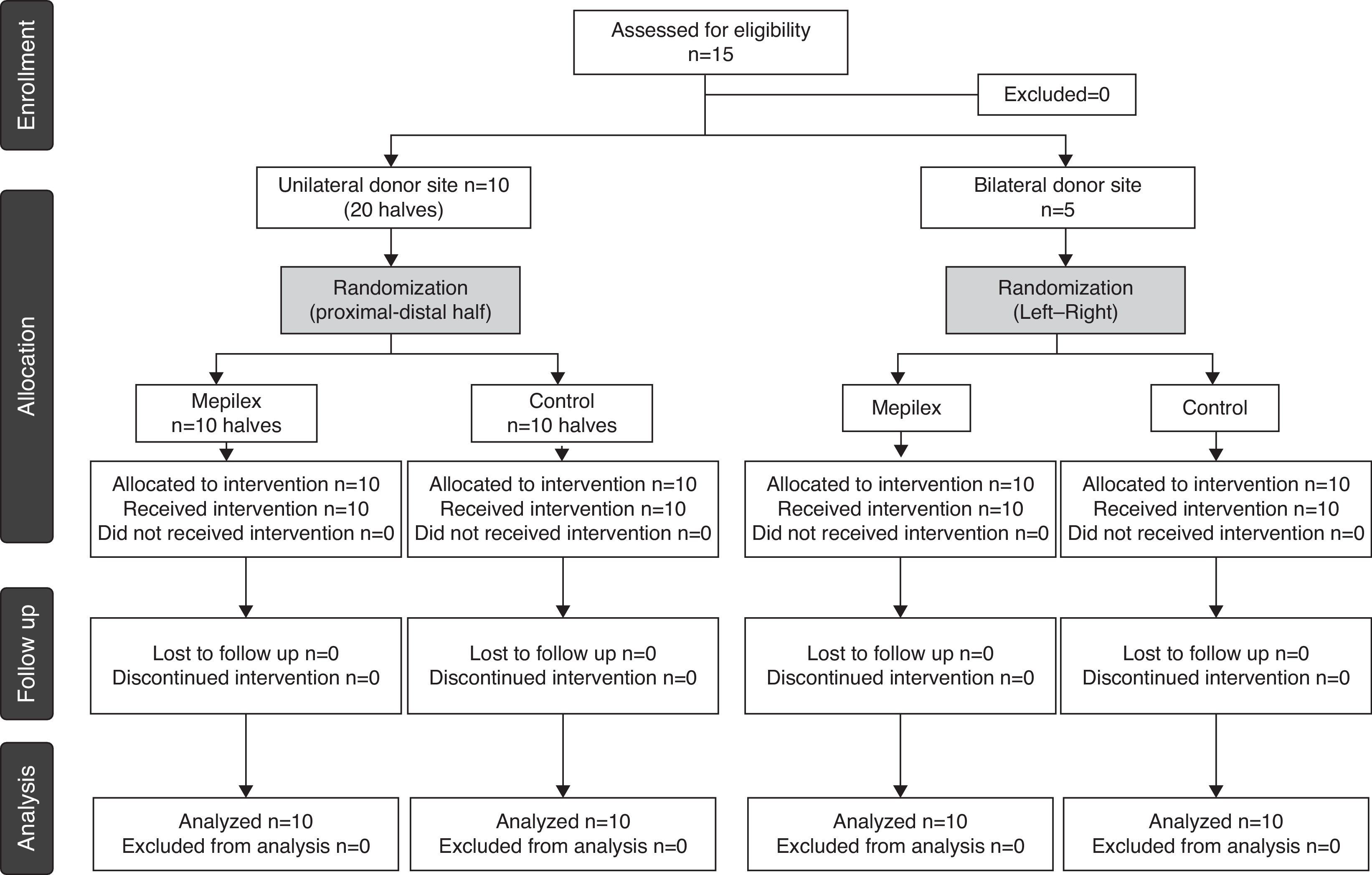
Patients and methodsWe conducted a prospective, comparative and randomized clinical trial between January and August, 2012. The Ethics Committee of our hospital approved the study protocol. All patients enrolled in the study signed an informed consent. Inclusion criteria included patients requiring split-thickness skin grafts secondary to any etiology. The patients were assigned to two groups. The first group included ten patients with a donor site of at least 20cm×10cm on one thigh. The second group included five patients who required bilateral harvest of STSGs of at least 10cm×10cm on each thigh. Exclusion criteria included pregnant women, immunosuppressed patients, a known allergy to any component of the dressings, dermatological diseases, and anticoagulant or corticosteroid treatment.
