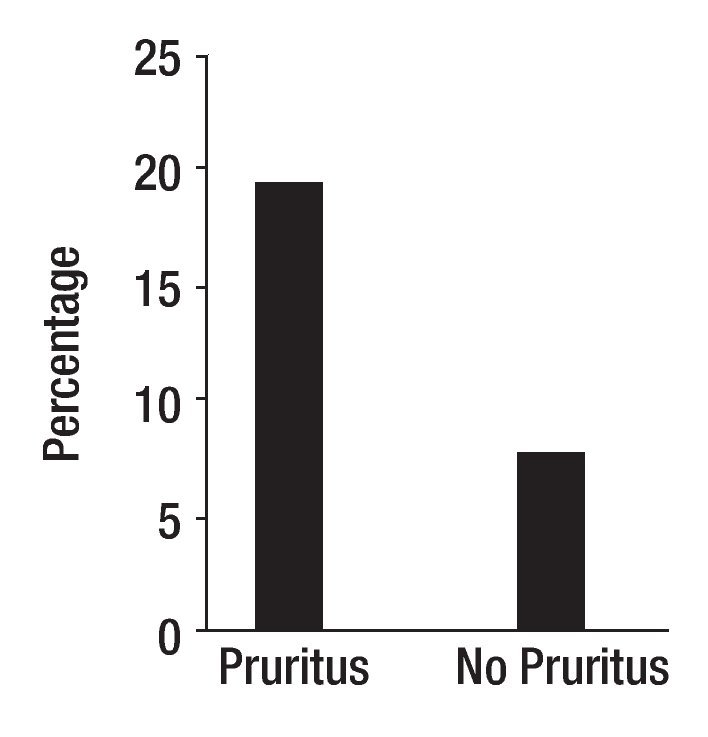
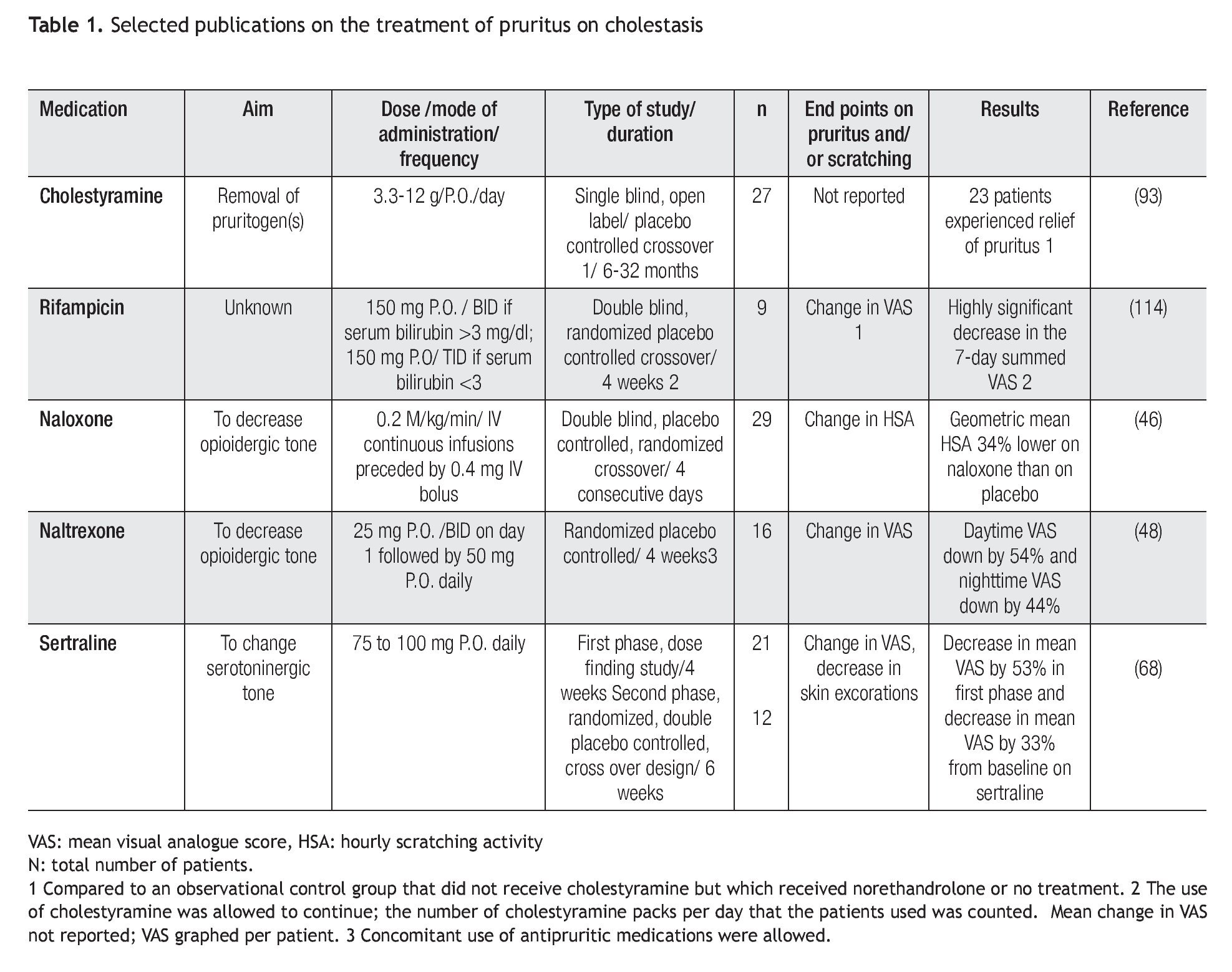
Cholestasis is defined as impaired secretion of bile.1 It is a consequence of all types of liver diseases. Pruritus is one of the symptoms experienced by patients with liver disease; it is hypothesized that the pruritus of cholestasis is mediated by increased opioidergic tone by a central mechanism.2 Pruritus is more common in patients with liver diseases characterized by bile duct injury (intra or extrahepatic) and ductopenia such as primary biliary cirrhosis and primary sclerosing cholangitis, than in patients with diseases characterized by hepatocellular injury such as chronic viral hepatitis likely due to a greater degree of cholestasis in the former. Patients with severe cholestasis, as measured currently (e.g. high fasting and two hour postprandial serum bile acids) do not consistently report pruritus and patients with perceived minimal cholestasis may report severe pruritus, however. This may suggest that the degree of cholestasis alone, does not determine the presence or absence of pruritus. The question arises then, why do some patients with cholestasis report pruritus and some do not? The answer may be related to the individual's ability to perceive pruritus. In this context, there is a rationale to explore genetic changes that may have an impact on how substances are transported from the hepatocyte to the bile canaliculus and on the perception of pruritus. The first idea was explored by studying genetic polymorphisms in the gene that codes for MRP2, a protein localized on the canalicular domain of the hepatocyte in patients with primary biliary cirrhosis.3 MRP2 (ABCC2) is a member of the family of ATP binding cassette (ABC) transporters expressed in various organs including the liver and the blood brain barrier.4,5 In the hepatocyte, MRP2 mediates the transport of conjugated compounds, including dianionic conjugated bile salts into bile;6 in addition, opioid ligands have been reported to be substrates of MRP2.7 Exon 25 of the MRP2 is one of the regions more frequently associated with MRP2 mutations.8 Samples from one hundred and one patients with primary biliary cirrhosis from Italy, from 76 patients from the United States, and 101 blood donors, matched for sex, age and geographical area with the Italian patients as control, were studied. The sequencing the exon 25 of MRP2 gene revealed a novel mutation (t3563a) in codon 1188. This mutation resulted from the substitution of valine by glutamate (V1188E). The mutation (wt/mutation) was present in heterozygosity in 19 of 101 (18.8%) of Italian patients