Introduction
Insulin resistance and a relative deficiency in insulin secretion contribute to the pathogenesis of type 2 diabetes mellitus.1 Insulin resistance has a major role in the early stages of the disease and is associated with cardiovascular risk factors. Therefore, amelioration of insulin resistance by pharmacological means yields benefits in glycemic control and in the cardiovascular risk profile.2
A logical approach for the treatment of type 2 diabetes mellitus would be the use of a combination of agents that reduce insulin resistance. Pioglitazone, a peroxisome proliferator-activated receptor-g agonist, increases insulin sensitivity in peripheral tissues and in the liver, decreases serum free fatty acid levels and improves diabetic dyslipidemia (low high density lipoprotein [HDL]-cholesterol and hypertriglyceridemia).2,3 Metformin, a biguanide, reduces hepatic insulin resistance by decreasing hepatic glucose production and gluconeogenesis. It also reduces peripheral insulin resistance and has modest effects on body weight and serum lipid levels.
The most recent algorithm proposed by the American Diabetes Association and the European Association for the Study of Diabetes for the treatment of type 2 diabetes mellitus recommends as a first step, besides life-style interventions, the prescription of metformin as initial monotherapy, given its effect on glycemia, the absence of weight gain or hypoglycemia, its tolerability, low incidence of adverse reactions, and low cost.4 In the present study, knowing the potential advantages of pioglitazone and metformin in the pathophysiology of the disease, we compared the efficacy at three months of a combined treatment (pioglitazone-metformin) versus monotherapy with metformin on glycemic control (measured as glycated hemoglobin A1c [A1c] levels) in new-onset type 2 diabetes mellitus patients naive to oral antidiabetic agents.
Methods
This was a three months, randomized-controlled trial that included new-onset type 2 diabetes mellitus patients (with less than one year of duration) naive to oral treatment for this condition. Exclusion criteria were liver disease and/or serum transaminase levels > 2.5 times upper normal values, a previous kidney transplant, kidney disease or dialysis therapy, a history of lactic acidosis, congestive heart failure class III or IV of the New York Heart Association Classification, human immunodeficiency virus seropositivity, a body mass index (BMI) < 25 kg/m2, anemia, treatment with nicotinic acid or glucocorticoids, treatment with angiotensin-converting enzyme inhibitors or angiotensin II-receptor antagonists within the previous 30 days, or the simultaneous participation in another clinical trial.
Patients were only instructed about reducing their carbohydrate intake. They were randomized to receive a pioglitazone-metformin combination (PGZ-MF) or metformin alone (MF). The PGZ-MF group started pioglitazone 30 mg-metformin 850 mg once daily during the first month, titrating metformin 850 mg to twice daily thereafter. The MF group started metformin 850 mg once daily during the first month, titrating this dose to twice daily thereafter. Anthropometric measures, blood pressure, the criteria for the diagnosis of type 2 diabetes mellitus, serum glucose, insulin, A1c levels and a lipid profile were determined for both groups at the beginning of the study and at three months. Serum insulin levels were obtained by radioimmunoassay (sensitivity 1.2 μU/mL, intraessay coefficient of variation 4.9 - 10%). Serum glucose and insulin levels were used to calculate HOMA-%S and HOMA-%B from the HOMA calculator version 2.2 (available at www.dtu.ox.ac.uk), introducing the minimal insulin value allowed by the calculator (2.9 μU/mL) for serum insulin levels below this value.
Population sample was calculated to demonstrate that the PGZ-MF group would have an A1c value 1.5% less at three months than that obtained by the MF group. We proposed this A1c value based on the reported additive reduction in A1c achieved by these agents. Using an α = 0.05 and β = 0.2, a variance of 2.25 and a two-tailed hypothesis, we obtained a requirement of 17 subjects for each group. We did not adjust to losses due to the high probability of follow-up. Values were transformed logarithmically if they did not suit for a normal distribution. Continuous variables comparisons were made by the Mann-Whitney test, and paired comparisons were made by the Student´s t and Wilcoxon tests (according to their distribution); categorical variables comparisons were made by the Fisher´s exact test. We used MedCalc version 11 statistical software. Data in tables are expressed as the mean ± 95% confidence interval of the mean. Significant statistical differences are described with a p value <.05.