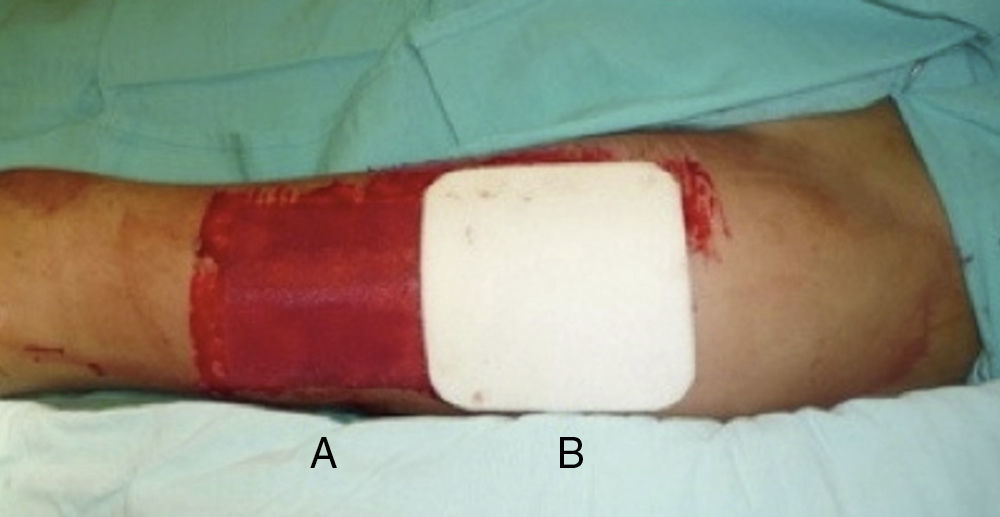
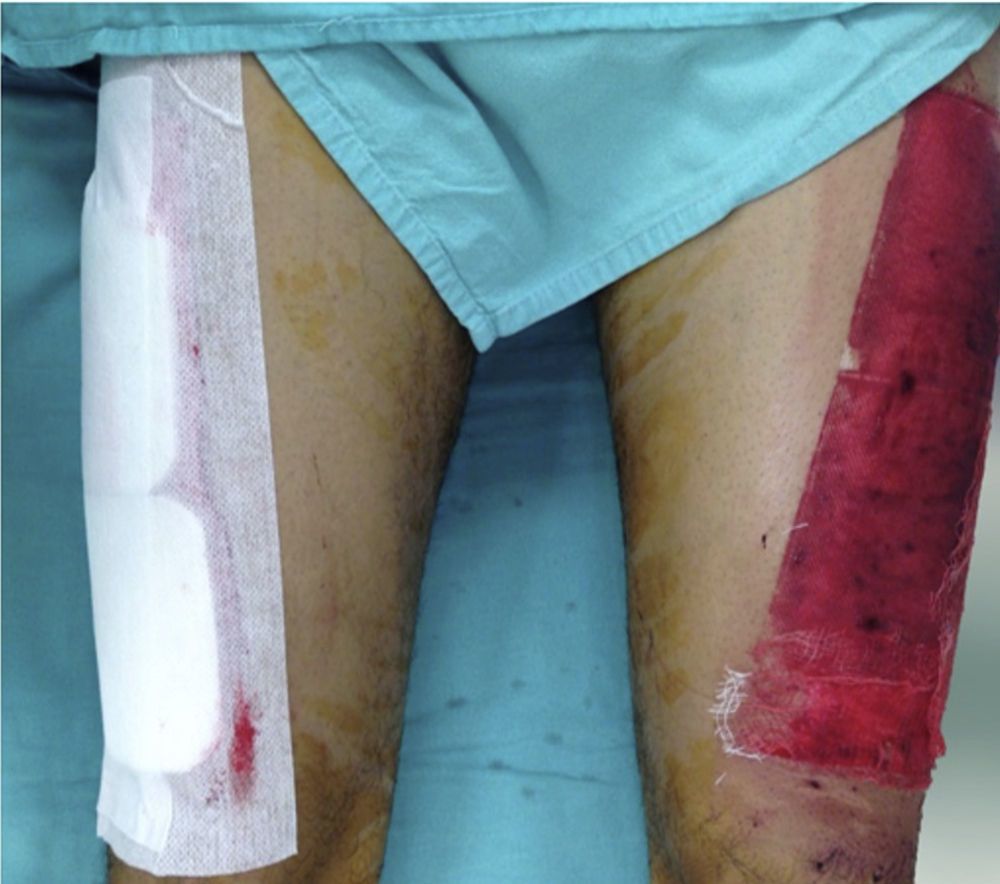
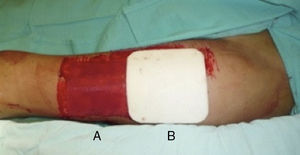
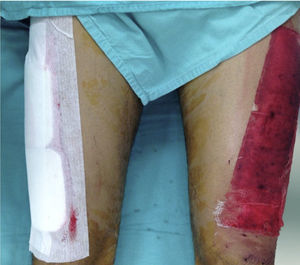
Donor site managementAll skin grafts were harvested from the proximal-anterolateral thigh by the same surgeon. The grafts were harvested with a dermatome to produce a homogeneous thickness of 0.4mm. In patients with a unilateral donor site, the wound was divided into proximal and distal halves and randomly assigned to be covered with either a non-adherent dressing (Adaptic®, Johnson & Johnson, Inc., New Brunswick, NJ), our standard method, or Mepilex®. The area covered with non-adherent gauze was managed in a semi-open fashion, with no secondary dressing. The Mepilex® patch was secured with an adhesive bandage (Hypafix®, BSN medical, Inc., Charlotte, NC) and left on site until the 8th day (Fig. 1). In the patients with bilateral donor sites, one side was covered with Mepilex® and the other with Adaptic® as previously described (Fig. 2). This assignment was randomly made.