with primary biliary cirrhosis, in 12 of 101 (11.9%) of Italian controls (p = 0.241), and in five of the 76 (6.6%) of patients from the United States. There was a significant association between genotypes and the origin of the samples (i.e. Italy and United States) (p = 0.02, OR = 3.29, 95%CI: 1.17-9.26) (Figure 1). All other patients were homozygous wt/wt carriers. V1188E was identified in 17 of 87 (19.5%) patients who reported pruritus and in 7 of the 90 patients, who did not (7.8%), (p = 0.02, RR = 2.51, 95% CI: 1.13-5.69) (Figure 1). The mutation was found in more samples from patients with pruritus from Italy, 13 of 39 (33.3%), than in samples from patients without pruritus, six of 62 (9.7%) (p = 0.003, RR = 3.44, 95% CI: 1.47-8.20). In the group of samples from patients from the United States, the mutation was found in four of the 48 samples from patients with pruritus (8.3%) and in one of the 28 patients (3.6%) without pruritus (p = 0.42, RR = 2.33, 95%CI: 0.37-15.4).3 It was reported that the presence of some single nucleotide polymorphisms in the MRP2 gene may be associated with a decrease in the in vivo function of the protein.9 Accordingly, the novel V1188E mutation found in this study may alter the ability of the transporter to transport substrates into the biliary canaliculus and it may lead to increased accumulation of pruritogens in plasma or to an increased availability of pruritogens in the central nervous system.3 The second idea, the ability to perceive pruritus, was explored by studying the polymorphism, Al18G found Exon 1.10 This polymorphism predicts an Asn-to-Asp change in amino acid residue 40 in the extracellular domain of the receptor,11 at a putative N-glycosylation site. Asn40Asp in the OPRM1 gene has been reported to be associated with behavioral changes in human beings,12,13 and changes in the binding profile of at least one endogenous opioid, beta-endorphin, to the receptor.14 The samples studied were from the same group of patients with primary biliary cirrhosis with and without pruritus described above. For this study, samples from 101 patients from Italy, 40 (63.5%) of which were from patients with pruritus and samples from 74 patients from the United States, 47 (63.5%) of which came from patients who had pruritus were available.10 A118G was found in 51 of 175 samples (29%) in heterozygosity and in homozygosity, in one sample from a patient without pruritus from Italy (0.5%). All other samples had sequences consistent with the common wild type. In the combined group, A118G was found in 24 (27.6%) samples from the group of patients with pruritus, and in 27 (30.6%) from the group of patients without pruritus (p = 0.7). In the samples from Italy, A118G was detected in eleven of the 40 (27.5%) from patients with pruritus and in sixteen of the 61 (26.2%) from patients without pruritus (p = 0.9). In the samples from the USA, A118G was found in thirteen of the 47 (27.6%) samples from the patients with pruritus and in eleven of the 27 (40.7%) from the patients without pruritus (p = 0.3). A118G was more common in the samples from the patients without pruritus from the United States, suggesting that its presence may be protective from pruritus in cholestasis. In this context, published data support the idea that A118G changes the effect of receptor activation, as suggested by the report of a patient heterozygote for A118G who did not respond as expected to the effects of morphine.15 Although the presence of A118G was not significantly different from that in patients with pruritus, the small sample size clearly limited this study. Indeed, as the A118G mutation has also been reported in other mammals,16 behavioral studies in primates could be conducted to explore the idea of A118G conferring protection against opioid receptor mediated scratching, which would have relevance in the genetic make up of patients with cholestasis. The novel findings generated from these two studies with a relatively small sample size provide support to the idea that genetic predisposition may play a role in the perception of pruritus in cholestasis, and calls for the conduct of international studies with large number of patients.
Figure 1. Samples from patients from Italy and from the United States with primary biliary cirrhosis with pruritus and without pruritus were studied for the presence of mutations in the gene that codes for MRP2. The sequencing of exon 25 of MRP2 gene revealed a novel mutation (t3563a) in codon 1188, which resulted from the substitution of valine by glutamate (V1188E). V1188E was identified in heterozygosity in 19.5% of patients who had reported pruritus, and in 7.8% who did not have pruritus (p = 0.02, RR = 2.51, 95% CI: 1.13-5.69) (3).
The magnitude of the problem of the pruritus of cholestasis
The pruritus of cholestasis is usually generalized and intermittent. It can be severe, leading to the sleep deprivation and even suicidal ideations. Intractable pruritus is an indication for liver transplantation.17 There are inconsistent reports on the predictability of the presence of pruritus based on serum liver profile or liver histology.18-20 Patients can experience pruritus regardless of the degree of their liver disease, as measured by current tests.