This study was approved by the Institutional Ethics Committee of our hospital. All participants provided written informed consent to the study.
Results
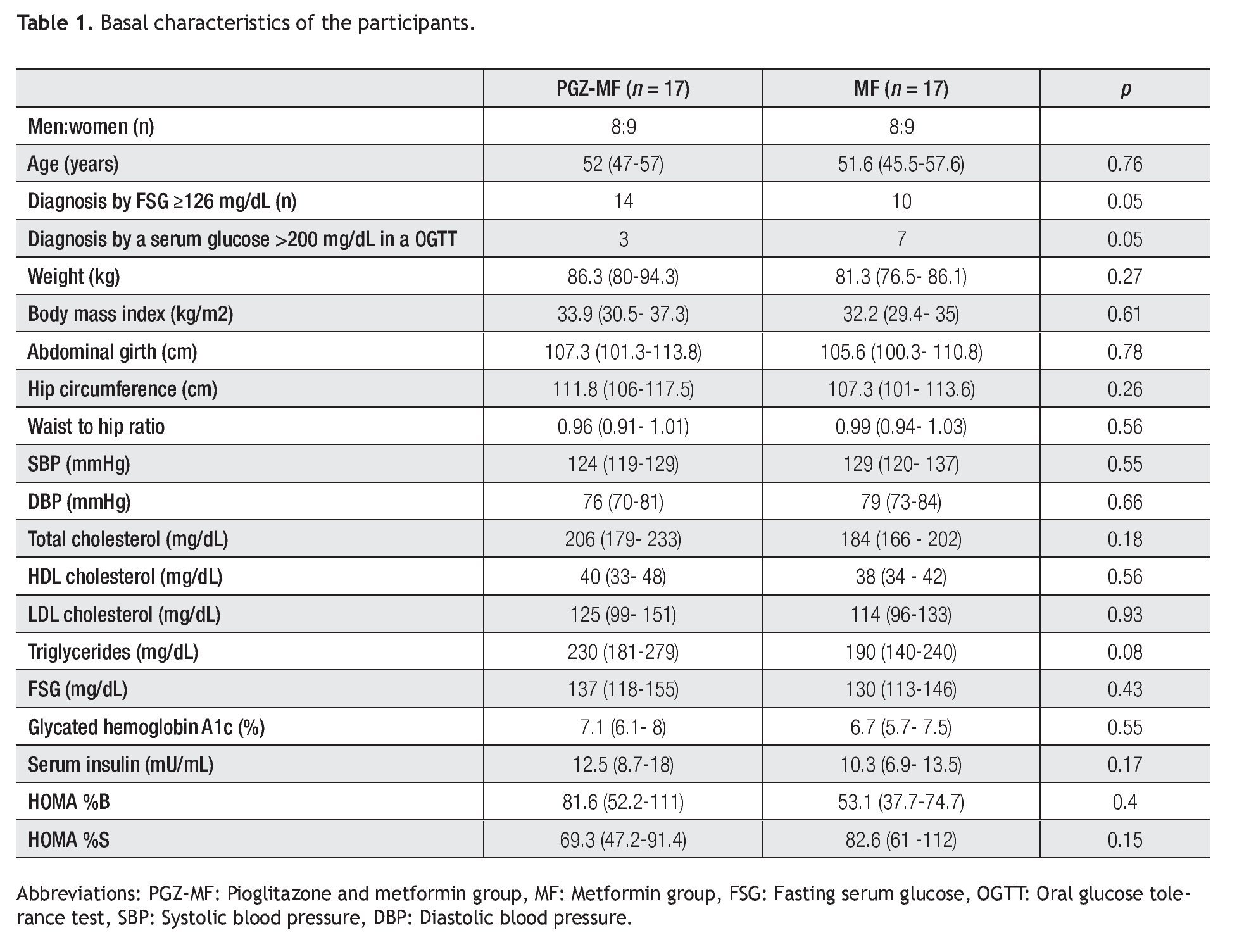
Thirty four patients were included, 17 for each group (Table 1). All patients finished the study. The majority of the patients in both groups were diagnosed with a fasting serum glucose ≥126 mg/dL in two separate occasions, but between groups there were significantly more patients diagnosed with a fasting serum glucose ≥ 126 mg/dL in the PGZ-MF group, while there were significantly more patients diagnosed with a serum glucose > 200 mg/dL in an oral glucose tolerance test in the MF group. The rest of their basal characteristics did not show major differences.