Pain of donor sites was assessed with a visual analog scale (VAS) using a scale from 0 to 10 on days 3, 5, 7 and 9 after grafting.
Assessment of epithelializationEpithelialization was assessed on the 8th and 10th days in the Mepilex®-covered areas and on the 10th day in the areas covered with non-adherent gauze. To remove the Adaptic gauze, a thick layer of petrolatum was applied over the area on the 9th day and left overnight. The next morning the adaptic gauze was removed at the patient's bedside. This procedure was painless and no analgesic was required.
Mepilex® was removed on the 8th day at the patient's bedside. If the Mepilex®-covered area was not fully healed by the 8th day, it was covered again, as previously explained for the Mepilex®-covered areas. Photographs were taken of both areas with a digital camera (Fuji Finepix S, Super EBC Fujinon lens: 26× Optical zoom f=4.3–111.8, 1:3.1–5.9) using standardized photo settings (30cm distance, f30, 1/30 and ISO 100) under standard daylight fluorescent lamps (GE T8 Standard linear fluorescent lamp 36W, 2750lm).
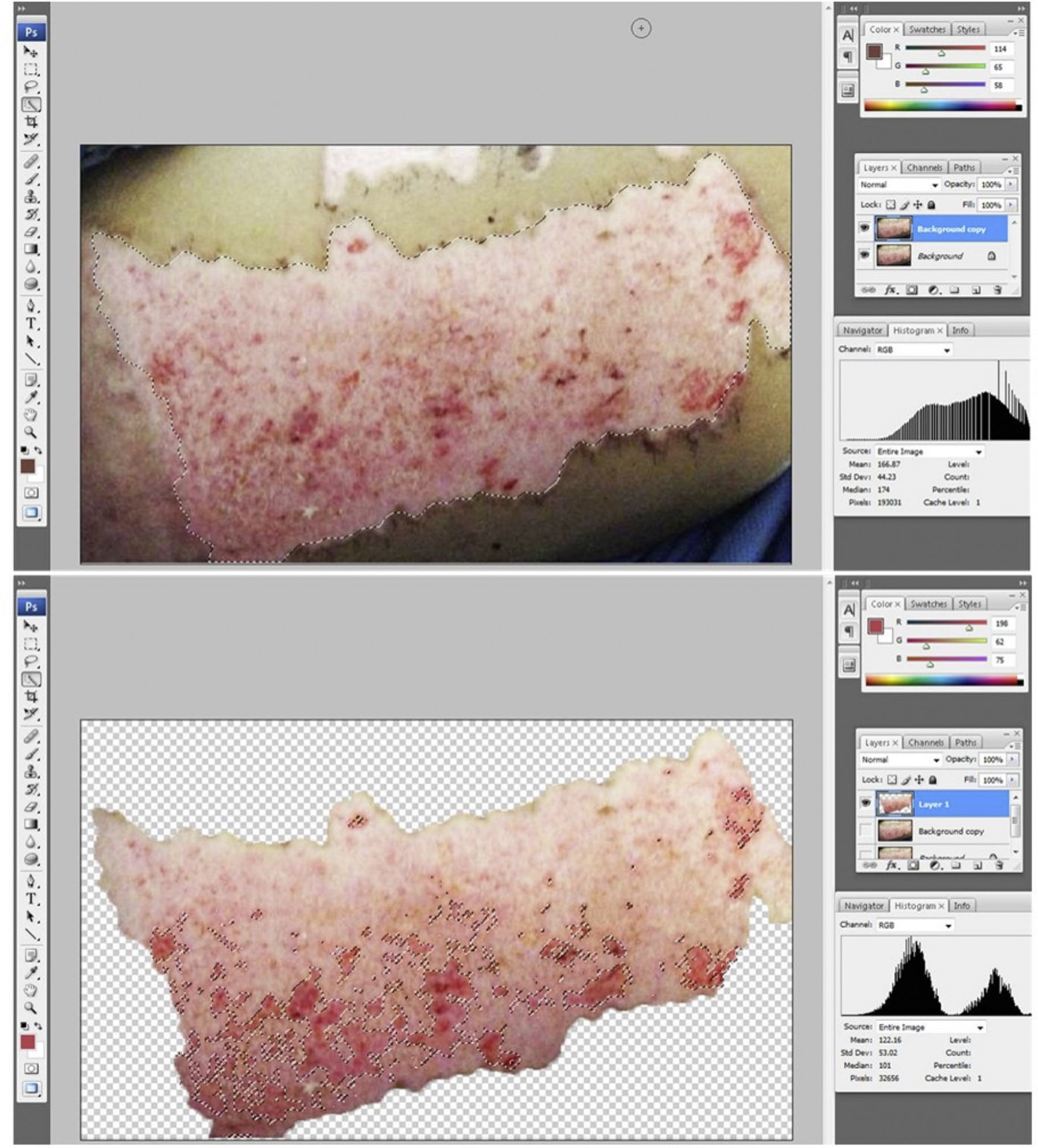
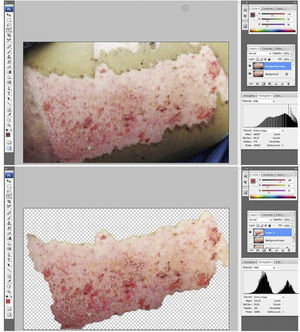
The photographs were assessed by a plastic surgeon blinded to the group and to the time point after grafting. Epithelialization percentage was calculated by analyzing the photographs of the donor sites using image software (Adobe Photoshop®). Briefly, the donor site area was selected and the amount of pixels within the selection was determined. Then, the unhealed areas were selected and the respective size of this selection was determined. The percentage of epithelialization was calculated by dividing the non-epithelialized area by the total donor site surface area (Fig. 3a and b).
