To determine the relationship between lactic acid levels and neonatal mortality in the first week of life, in patients admitted to the Neonatal Intensive Care Unit (NICU) of the “Dr. José E. González” University Hospital.
Material and methodsProspective, observational and diagnostic test performed in the neonatal ward of the “Dr. José Eleuterio González” University Hospital. We included all live preterm infants on mechanical ventilation who were admitted to the NICU from November 1, 2011 to October 31, 2012.
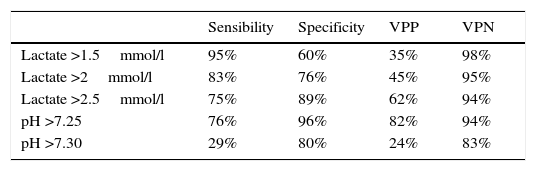
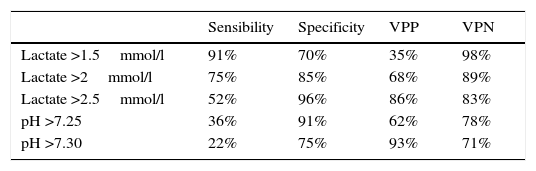
ResultsOne hundred and fifty four patients met the inclusion criteria. At 72h, we found that the best sensitivity (95%) was when lactate was less than 1.5mmol/l and the best specificity (89%) was present when lactate levels were greater than 2.5mmol/l. The pH <7.25 had a sensitivity of 76% and specificity of 96%. At 168h (7 days) we found that the best sensitivity (91%) was when lactate levels were less than 1.5mmol/l, and that the best specificity (96%) was when lactate levels were greater than 2.5mmol/l. We found that the pH <7.25 had a sensitivity of 36% and specificity of 91%.
ConclusionThe lactate serum, at 1.5mmol/l, has a sensitivity (to be killed) of 95% on the third day and 91% on the seventh day. When the pH is greater than 7.25 there is a specificity (to be alive) of 96% on the third day and 91% on the seventh day. 67% of the dead were under 1500g, and were 61% under 28 gestational weeks.
Lactic acid is a terminal product of the anaerobic metabolism of glucose, and it is obtained by a reduction of pyruvic acid in a reaction catalyzed by the lactic dehydrogenase enzyme, where a dinucleotide adenine nicotinamide (NAD) coenzyme goes from its reduced form to its oxidized form.1–3
Under normal conditions, serum levels are at 2mEq/L or lower. However, exercise can elevate it up to 4mEq/L. Most lactate is efficiently eliminated by the liver and is utilized in gluconeogenesis or in the production of energy. When considerable increases in lactate serum numbers are produced, with a reduction in lactate to pyruvate metabolism conversion, metabolic acidosis occurs, which is usually severe and may lead to patient death.4
Lactic acid is increased in situations of tissue hypoxia–ischemia. Different studies conducted in adults,5 as well as in children6,7 and newborns,8 have shown that in critical situations, lactic acid levels at the moment of the patient's admittance to the ICU and its evolution have a positive correlation with the risk of death.9
Lactic acid can increase for several causes other than tissue hypoxia. Within the context of the ICU, in order of frequency, amongst the most probable are those of hemodynamic causes,10 which determine tissue hypoxia,11 as in the case of sepsis, heart failure, shock (cardiogenic and septic) and multiple organ dysfunction.11 Amongst the non-hemodynamic causes there are12,13: the use of biguanides in diabetic patients, the use of pharmaceuticals such as adrenalin and nitroprusside, seizures, intestinal infarction, or in general, patients with intestinal hypomotility, short bowels, thiamine deficiency, liver diseases in general, ethanol overdoses, methanol, salicylates and ethylene glycol.
Lactate has proven to be a good prognostic indicator in hospitalized adults and children14,15 and different types of patients, including critical,16–18 and surgical,19 with sepsis20,21 and trauma.22,23 Its main advantage is how fast and easy its determination is, and its evolutionary prognosis capability.24,25
The objective of this study is to determine the relationship of lactic acid levels with neonatal mortality rates in the first week of life, in patients admitted to the Neonatal Intensive Care Unit at the “Dr. Jose E. Gonzalez” University Hospital, as well as to quantify neonatal mortality rate prevalence in the first week of life in relation to lactic acid levels, to know non-pathological and pathological maternal backgrounds, learn the newborn's characteristics, to identify the main in-hospital morbidities, document the main causes of associated death, and identify lactate levels associated and a higher mortality rate.