Although there were more patients achieving a 1.5% decrease in A1c levels in the PGZ-MF group (52%) compared to the MF group (17%), we did not find a difference between both groups (p = 0.07) nor we did find a difference between groups for obtaining an A1c level ≤ 7% at three months (47% from the PGZ-MF group versus 82% from the MF group, p = 0.07). The PGZ-MF group did not reach an A1c level 1.5% less than that obtained by the MF group at three months.
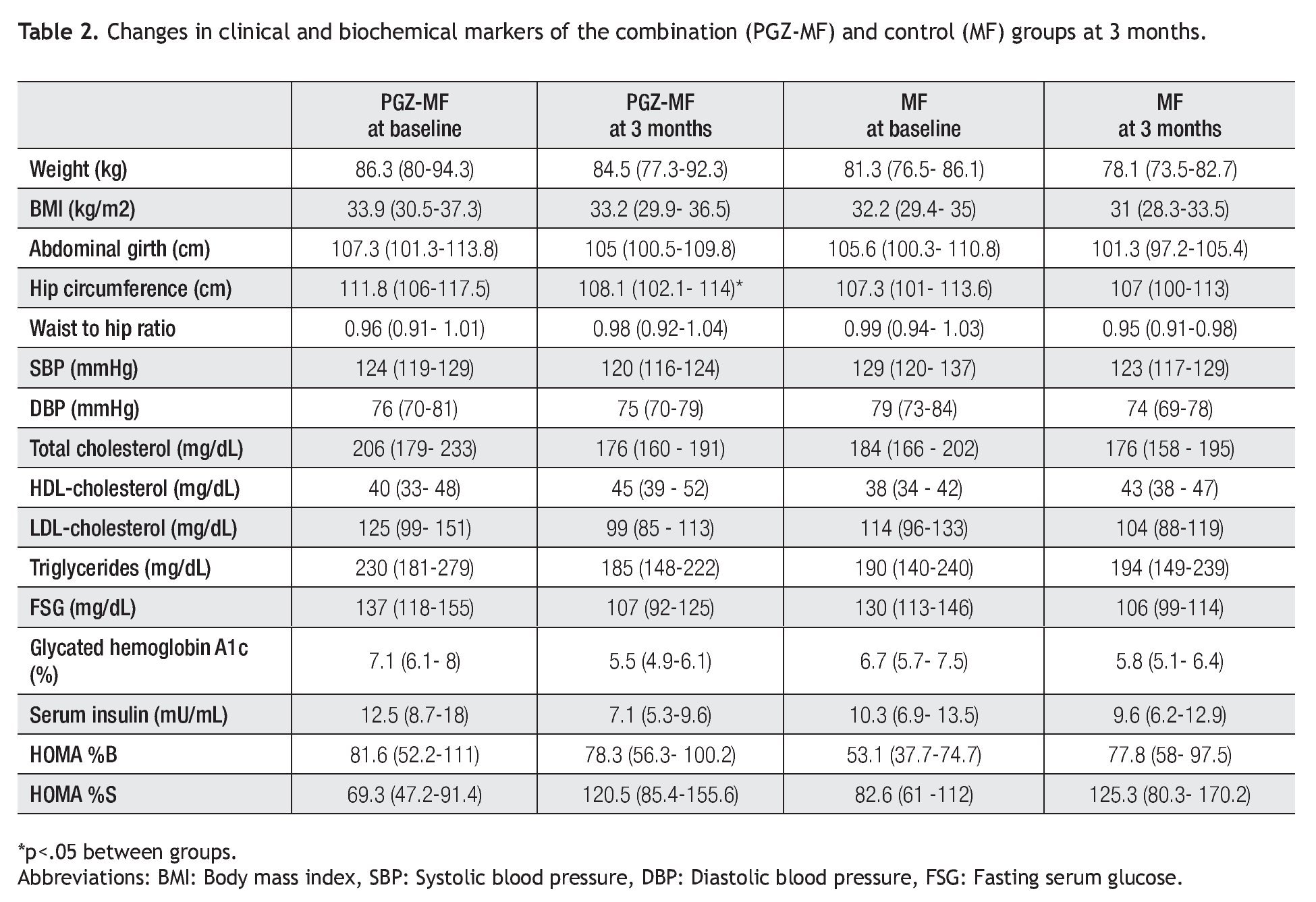
Both groups achieved a decrease in body weight, abdominal girth, hip circumference and systolic and diastolic blood pressure at three months (Table 2). Nevertheless, significant changes were a decrease in body weight (1.87 kg, 2.02% from baseline, 95% CI = -0.14, 3.88), abdominal girth (2.6 cm, 1.99% from baseline, 95% CI = 0.7, 5.9) and hip circumference (3.7 cm, 3.33% from baseline, 95% CI = 2, 5.4) in the PGZ-MF group, while the significant changes in the MF group were a decrease in body weight (3.17 kg, 3.81% from baseline, 95% CI = 1.33, 5), BMI (1.27 kg/m2, 3.81% from baseline, 95% CI = 0.57, 1.97), abdominal girth (4.2 cm, 3.61% from baseline, 95% CI = 0.5, 7.9) and diastolic blood pressure (5.8 mmHg, 6.15% from baseline, 95% CI = -1.2, 12.8). Although the waist to hip ratio increased in the PGZ-MF group and decreased in the MF group, the difference was not significant. Between groups, the sole significant difference was the change in hip circumference (a decrease of 3.33% from baseline in the PGZ-MF group versus a decrease of 0.1% from baseline in the MF group, p = 0.04).
