Set the sensitivity of the histopathological diagnosis of chorioamnionitis (CAMH) for early diagnosis of neonatal sepsis and the relationship between histological chorioamnionitis and premature rupture of membranes and neonatal sepsis.
Materials and methodsProspective, observational study and diagnostic test performed in the Neonatology Service of the “Dr. José Eleuterio González” University Hospital. Epidemiological variables were collected from mothers and newborns. The relationship between histological chorioamnionitis with premature rupture of membranes and early neonatal sepsis was established.
ResultsWe recorded 3694 births. Of these, 122 patients were studied as potentially infected, of whom 37 patients were excluded (2 by transfer to another hospital and 35 by not finding a histopathological study of the placenta). The study included 85 newborns. Of these, 43 (50.5%) developed clinical and laboratory data of early neonatal sepsis, the rest (n=42, 49.5%) were healthy newborns. The sensitivity of histological chorioamnionitis with premature rupture of membranes (PRM) of more than 24h was 81% for neonatal sepsis and 51% without. The risk factors for neonatal sepsis were: Mother with infection (p<0.001), weight <1500g (<0.001), gestational age <28 weeks (<0.05), APGAR score <6 in 5min (p<0.05).
ConclusionsPlacental chorioamnionitis with premature rupture of membranes > 24h has an 81% sensitivity for neonatal sepsis. A newborn with histological chorioamnionitis has a 51% sensitivity for neonatal sepsis.
Chorioamnionitis (CAM) is the inflammation of the amniochorionic membrane and the most frequent cause of premature delivery. Chorioamnionitis can be clinically defined based on maternal symptoms which include fever, abdominal pain, abnormal vaginal flow and leukocytosis. It can also be histologically defined with evidence of inflammation and necrosis in the entire chorionic plate and amnion. Chorioamnionitis is linked to significant maternal and neonatal morbidity and mortality rates.1–11 It often occurs through an ascending and multi-bacterial infection. CAM can cause fetal inflammatory response syndrome, which brings along greater risks of periventricular leukomalacia (PVL), cerebral palsy and chronic pulmonary disease.12–15 The objective of this study was to correlate histological chorioamnionitis sensitivity with neonatal sepsis and premature rupture of membranes (PRM).
Materials and methodsAn observational, prospective study and a diagnostic test were conducted at the neonatal service of the “Dr. Jose E. Gonzalez” University Hospital of the Autonomous University of Nuevo León, from August 1st, 2012 to July 31st, 2013. This study was accepted by the institution's Ethics Committee with the folio no. NE12-002.
The study included those patients admitted to the Neonatal Intermediate and Intensive Care Units with a diagnosis of potentially infected, with a history of premature rupture of membranes with 18h of evolution or longer, maternal fever, warm and/or fetid uterine cavity, and a histopathological study of the placenta. On the other hand, we excluded potentially infected newborns born in a different hospital and, lastly, we eliminated patients who did not have a histopathological study of the placenta.
The sample was made by convenience and included the potentially infected patients. After looking at in-hospital clinical evolution the patients were divided into two groups for analytical comparison: the first group included patients with neonatal sepsis and the second group was a control group which included healthy patients.
Studied variablesMaternal historyMaternal age, divided into, <18 years old, 19–30 years old and >30 years old; health, subdivided into healthy mothers and history of threatened abortion, premature delivery, infections, preeclampsia, eclampsia, gestational diabetes, chorioamnionitis, antepartum hemorrhages and premature rupture of membranes, with a subdivision of time intervals of: 18–24h, 25–48h, 49–72h and >72h.
Variables of newbornsWeight at birth in grams, divided into <1000, 1000–1500, 1501–2500, 2501–4000 and >4000; gestational age in weeks according to the following intervals: <28 weeks, 28–33 weeks, 34–36 weeks, 37–42 and >42 weeks. Trophism according to a percentile in weight divided into; adequate for gestational age, small for gestational age and large for gestational age; male or female, and APGAR score at 5min divided as follows: 0–3 points, 4–6 points and 7–10 points.
Qualitative variables were used in the statistical analysis. Hypothesis tests were the following: chi square test (non-parametric), an alpha value of 0.05 was used and the null hypothesis was rejected when the critical value was less than 0.05. For those variables representing statistically significant numbers, the odds ratios were obtained, and when they were based on a confidence interval of 95%, risk factors supporting the alternative hypothesis and protective factors were obtained. Sensitivity and specificity were obtained, as well as positive and negative predictive values using a 2×2 contingency table where the predictive or “independent” variable was the premature rupture of membranes and the outcome or “dependent” variable was neonatal sepsis.
Sensitivity and specificity were obtained, as well as positive and negative predictive values using chorioamnionitis with sepsis as a predictive variable and the ruptured sac >24h as another dependent variable.