PBCers is an organization that supports education and research in primary biliary cirrhosis (http://pbcers. org/). An internet survey via the website supported by the PBCers was conducted to understand how patients perceived pruritus. A total of 239 subjects with a diagnosis of primary biliary cirrhosis responded to the survey; of these, 232 were women, and 165 (69%) reported itch. Twenty-nine of 164 (17.6%) subjects stated that the itch was "relentless" or so severe that it led to wanting to "tear (the) skin off", and six responses (3.6%) stated that the subjects scratched until they bled. A total of 120 of 162 respondents (74%) reported that their itch affected their sleep, 107 of 164 respondents (65.2%) stated that their itch was worse at night, and 11% reported that nothing relieved the itch. The results of a subsequent survey currently posted on the PBCers website, reports that 282 respondents (38.6%) experienced itch and that 12% reported than nothing made a difference on the itch, similar to what was found in the prior survey.21
Pathogenesis of the pruritus of cholestasis
Pruritus is defined as an unpleasant sensation that triggers the need to scratch.22 Scratching is the behavioral manifestation of pruritus; scratching activity can be measured, and thus, it can be incorporated as an objective end-point into clinical trials of interventions to treat this symptom.23-26
It is inferred that the accumulation of pruritogenic substances in plasma and other tissues mediate the pruritus of cholestasis. The specific pruritogen(s) has not been identified; however, to date the only hypothesis in support of which evidence has been presented from clinical trials, is the hypothesis stating that the pruritus of cholestasis is mediated, at least in part, by increased opioidergic tone; a central mechanism has been proposed.2 Three lines of evidence support the idea that in cholestasis there is increased opioidergic tone in the central nervous system: (a) the opiate withdrawal like reaction experienced by patients with cholestasis upon the administration of opiate antagonists,27-29 (b) the state of antinociception, that is stereospecifically reversed by an opiate antagonist (i.e. naloxone) in an animal model of cholestasis30 and, (c) the altered expression of opioid receptors in the brain of rats with cholestasis.31,32 The pharmacological increase in opioidergic tone, as in the case of intrathecal administration of morphine and other drugs with affinity for the opioid receptors, is associated with pruritus, which can be prevented or effectively treated by opiate antagonists. Clinical observations33-38 and animal studies39,40 indicate that the pruritus and scratching behavior associated with the administration of morphine and opiate drugs is centrally mediated. The clinical suggestion that cholestasis is associated with increased central opioidergic tone (i.e. the opiate-withdrawal like syndrome precipitated by opiate antagonists in patients with cholestasis) supports idea that the pruritus is also centrally mediated. In addition, there is an emerging consensus suggesting that all conditions associated with chronic pruritus may be associated with central sensitization for itch. Patients with atopic dermatitis, who suffer from chronic pruritus and scratching, can experience noxious stimuli like heat, pressure, electrical and chemical stimulations as pruritus, in contrast to the painful perception reported by the control subjects. Also, in a model to mimic chronic itch by infusion of histamine into a defined skin area, a chemical stimulus was perceived as pain, and as itch; this finding suggests that chronic itch may allow non-pruritogenic stimuli to be perceived as pruritus. The timing of the appearance of itch in these studies is consistent with neurotransmission via C-nociceptors.41 These data have been interpreted to suggest that constant pruritogenic stimuli may result in central sensitization to itch, which does not permit nociceptive stimuli (i.e. stimuli that are normally associated with pain) to inhibit pruritus but to facilitate it. Furthermore, recordings obtained from a patient with chronic pruritus and prurigo nodularis secondary to chronic scratching revealed spontaneously active itch fibers;42 there may be constant stimuli of C-pruriceptors (i.e. fibers that respond to pruritic stimuli) in cholestasis from the accumulation of toxic substances, which may lead to central sensitization for pruritus. In further support of a central origin of the pruritus of cholestasis, are data from brain scans by single photon emission computed tomography and functional magnetic resonance imaging methodology in patients with pruritus of cholestasis during periods of itch and no itch. In this study, published in abstract form, itch was reported to be associated with sensory cortex activation and increasing itch severity was reported to correlate with activity in the prefrontal cortex, orbital frontal cortex, putamen, globus pallidus, insular cortex, and orbital anterior and posterior cingulated cortices, but not with activation of the sensory cortex. Based on the pattern of activation, the authors concluded that the limbic system is the primary central nervous system pathway involved in the perception of itch, and stated that the findings support a central origin for this type of pruritus.43 In summary, support for the hypothesis stating that the pruritus of cholestasis was centrally mediated, continues to emerge.44
In the context of opioid neurotransmission, if cholestasis is associated with increased opioidergic tone, and the pruritus of cholestasis is mediated, at least in part, by this type of neurotransmission, opiate antagonists should decrease the pruritus and its behavioral manifestation, scratching activity. This hypothesis was tested in clinical trials of opiate antagonists in patients with cholestasis and pruritus, in which decrease in scratching behavior, reported as hourly scratching activity, was incorporated as an end point. In short term studies, the administration of opiate antagonists was associated with some decrease in hourly scratching activity in an average of 89% of the patients (range, 82.8 to 100%).28,29,45,46 Opiate antagonists were reported to be associated with some decrease in the perception of pruritus, as measured by a visual analogue scale for pruritus in a range from 65.5% to 100% of patients studied.27,29,45-50 In a placebo controlled study, the opiate antagonist naltrexone was associated with 50% reduction in the visual analogue scale for pruritus in thirteen of 34 patients (38%) although, changes lower than 50% were not discernable from the publication.51 Five clinical trials of opiate antagonists to treat the pruritus of cholestasis were submitted to a meta analysis; three studies tested the effect of opiate antagonists administered orally (i.e. naltrexone and nalmefene) and two tested the effect of intravenous naloxone with a reported total of 84 participants. The report of this analysis documented that opiate antagonists were significantly more likely to decrease pruritus than the control intervention, with a standard mean difference (SMD) of - 0.68, 95% - 1.19 to - 0.17, and to decrease scratching activity with a SMD of - 0.64, 95% - 1.28 to 0.01.52 These results support the role of a specific group of substances with affinity for the opioid receptors, endogenous opioids, in the mediation of the pruritus of cholestasis.