Materials and methodsA prospective, observational and diagnostic test was performed in the neonatal ward of the “Dr. José Eleuterio González” University Hospital of the Autonomous University of Nuevo León. We included all live preterm infants on mechanical ventilation who were admitted to the NICU within the period of November 1, 2011 to October 31, 2012. The study was approved by the University's Ethics Committee with the folio no. NE12-003.
Exclusion criteria included all live preterm infants who did not require mechanical ventilation admitted to the NICU between November 1, 2011 and October 31, 2012, patients transferred from other hospital units, and those who did not meet the requirements of the file.
Variables were the following: the evaluated newborns’ characteristics were weight at birth, and were classified into <1000g, 1001–1500g, 1501–2500g and 2501–4000g categories. Also, gestational age was evaluated and classified according to the number of weeks of gestation as <28 weeks, 28–33 weeks, and 34–36 weeks of gestation. Trophism was evaluated and classified as adequate weight for gestational age and low weight for gestational age.26 They were divided by male and female, according to gender, and were evaluated and classified according to their APGAR score at 5min in 0–3, 4–6 and 7–10 points.
Within these variables, the study included pathological and non-pathological maternal family history: no family history, smoking, alcoholism, drug addiction and tattoos on the mothers of the newborns, the number of sexual partners was studied, divided into one, two, three or more. According to the mother's health during pregnancy, they were divided into healthy, or whether the mother presented an ailment, such as high blood pressure, infections, preeclampsia, eclampsia or gestational diabetes, among others. Also, it was determined whether or not the patients carried out proper prenatal care.
Additionally, the mothers’ age was evaluated and divided as follows: under 18 years old, from 19 to 30 years old, and over 30 years old. They were divided into single, married and the consensual union, according to their marital status. Regarding schooling, the classifications were illiterate, elementary, junior high school, high school, and bachelor's degree. Also, a classification according to their occupation was made, dividing them as follows: housewife, worker and student. Among the evaluated variables were causes of in-hospital morbidity and mortality, from which the following pathologies were studied: hyaline membrane disease grades 1 through 4, pulmonary edema, meconium aspiration syndrome, septic shock, sepsis, extreme prematurity and perinatal asphyxia.
Lactate and pH were studied at 72h (3 days), assessing sensitivity, specificity, positive predictive value and negative predictive value of lactate levels >1.5mmol/l, 2mmol/l and lactate at 2.5mmol/l, as well as pH <7.25 and <7.30.
Similarly, lactate and pH were studied at 168h (7 days), assessing sensitivity, specificity, positive predictive value and negative predictive value of lactate levels >1.5mmol/l and 2mmol/l and lactate at 2.5mmol/l, as well as pH <7.25 and <7.30.
Qualitative variables were used in the statistical analysis. The chi square (non-parametric) tests were utilized to test the hypothesis. An alpha value of 0.05 was used and the null hypothesis was rejected when the critical value was under 0.05.
Sensitivity, specificity, and both positive and negative prediction values were determined with a 2×2 contingency chart where the predictive or independent variable was alive or dead and the outcome or dependent variables were lactate levels >1.5mmol/l, 2mmol/l and lactate at 2.5mmol/l, as well as pH<7.25 and <7.30.
ResultsWithin the period of time outlined in this study, there were 2657 births. 363 (13.6%) of these were admitted to the NICU. Out of the 363 admitted to the NICU, 226 (67.2%) were premature. Out of the premature ones, 154 met the inclusion criteria, from which 36 died and 118 survived.