Donor site assessment: (a) donor site area to be assessed. The surgeon assessing the epithelialization rate was blinded to group and time point of the area; (b) extension (in pixels) of the non-healed area was determined by diviiding the unhealed area over the total donor site area.
Difference in scores of the visual analog scale was assessed by ANOVA on Ranks. The difference of epithelialized areas was assessed with one-way ANOVA (Sigma Stat 3.5, Germany). Significance was defined as p<0.05.
ResultsFifteen patients requiring skin grafting for the management of trauma injuries were included (Fig. 4). There were 11 men and 4 women with a median age of 27 years (range 10–68) (Table 1).
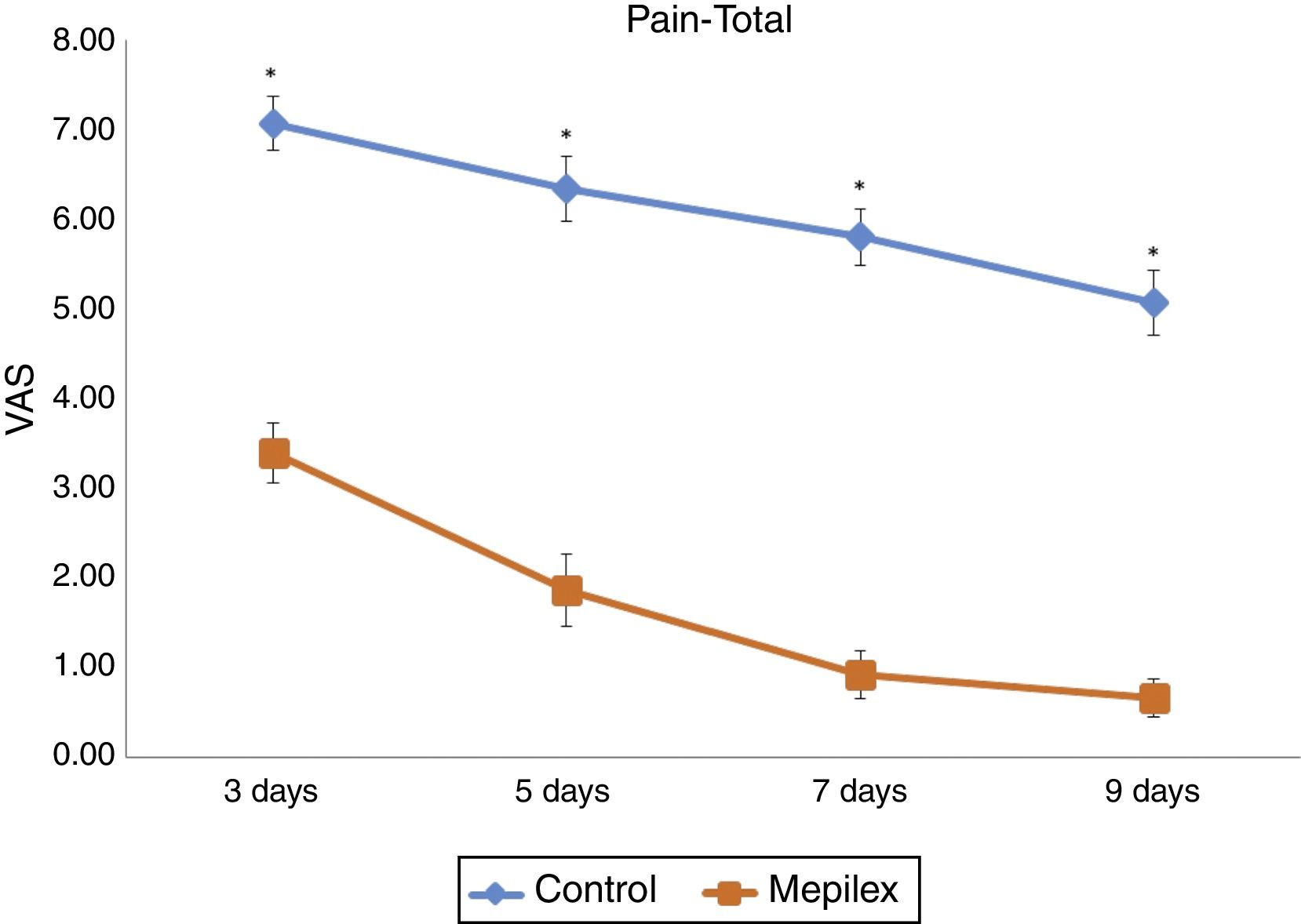
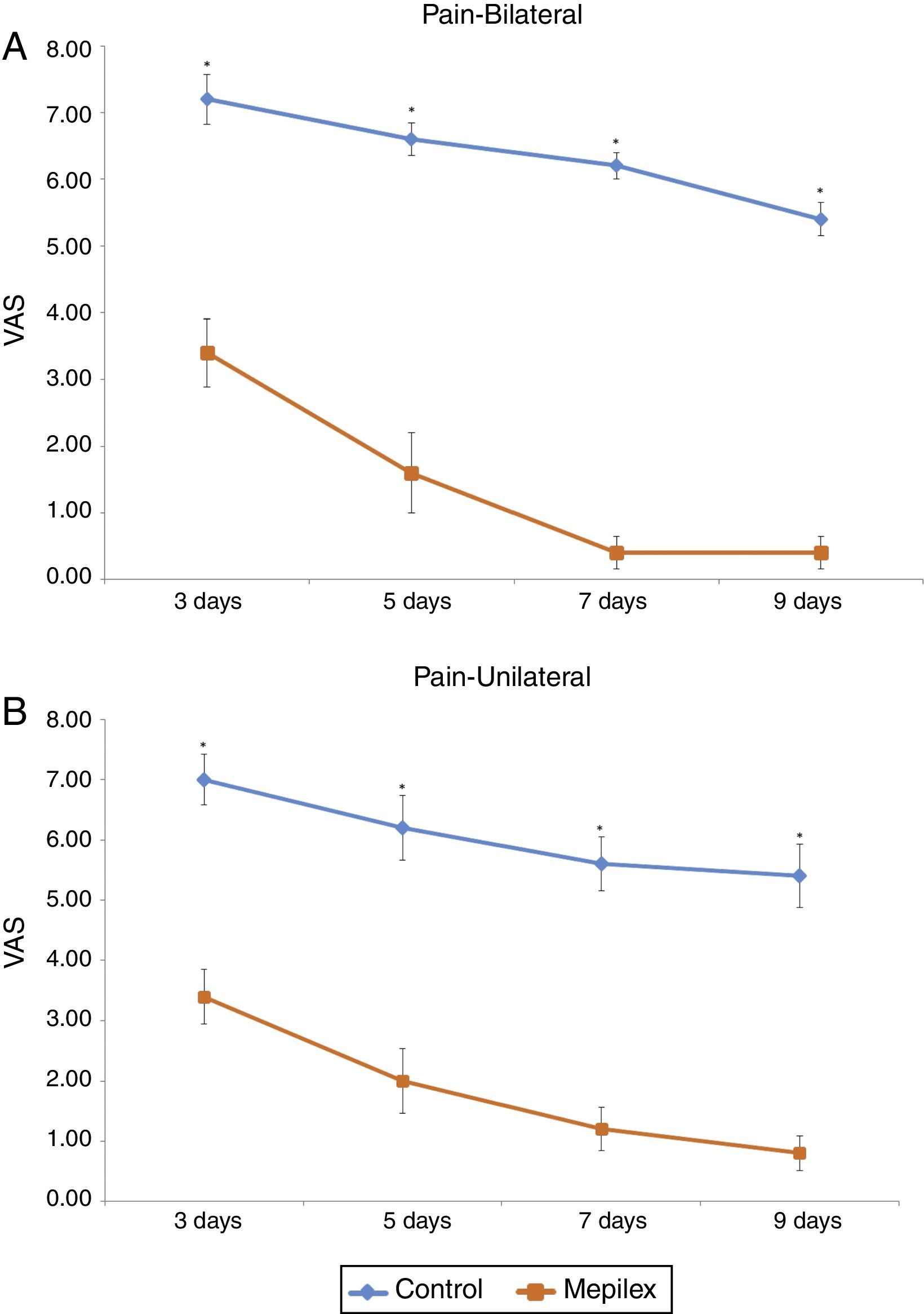
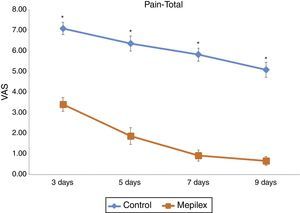
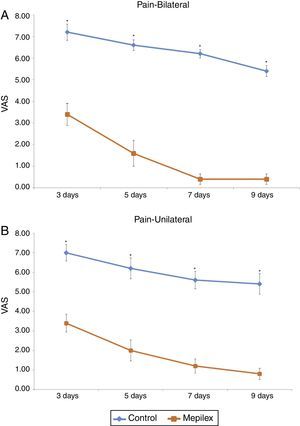
Postoperative donor site pain was consistently lower in the areas covered with Mepilex® during the study. The mean pain scores for the entire treatment period were 6.07±1.46 for control and 1.72±1.6 for Mepilex®. The analysis of each time point showed similar results: on day three, patients reported a mean VAS score of 3.4±1.3 for the Mepilex® group and 7.07±1.16 for the control group; at day five 1.87±1.55 in the Mepilex® group and 6.33±1.4 in the control group; at day seven 0.93±1.03 in the Mepilex® group and 5.8±1.21 in the control group, and on day nine 0.67±0.82 in the Mepilex® group and 5.07±1.39 in the control group (p<0.001) (Fig. 5). Further analysis revealed similar results in the patients with unilateral treatment (both for Mepilex® and Adaptic on the same thigh) and those treated bilaterally (Mepilex® on one thigh and Adaptic on the contralateral one) (p<0.001) (Fig. 6a and b).