ResultsThere were 3694 births in the studied period. Of these, 122 patients were studied as potentially infected. 37 patients were excluded (2 because they were moved to a different hospital and 35 because they did not have the histopathological study of the placenta). In the end, the studied sample was 85 newborns. Out of these, 50.5% (n=43) developed clinical and laboratory data of early neonatal sepsis, the rest 49.5% (n=42) were reported as healthy.
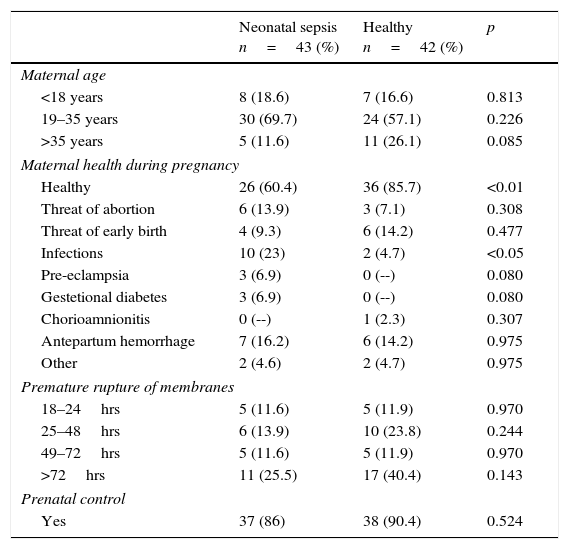
When comparing maternal history in both groups (neonatal sepsis vs. healthy) the findings were the following: maternal age of the study groups was the same, with the highest number of mothers in the 19–35 years old group. Regarding maternal health during pregnancy, mothers of the neonatal sepsis group had a higher morbidity p<0.001, among which infections standout (23% vs. 4.7%) p<0.05. The rest of the diseases registered without a significant number. Regarding ruptured sac time, in both groups, the highest percentage was found in rupture over 72h, in the statistical analysis, both groups were the same. In prenatal care, both groups were similar, with 86% of the patients in the neonatal sepsis group and 90.4% in the healthy group (Table 1).
Maternal antecedents.
| Neonatal sepsis n=43 (%) | Healthy n=42 (%) | p | |
|---|---|---|---|
| Maternal age | |||
| <18 years | 8 (18.6) | 7 (16.6) | 0.813 |
| 19–35 years | 30 (69.7) | 24 (57.1) | 0.226 |
| >35 years | 5 (11.6) | 11 (26.1) | 0.085 |
| Maternal health during pregnancy | |||
| Healthy | 26 (60.4) | 36 (85.7) | <0.01 |
| Threat of abortion | 6 (13.9) | 3 (7.1) | 0.308 |
| Threat of early birth | 4 (9.3) | 6 (14.2) | 0.477 |
| Infections | 10 (23) | 2 (4.7) | <0.05 |
| Pre-eclampsia | 3 (6.9) | 0 (--) | 0.080 |
| Gestetional diabetes | 3 (6.9) | 0 (--) | 0.080 |
| Chorioamnionitis | 0 (--) | 1 (2.3) | 0.307 |
| Antepartum hemorrhage | 7 (16.2) | 6 (14.2) | 0.975 |
| Other | 2 (4.6) | 2 (4.7) | 0.975 |
| Premature rupture of membranes | |||
| 18–24hrs | 5 (11.6) | 5 (11.9) | 0.970 |
| 25–48hrs | 6 (13.9) | 10 (23.8) | 0.244 |
| 49–72hrs | 5 (11.6) | 5 (11.9) | 0.970 |
| >72hrs | 11 (25.5) | 17 (40.4) | 0.143 |
| Prenatal control | |||
| Yes | 37 (86) | 38 (90.4) | 0.524 |
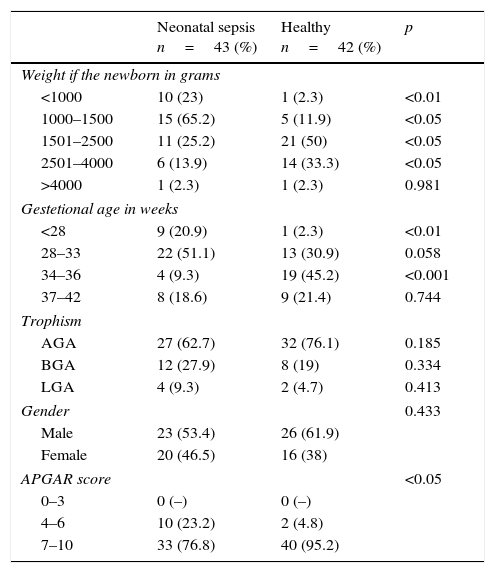
When comparing weight at birth, most patients with sepsis weighed under 2500g (<0.05). Regarding age groups, the <28 weeks group had 20.9% of newborns with sepsis and 2.3% of the healthy ones (p<0.01). We identified the largest group in the 28–33 weeks interval, with 51.1% of the patients with sepsis and 30.9% of the healthy ones, a non-significant number compared with the group of 34–36 weeks with 9.3% septic patients and 45.2% healthy ones (p<0.001); gestational ages of 37–42 and more weeks were without significant numbers. Regarding trophism, the largest number corresponded to an adequate weight for gestational age in both groups (62.7% and 76.1%, septic and healthy respectively), a non-significant number. Male patients were 53.4% of the neonates within the sepsis group and 61.9% of the healthy group (NS). Regarding the APGAR score, there were more <6 scores in the sepsis group (p<0.05) (Table 2).