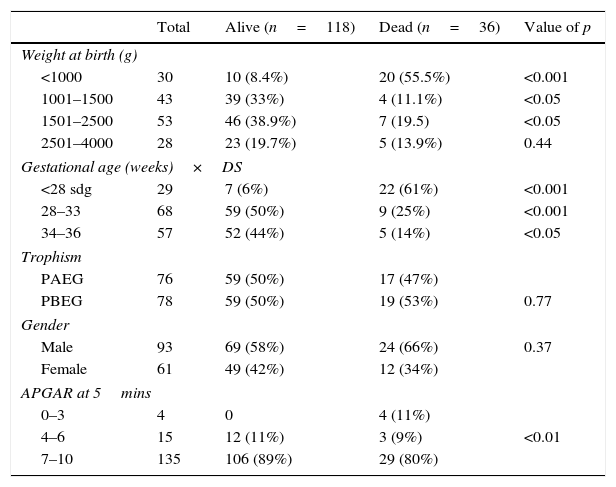
When comparing their weight at birth, we are able to find that there were more children under 1000g in the dead group (p<0.001) and there were more children between 1000 and 2500g in the alive group (p<0.05). Regarding gestational age, there were more children under 28 weeks of gestation in the dead group with (p<0.001) and more children with a gestational age of 28–33 weeks of gestation (p<0.001) and 34–36 weeks of gestation (<0.05) in the alive group. Concerning trophism and gender, both groups were similar (NS). When comparing APGAR scores, there were more dead children with a low APGAR (p<0.001) (see Table 1).
Newborn characteristics.
| Total | Alive (n=118) | Dead (n=36) | Value of p | |
|---|---|---|---|---|
| Weight at birth (g) | ||||
| <1000 | 30 | 10 (8.4%) | 20 (55.5%) | <0.001 |
| 1001–1500 | 43 | 39 (33%) | 4 (11.1%) | <0.05 |
| 1501–2500 | 53 | 46 (38.9%) | 7 (19.5) | <0.05 |
| 2501–4000 | 28 | 23 (19.7%) | 5 (13.9%) | 0.44 |
| Gestational age (weeks)×DS | ||||
| <28 sdg | 29 | 7 (6%) | 22 (61%) | <0.001 |
| 28–33 | 68 | 59 (50%) | 9 (25%) | <0.001 |
| 34–36 | 57 | 52 (44%) | 5 (14%) | <0.05 |
| Trophism | ||||
| PAEG | 76 | 59 (50%) | 17 (47%) | |
| PBEG | 78 | 59 (50%) | 19 (53%) | 0.77 |
| Gender | ||||
| Male | 93 | 69 (58%) | 24 (66%) | 0.37 |
| Female | 61 | 49 (42%) | 12 (34%) | |
| APGAR at 5mins | ||||
| 0–3 | 4 | 0 | 4 (11%) | |
| 4–6 | 15 | 12 (11%) | 3 (9%) | <0.01 |
| 7–10 | 135 | 106 (89%) | 29 (80%) | |
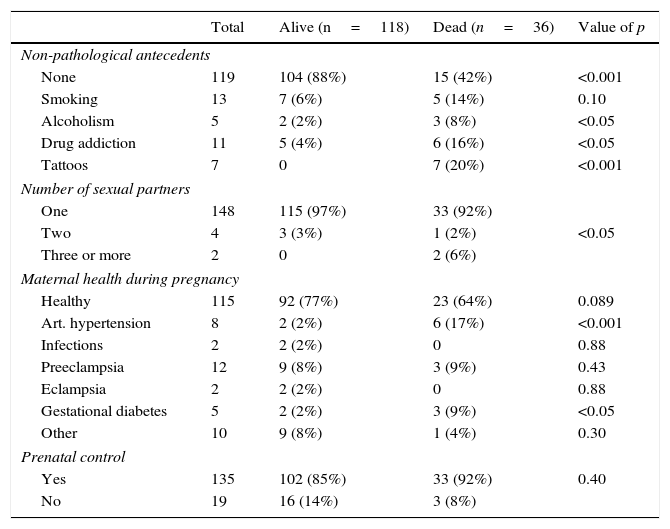
When comparing non-pathological and pathological maternal history, we are able to find that there were more mothers without any history in the alive group (p<0.001) and there was a greater link of alcoholism (<0.05), drug addiction (<0.05) and tattoos (<0.001) in mothers of those patients who died. Regarding the number of sexual partners, those with 3 or more sex partners were higher in the dead group (<0.05).