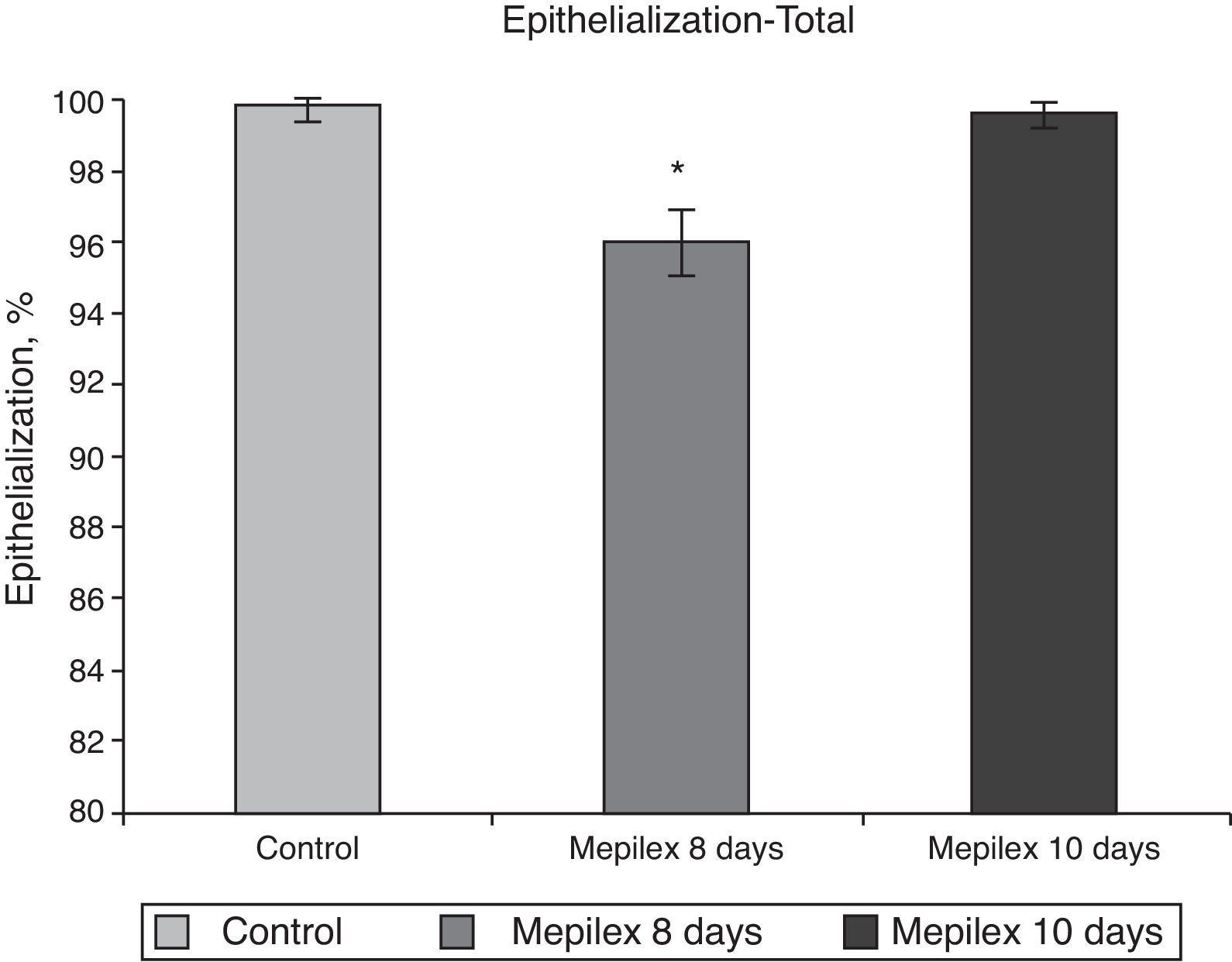
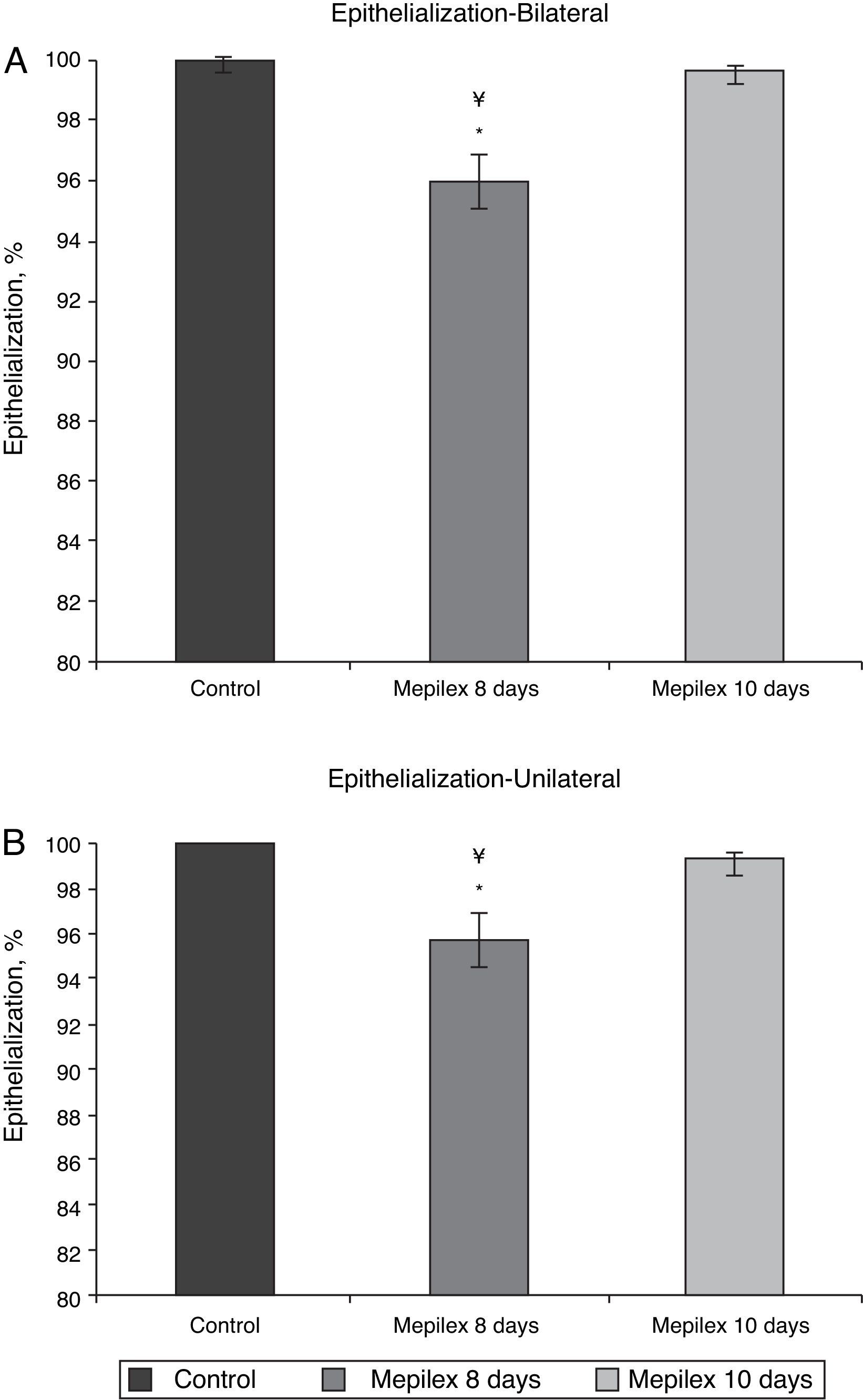
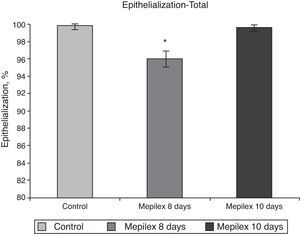
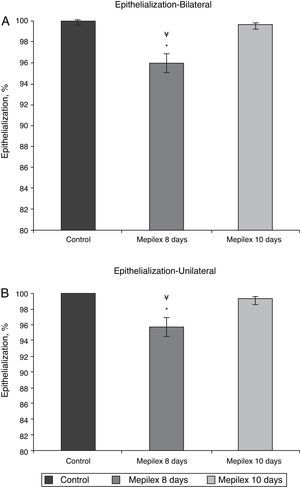
Regarding epithelialization, there was no significant difference between both groups at day ten, (99.55±0.09% in the Mepilex®-covered areas vs. 99.91±0.18% in the control group; p=0.69). At day eight, the areas covered with Mepilex® were only 95.94% epithelialized, a finding that was statistically different with both groups on the 10th day (p=0.008 vs. control and p=0.008 vs. Mepilex® at day ten) (Fig. 7). The comparison between unilateral and bilateral treatments showed practically identical results: Mepilex®-covered areas were 95.74% epithelialized at day 8 in the unilateral group vs. 99.38% at day 10 (p=0.003) and vs. 99.92% in the control group (p<0.001) (Fig. 8a). In the bilateral group, the areas covered with Mepilex® were 95.94% healed at day 8 vs. 99.55% at day 10 (p=0.008) and vs. 99.90% in the control group (p=0.008) (Fig. 8b).