Newborn characteristics.
| Neonatal sepsis n=43 (%) | Healthy n=42 (%) | p | |
|---|---|---|---|
| Weight if the newborn in grams | |||
| <1000 | 10 (23) | 1 (2.3) | <0.01 |
| 1000–1500 | 15 (65.2) | 5 (11.9) | <0.05 |
| 1501–2500 | 11 (25.2) | 21 (50) | <0.05 |
| 2501–4000 | 6 (13.9) | 14 (33.3) | <0.05 |
| >4000 | 1 (2.3) | 1 (2.3) | 0.981 |
| Gestetional age in weeks | |||
| <28 | 9 (20.9) | 1 (2.3) | <0.01 |
| 28–33 | 22 (51.1) | 13 (30.9) | 0.058 |
| 34–36 | 4 (9.3) | 19 (45.2) | <0.001 |
| 37–42 | 8 (18.6) | 9 (21.4) | 0.744 |
| Trophism | |||
| AGA | 27 (62.7) | 32 (76.1) | 0.185 |
| BGA | 12 (27.9) | 8 (19) | 0.334 |
| LGA | 4 (9.3) | 2 (4.7) | 0.413 |
| Gender | 0.433 | ||
| Male | 23 (53.4) | 26 (61.9) | |
| Female | 20 (46.5) | 16 (38) | |
| APGAR score | <0.05 | ||
| 0–3 | 0 (–) | 0 (–) | |
| 4–6 | 10 (23.2) | 2 (4.8) | |
| 7–10 | 33 (76.8) | 40 (95.2) | |
AGA, adequate to gestational age; BGA, below gestational age; LGA, larger than gestational age.
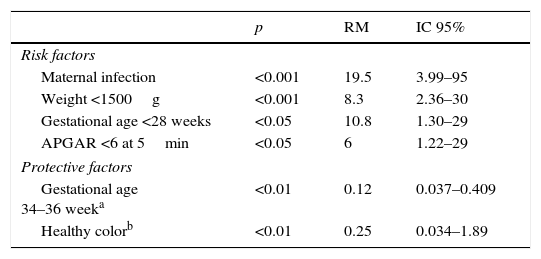
The odds-ratio (OR) analysis, with a confidence level of 95%, considering risk factors for neonatal sepsis development present the following: presence of maternal infection OR=19.5, weight at birth <1500g OR=8.3, for the gestational age <28 week group OR=10.8 and APGAR <6 at 5min OR=6. Protective factors to avoid getting sick are: gestational age of 34–36 weeks OR=0.12, which establishes a protective factor of 8 and the corresponding variable of a healthy mother OR=0.25 with a protective factor of 4 (Table 3).
Odds ratio (OR) for neonatal sepsis of statistically significant value.
When correlating histological chorioamnionitis with sepsis, we were able to find a sensitivity of 51% and when relating chorioamnionitis and ruptured sac >24h we were able to find a sensitivity of 81% with a specificity of 31%, a positive predictive value of 45% and a negative predictive value of 71% (Table 4).
Chorioamnionitis with sepsis and PRM relation >24h.
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|
| Chorioamnionitis and neonatal sepsis | 51 | 35 | 44 | 41 |
| Chorioamnionitis and ruptured sac >24h | 81 | 31 | 45 | 71 |
PRM, premature rupture of membranes; PPV, positive predictive value; NPV, negative predictive value.