When comparing the mothers’ health during pregnancy, results show that in the dead group there were more mothers with high blood pressure (<0.001) and gestational diabetes (p<0.05) and the presence of infections, preeclampsia, eclampsia and other diseases was not significant (NS). Prenatal care was similar in both groups (NS) (see Table 2A).
Pathological and non-pathological maternal antecedents.
| Total | Alive (n=118) | Dead (n=36) | Value of p | |
|---|---|---|---|---|
| Non-pathological antecedents | ||||
| None | 119 | 104 (88%) | 15 (42%) | <0.001 |
| Smoking | 13 | 7 (6%) | 5 (14%) | 0.10 |
| Alcoholism | 5 | 2 (2%) | 3 (8%) | <0.05 |
| Drug addiction | 11 | 5 (4%) | 6 (16%) | <0.05 |
| Tattoos | 7 | 0 | 7 (20%) | <0.001 |
| Number of sexual partners | ||||
| One | 148 | 115 (97%) | 33 (92%) | |
| Two | 4 | 3 (3%) | 1 (2%) | <0.05 |
| Three or more | 2 | 0 | 2 (6%) | |
| Maternal health during pregnancy | ||||
| Healthy | 115 | 92 (77%) | 23 (64%) | 0.089 |
| Art. hypertension | 8 | 2 (2%) | 6 (17%) | <0.001 |
| Infections | 2 | 2 (2%) | 0 | 0.88 |
| Preeclampsia | 12 | 9 (8%) | 3 (9%) | 0.43 |
| Eclampsia | 2 | 2 (2%) | 0 | 0.88 |
| Gestational diabetes | 5 | 2 (2%) | 3 (9%) | <0.05 |
| Other | 10 | 9 (8%) | 1 (4%) | 0.30 |
| Prenatal control | ||||
| Yes | 135 | 102 (85%) | 33 (92%) | 0.40 |
| No | 19 | 16 (14%) | 3 (8%) | |
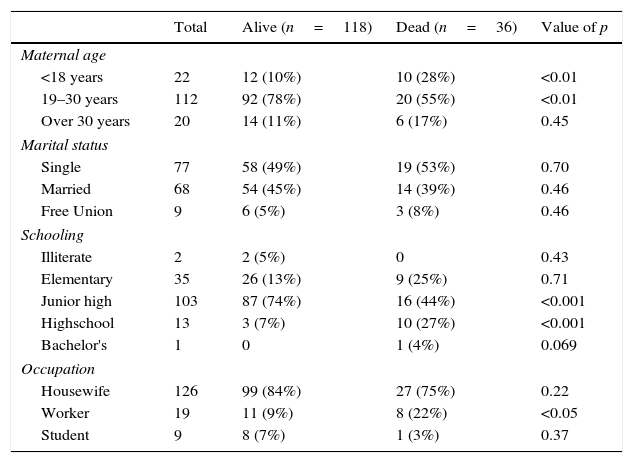
When comparing associated maternal age, we are able to observe more mothers <18 years old in the dead group (p<0.01) and more mothers between 19 and 30 years old (p<0.01) in the alive group. Marital status was similar in both groups (NS). Regarding schooling, there were more children in the dead group whose mothers has completed high school (p<0.001), whereas in the alive group elementary school was predominant (p<0.001). There were more working mothers in the dead group (p<0.05) (see Table 2B).
Pathological and non-pathological maternal antecedents.