In the lipid profile, both groups showed a decrease at three months in serum total cholesterol (10.46% from baseline in the PGZ-MF group versus 2.99% from baseline in the MF group) and low-density lipoprotein (LDL) cholesterol levels (4.37% from baseline in the PGZ-MF group versus 4.01% from the baseline in the MF group), besides an increase at three months in HDL-cholesterol levels (27.28% from baseline in the PGZ-MF group versus 15.39% from baseline in the MF group). The PGZ-MF group achieved a significant decrease in serum triglyceride levels (14.97% from baseline, 45 mg/dL, 95% CI = 2, 87), while the MF group showed an increase in triglyceride levels (12.87% from baseline) and a significant increase in HDL-cholesterol (5 mg/dL, 95% CI = 1, 8).
At three months both groups showed a decrease in fasting serum glucose (15.82% from baseline in the PGZMF group versus 13.76% from baseline in the MF group) and A1c levels (18.62% from baseline in the PGZ-MF group versus 10.14% from baseline in the MF group), besides an increase in HOMA-%S (34.4% from baseline in the PGZ-MF group versus 13.03% from baseline in the MF group). The PGZ-MF group achieved a significant decrease in fasting serum glucose (24 mg/dL, 95% CI = 3, 44), A1c (1.5%, 95% CI = 0.8, 2.2), serum insulin levels (7.7 mU/mL, 95% CI = 2.4, 13) and a significant increase in HOMA-%S (51.1, 95% CI = 14.9, 87.4). The MF group significantly achieved a decrease in fasting serum glucose (23 mg/dL, 95% CI = 5, 42) and A1c levels (0.8%, 95% CI = 0.2, 1.4). The PGZMF group showed a non-significant decrease in HOMA-%B (-3.38, 15.44% from baseline, 95% CI = 27.9, 21.13) while the MF group showed a non-significant increase (12.1, 11.9% from baseline, 95% CI = -8.5, 32.9).