Chorioamnionitis (or intra-amniotic infection) refers to the inflammation of the amniochorionic membrane (in chorion and amnion). It is estimated to be present in approximately 2–4% of full-term pregnancies,1,2 and in approximately 40–70% of women with premature deliveries.3,4 CAM can be defined clinically based on maternal symptoms, including fever, abdominal pain, abnormal vaginal flow and leukocytosis,5–7 as well as histologically, with evidence of inflammation and necrosis in the entire chorionic plate and amnion.1,8
Chorioamnionitis is linked to significant morbidity and mortality maternal and neonatal rates.9 Neonatal morbidities include a greater risk of neonatal sepsis,10 pneumonia,11 and neurological damage.12,13 On the other hand, chorioamnionitis can lead to fetal inflammatory response syndrome, which brings along greater risks of periventricular leukomalacia, cerebral palsy14,15,34 and chronic pulmonary disease.16,17
The more premature the child is, the greater the probability of detecting histological chorioamnionitis. A study found that out of those cases which gave birth between 21 and 24 weeks, 67% showed evidence of infection and histological inflammation, compared to 22% of those who gave birth between weeks 33 and 36.18 In our study, out of a sample of 85 patients, 58% had histologic chorioamnionitis (HCAM) and of the septic 51% CAMH.
Chorioamnionitis occurs more frequently as a result of ascending vaginal and cervix bacteria, it is more frequently observed as a secondary complication to a prolonged rupture of membranes.19 Less common ways of infection include hematogenous dissemination or transmission posterior to an invasive procedure (in other words, amniocentesis, chorionic villus sampling, or other fetal procedure). Bacterial, viral and in rare occasions fungal agents have been related to the pathogenesis of chorioamnionitis and premature delivery. Some of the commonly identified pathogens include Ureaplasma urealyticum, Chlamydia trachomatis, Neisseria gonorrea, Mycoplasma hominis, group B strephtococcus and Trichomonas vaginalis. Additional bacteria include gram-negative anaerobes, including Gardnerella vaginalis and Bacteroides spp.20–22
Fungal organisms, including several Candida species (Candida albicans, Candida tropicalis and Candida glabrata), have also been associated with chorioamnionitis. These infections have been reported in pregnant women with in vitro fertilization, in those with retained intrauterine devices after amniocentesis and in those with a prolonged rupture of membranes.23–27 Organisms like Mycoplasma are typically low virulence organisms, which explains why women with histological chorioamnionitis frequently do not present clinical symptoms.27
Aside from colonization with bacteria and virus, there are several risk factors for the development of chorioamnionitis. As previously discussed, prematurity and premature rupture of membranes are commonly linked to chorioamnionitis. In our casuistry, 86.5% of children with neonatal sepsis had a ruptured sac of over 24h. In full-term babies, risk factors included: long duration of delivery and of the rupture of the membrane, and nulliparity.28 Additionally, women with early rupture of membranes at term who receive multiple digital vaginal tests, having a prolonged labor or with meconium-stained liquid have a greater risk of developing chorioamnionitis.29 In our study, we were able to find that 81% of children who had ruptured sac of over 24h and placenta chorioamnionitis had neonatal sepsis.
CAM can be diagnosed with the use of histological or clinical criteria. Clinical diagnosis is based in local or systemic infection signs and symptoms. Common definition includes maternal fever (fever>37, 5–38°C) and one of the following: abdominal pain, uterine pain, fetid vaginal flow, maternal tachycardia (>100heartbeats/min), fetal tachycardia (persistent elevation of fetal cardiac frequency >160heartbeats/min) and an elevated count of maternal white blood cells (>15,000cells/mm3). The cut-off point for maternal fever varies through several studies. However, the most recent literature cites a value of >38°C, thus excluding the many women with a low degree fever during delivery, which is not related to an infectious process.6,30,31
Chorioamnionitis can occur histologically, and is organized on the base of specific criteria, with the increase in neutrophils infiltration and the development of necrosis, thickening of the amnion basement membrane and chorionic micro-abscesses which are seen with the increase of the severity of the disease. Moreover, fetal inflammatory response can progress from a chorionic vasculitis/umbilical (infiltration of neutrophils into chorionic or umbilical vessels) to necrotizing funisitis (inflammation of the umbilical cords conjunctive tissue).8,13 Clinical diagnosis of chorioamnionitis, however, is not always confirmed by histological or microbiological studies. In a study of 139 pregnancies with clinical findings of chorioamnionitis, the histological exam of the placenta did not support the clinical diagnosis in approximately a third of the cases.32 In our study, 49% of children with sepsis had a histologically normal placenta. Chorioamnionitis, defined by positive cultures of amniotic liquid, was found in 36% of women with premature rupture of membranes.33
Conclusions51% of newborns with histologic chorioamnionitis had sepsis. Placenta chorioamnionitis with premature rupture of membranes of more than 24h has an 81% sensitivity for a neonatal sepsis diagnosis.
Limitations of the study- •
A larger population is required for the findings in our samples to be real for the general population.
- •
In this sample, it was not possible to perform a ROC curve, which could have been helpful.
No financial support was provided.
Conflict of interestThe authors have no conflicts of interest to declare.