| Total | Alive (n=118) | Dead (n=36) | Value of p | |
|---|---|---|---|---|
| Maternal age | ||||
| <18 years | 22 | 12 (10%) | 10 (28%) | <0.01 |
| 19–30 years | 112 | 92 (78%) | 20 (55%) | <0.01 |
| Over 30 years | 20 | 14 (11%) | 6 (17%) | 0.45 |
| Marital status | ||||
| Single | 77 | 58 (49%) | 19 (53%) | 0.70 |
| Married | 68 | 54 (45%) | 14 (39%) | 0.46 |
| Free Union | 9 | 6 (5%) | 3 (8%) | 0.46 |
| Schooling | ||||
| Illiterate | 2 | 2 (5%) | 0 | 0.43 |
| Elementary | 35 | 26 (13%) | 9 (25%) | 0.71 |
| Junior high | 103 | 87 (74%) | 16 (44%) | <0.001 |
| Highschool | 13 | 3 (7%) | 10 (27%) | <0.001 |
| Bachelor's | 1 | 0 | 1 (4%) | 0.069 |
| Occupation | ||||
| Housewife | 126 | 99 (84%) | 27 (75%) | 0.22 |
| Worker | 19 | 11 (9%) | 8 (22%) | <0.05 |
| Student | 9 | 8 (7%) | 1 (3%) | 0.37 |
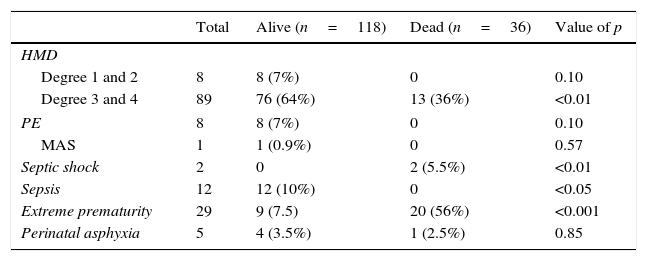
When comparing in-hospital morbidity and mortality causes, we are able to observe that there were more dead children with grade 3 and 4 hyaline membrane disease diagnosis (p<0.001), septic shock (p<0.01) and prematurity (p<0.001). On the other hand, sepsis was higher in the alive group (p<0.05). The presence of grade 1 and 2 HMD, pulmonary edema, meconium aspiration syndrome and perinatal asphyxia were not significant (NS) (see Table 3).
Causes of intrahospital morbidity.
| Total | Alive (n=118) | Dead (n=36) | Value of p | |
|---|---|---|---|---|
| HMD | ||||
| Degree 1 and 2 | 8 | 8 (7%) | 0 | 0.10 |
| Degree 3 and 4 | 89 | 76 (64%) | 13 (36%) | <0.01 |
| PE | 8 | 8 (7%) | 0 | 0.10 |
| MAS | 1 | 1 (0.9%) | 0 | 0.57 |
| Septic shock | 2 | 0 | 2 (5.5%) | <0.01 |
| Sepsis | 12 | 12 (10%) | 0 | <0.05 |
| Extreme prematurity | 29 | 9 (7.5) | 20 (56%) | <0.001 |
| Perinatal asphyxia | 5 | 4 (3.5%) | 1 (2.5%) | 0.85 |
At 72h, findings show the best sensitivity (95%) was when lactate was lower than 1.5mmol/l and the best specificity (89%) was with lactate levels higher than 2.5mmol/l; pH <7.25 had a sensibility of 76% and a specificity of 96% (see Table 4).
At 168h (7 days), findings show the best sensitivity (91%) was when lactate was lower than 1.5mmol/l and the best specificity (96%) was with lactate levels higher than 2.5mmol/l; pH <7.25 had a sensibility of 36% and a specificity of 91% (see Table 5).
DiscussionLactic acid is a terminal product of the anaerobic metabolism of glucose.1–3 Under normal conditions serum levels are at 2mEq/L or lower.4 Lactic acid is increased in situations of tissue hypoxia–ischemia,5–8 and its levels have a high correlation with mortality.9–11 The causes which provoke an increase of lactic acid can be hemodynamic or non-hemodynamic.12–25
The number of premature births continues to increase in developed countries, as well as the related low weight, with an increased mortality rate for patients that weigh less than 1500g.27,28 Within the findings of our study, when comparing weight at birth, we found that there were more children that weighed less than 1000g in the dead group.
A very important relation between a high number of deaths and a gestational age of 28–31 weeks has been reported in various studies, showing that they present a higher risk of complications and mortality during their time in the NICU.28,29 Within our findings, upon comparison of gestational ages, we found that there were more children at less than 28 weeks (p<0.001) in the dead group, and more children at 28–33 weeks and 34–36 weeks in the alive group (p<0.001).