There were no adverse reactions or increases in the transaminase levels in any patient.
Discussion
In a three months period, we found that the combined use of pioglitazone and metformin produced similar changes than metformin monotherapy in patients with new-onset type 2 diabetes mellitus naive to treatment, but we found an additional benefit in the decrease in hip circumference in the former group. Our findings at three months disagree with the use of this combination in other ethnic groups with new-onset type 2 diabetes mellitus. Pavo et al 2 found, in a Hungarian-Russian population sample, that the pioglitazone-metformin combination at 32 weeks produced a significant decrease in serum insulin levels, and an increase in HOMA-%S (14.9% in the pioglita-zone group) compared with metformin monotherapy. This finding was reproduced by Tan et al,3 who found in an American population sample an increase in HOMA-%S of 17% (66.2% to 82.2%, p <.0001) at 16 weeks in the combined treatment group versus no change in the metformin monotherapy group. Einhorn et al,5 in a 16 week-study, showed that the pioglitazone-metformin combination, compared to metformin monotherapy, produced a significant decrease in A1c (0.83%), fasting serum glucose (37 mg/dL) and serum triglycerides (18.2% from baseline) levels, and an increase in HDL-cholesterol (8.7% from baseline) levels. Of consideration, Pavo et al,2 mentions that the combined therapy group gained body weight, a finding that was not reproduced in our study.
Regarding A1c, although we did not demonstrate a difference greater than 1.5% between the decreases in A1c obtained at the end of the study for both groups, all patients obtained a significant reduction in their A1c levels at three months. We report a greater decrease with the combined therapy than that obtained by Einhorn in a longer study,5 perhaps because in his study patients with initial A1c values >8% with metformin monotherapy for more than 30 days were included.
We are aware that our findings rely on a very small sample of patients. Nevertheless, we believe that early treatment with this combination offers benefits in the short term, since we found that it was effective, reached the A1c proposed values for the prevention of micro and macro-vascular complications, and particularly, decreased hip circumference in patients with new-onset type 2 diabetes mellitus. Also, we found that this initial combined treatment was not associated with weight gain and did not induce adverse reactions. Our data can support the findings of Chalmers et al,.6 who described, in a three year study which included American patients with new-onset type 2 diabetes mellitus and A1c > 7%, that the decreasing slope of A1c with the combined therapy was less (0.1% per year) than the combined therapy of metformin plus glicazide or repaglinide (0.5% per year).
In summary, initial treatment of new-onset type 2 diabetes mellitus with a combination of pioglitazone and metformin produced, at three months, similar changes in body weight, abdominal girth, serum triglycerides, insulin levels and an increase in peripheral insulin sensitivity compared with metformin monotherapy. The only significant difference between groups was a greater reduction in hip circumference with the combination therapy. We did not find any difference between groups in their ability to decrease A1c levels at three months, but we found a greater decrease in the combined therapy group.
Author disclosure statement: No competing financial interests exist.
Acknowledgment: This information was previously published as an abstract (2075-PO, Pioglitazone + metformin, combined therapy in recently diagnosed type 2 diabetes mellitus) in the 2006 American Diabetes Association meeting in Washington, D.C.
Correspondence author: Fernando Javier Lavalle González M.D.
Departamento de Endocrinología Hospital Universitario "Dr. José Eleuterio González". Avenida Madero y Gonzalitos S/N Col. Mitras Centro C.P. 64460 Monterrey, Nuevo León, México.
Telephone: (+52) 81-83487871.
E-mail:fjlavallegzz@hotmail.com
Received: Noviembre, 2010.
Accepted: Enero, 2011