The choice of the dressing for the donor site can have a major impact on patient satisfaction and recovery.15 Skin-graft harvesting results in large open surface areas that are prone to pain, infection or other morbidities.16,17 Donor site management plays an important and critical role in the evolution and rehabilitation of patients because early rehabilitation and mobilization is critical for achieving optimal outcomes in burn patients.18,19
Pain impairs prompt rehabilitation, since pain related to donor sites is the most important complaint within the first ten days after graft harvesting in burn patients.7 Therefore, by decreasing pain, we could potentially help the early mobilization of patients, especially in pediatric populations.
All kinds of dressings are reported in the literature for donor site management, but they all have different drawbacks and the ideal dressing has yet to be described.20,21 In our continuous search for available material that best suits the needs of our burn patients, we decided to assess Mepilex® for its potential advantage of pain reduction without affecting the epithelium. The principal aim of our study was to assess the reduction of pain of donor sites. Since pain is a subjective parameter and varies from individual to individual, it is very difficult to make objective and reliable measurements.22 Therefore, we used the VAS, which is a widely-used and accepted tool for pain assessment.15,23
Donor areas were located in the anterolateral thigh in all cases to reduce variability of pain related to the topographic area. We decided to include patients with unilateral and bilateral donor sites to compare the effect of the dressing in reducing pain because either approach has its pros and cons. In both cases, the patient serves as his own control, which is desirable since using different patients for control and treatment groups makes elimination of the psychological effect of every individual on the perception of pain very difficult, increasing the bias of the study. By dividing the donor sites into halves (proximal and distal) we are assessing the same anatomic area, decreasing variability for a different topographic area. Even though there is a reasonable possibility for poor discrimination of pain between adjacent zones, the scores were significantly different in both areas, confirming that the patient is capable of discriminating the pain in two adjacent areas. The bilateral treatment allowed removal of the theoretical difficulty of effectively discriminating pain in two adjacent areas, but it includes the bias of two topographic areas. Nevertheless, since pain was effectively reduced with Mepilex® in both cases, we can state its superiority over the standard management in pain reduction.
We usually remove the non-adherent gauze of donor sites at day 10 because we have found that epithelialization is complete by that time, and previous attempts to remove the gauze earlier led almost invariably to unhealed wounds. Therefore, we decided to remove the dressings on that day and chose it as the final time point for comparison.
Since epithelialization was practically completed in both groups by day 10 and this was not statistically different (p=0.29), we can conclude that Mepilex® does not impair epithelialization of donor sites. We decided to remove Mepilex® before the 10th day because we wanted to see if it would improve epithelialization. It was arbitrarily decided to set day 8 to assess epithelialization in the Mepilex® group, because if it actually did improve epithelialization, this would be clinically relevant. Since epithelialization was statistically and clinically-relevant incomplete by day 8, we do not advise removal on that day. A new study would be needed to assess if Mepilex® can induce full epithelialization by day 9, but we believe that this is unlikely and clinically irrelevant. An exception for this would be in severely burned patients who typically require several graft takes and one day saved with each harvest would be advantageous.
Even though the sample size is relatively small (15 patients), statistical significance and power was achieved (p<0.001, power 1.00). Therefore, we do not believe that increasing the sample size would influence outcome. Another limitation of this study is the fact that the patient was not blinded to the treatment and it is difficult to eliminate the placebo effect. It was not possible to get dressing material without Safetac Technology. Perhaps future studies can compare other occlusive dressing material to determine if this type of technology is responsible for the observed pain reduction or the occlusive method per se.
However, we found that Mepilex® was an easy-to-use dressing, painless at removal, and maintained its position during the whole treatment period, which is not always possible with other dressing materials; e.g., hydrocolloids. Another advantage was that in this type of moderately exudating wound, Mepilex® efficiently absorbed exudates without the need of secondary dressings. It was not necessary to change the patch due to fluid saturation at any time. The fact that there was no impairment in the rate of epithelialization in the covered donor site encourages its use in cases where the donor area is required to be in contact with the bed; e.g., posterior thigh or patients in a prone position. It is well known that this practice may complicate these areas with conventional management. There were no reports of adverse effects with the use of Mepilex® in any patient and they preferred the use of Mepilex® dressing instead of standard management in all cases.
Therefore, we can conclude that the use of Mepilex® translates into a significant and relevant reduction of pain in donor sites without affecting epithelialization.
DisclosureThe authors declare that they have not published any manuscript similar to this study that might be regarded as redundant or duplicated work.
FundingNo financial support was provided.
Conflict of interestThe authors have no conflicts of interest to declare.