It is worth considering that some studies have found an association between the masculine gender and a risk of death.2,14,16,23–25,27,28 The studies say that when comparing genders in relation to mortality, it is commonly observed that the death rate of males is higher than that of females at all ages. In our study, when comparing genders, both groups were similar (NS).
The APGAR score has been considered an important predictive variable of neonatal mortality. This variable is more directly related to the quality of attention at delivery, in spite of the influence of previous conditions of the newborn during the intrauterine period, which determines its vitality at birth. This score has been mentioned by various authors23–25,27,28 as an important variable which is closely related to the risk of death when scores of less than 7 are obtained at the first and fifth minutes. Within our findings, we observed that there were more dead in the low APGAR category (p<0.001).
The important increase of neonatal death rates due to the high prevalence of premature births and low weight at birth has been mentioned in various studies.27,28 These are closely related to other indicators in the mothers, such as the intake of alcohol, tobacco and illicit drugs. Within our pathological and non-pathological findings on the mothers, we found that the mothers of deceased patients presented a greater relation to alcoholism (<0.05), drug addiction (<0.05) and tattoos (<0.001).
Regarding prematurity, various studies have reported that it is a factor in risks associated with neonatal death, malnutrition, infections, iatrogenic prematurity, hypertension and the premature rupture of membranes, among other things.17,27–29 In our study, we observed that there were more mothers with arterial hypertension (<0.001) and gestational (p<0.05) diabetes in the dead group. The presence of infections, preeclampsia, eclampsia and other diseases were not significant (NS).
The frequency of prenatal attention is one of the most important things to prevent neonatal death, because when the frequency of prenatal attention is greater, the chance that fetal and maternal illnesses can be detected and early intervention applied is also greater.23 Regarding prenatal consultations, it has been found that the risk of mortality is 2.32 times greater in the group of mothers which had been to up to 3 consultations, and up to 3.56 times greater in the group of mothers which had not gone to any consultation.28 Some authors12,27,28 corroborate these results, finding that the mothers with less than 5 consultations have a 2.5 times higher risk than the others. In the findings of our group, upon comparison of prenatal control, both groups were similar (NS).
de Fátima Almeida et al.,28 in their study performed at the Federal University of Espíritu Santo in Brazil, studied neonatal deaths as well as the risk factors associated with them, finding a risk of death 3 times higher in mothers that were less than 15 years old (OR=2.97; IC 95%). This bears a close resemblance to our study, which found that there were more mother that were less than 18 years old in the dead group (p<0.01).
Maternal education is considered an indicator of social status. The mother's level of education can be considered a factor related to her cultural profile and conduct of health care, which has an important effect on the determination of mortality.23,27,28 This investigation has demonstrated, through an analysis of the correlation between maternal education and obstetrical indicators, that there is a statistically significant association between a low level of education and low weight at birth, an elevated number of premature births and a lesser number of prenatal consultations. Maternal education can be considered to be an obstetrical risk factor for pregnant women and newborns. The analysis also signified that the significant association between neonatal mortality and an inferior education with these women may be due to lower social conditions and limited access health services.28
Within the findings of our study, upon comparison of educational levels, we found that there were a greater number of children in the dead group of mothers with a high school education (p<0.001) and there were more children in the alive group of mothers with an elementary school education (p<0.001). Within the findings of our study, we found that, at 72h, the best sensitivity (95%) was when lactate was lower than 1.5mmol/l and the best specificity (89%) was with lactate levels higher than 2.5mmol/l, which is in accordance with medical literature.
ConclusionAccording to our findings, we can conclude the following: the study of risk factors in mothers is important in order to prevent premature births as well as its strong connection with low weight at birth and a lower gestational age, since results show that 67% of deaths were under 1500g and that 61% were under 28 weeks of gestation.
Lactate over 1.5mmol/l has a sensitivity (to be dead) of 95% at day 3 and 91% at day 7.
When pH is higher than 7.25 there is a specificity (to be alive) of 96% at day 3 and 91% at day 7.
Conflict of interestThe authors have no conflicts of interest to declare.
FundingNo financial support was provided.