An increased availability of endogenous opioids at the opioid receptor would result in increased opioidergic tone. There is evidence to suggest that the liver is a source of endogenous opioids in liver disease, as follows: (a) there is increased expression of Met-enkephalin immunoreactivity in the liver of patients with primary biliary cirrhosis, a liver disease characterized by cholestasis and pruritus,53 and also, in the liver of patients with chronic liver disease from viral and autoimmune hepatitis.54 In addition, in a rat model of cholestasis, the mRNA of preproenkephalin, the gene that codes for Met-enkephalin and Met-enkephalin containing peptides, is detected, in contrast to its absence from control livers; furthermore, Met-enkephalin immunoreactivity is strongly expressed, suggesting that the protein is being made, in contrast to its absence in the control livers.55 In addition, in some animal models of cholestasis56 and in some patients with liver disease, including primary biliary cirrhosis, the concentration of Met-enkephalin, one of the endogenous opioid peptides, is higher than that of control subjects.27,57 It is not known whether opioids derived from the liver in cholestasis go to the brain to mediate what has been interpreted as centrally increased opioidergic neurotransmission; however, transport proteins found in the basolateral domain of the hepatocyte are also found in the choroid plexus and in the blood brain barrier and can transport opiates in vitro58 and they may potentially transport periphery-derived opioids into the central nervous system. Furthermore, increased availability of opioid peptides in the periphery may facilitate their entrance into the central nervous system.59 Thus, an increased in the concentration of endogenous opioids in the periphery may lead to increased opioidergic tone, centrally. The increased concentration of particular endogenous opioids (i.e. Met-enkephalin) should not be interpreted as evidence to support that this particular peptide specifically mediates the pruritus of cholestasis, as there may be other opioids or combination of opioid peptides that could exert that effect. What identifies a role of endogenous opioids in the pathophysiology of cholestasis is the behavior suggesting increased opioidergic tone: (a) in patients, the opiate withdrawal like syndrome when given opiate antagonists,27 and (b) in animal models of cholestasis, the state of naloxone reversible antinociception.30
Other neurotransmitter systems have been explored in the context of cholestasis and pruritus.60 Serotonin participates in the mediation of nociceptive stimuli, which provided a rationale for the testing of ondansetron, a serotonin type 3 antagonist, in the treatment of pruritus in patients with liver disease, including some with cancer with metastasis to the liver;61-65 however, its effect on the behavioral manifestation of pruritus, scratching, was not confirmed in studies that included behavioral methodology.63,66 Sertraline, a serotonin reuptake inhibitor, was also reported to be associated with relief of pruritus in patients with cholestasis.67,68 The use of selective serotonin reuptake inhibitors have also been reported to decrease pruritus in polycythaemia vera69 and in patients with malignancy whose pruritus was multifactorial.70,71 These limited experiences have not confirmed a role of the serotonin system in the pathogenesis of the pruritus of cholestasis.
Substance P is an excitatory neurotransmitter that acts through the NK-1 receptor synthesized by primary afferent nociceptors and released into the spinal cord after noxious stimuli. The central administration of substance P was reported to be associated with scratching behavior in laboratory animals.72,73 In addition, the sustained administration of morphine, which results in increased opioidergic tone, to laboratory animals, activates mechanisms that promote pain (e.g. nociception), mediated, in part, by the NK-1 receptor,74 instead of analgesia; furthermore, prolonged administration of opiates is associated with increased expression of substance P in the dorsal root ganglia, which are involved in the transmission of nociceptive stimuli.75 The increased opioidergic tone of cholestasis may contribute to a state of enhanced nociception that may be perceived as pruritus mediated, in part, by substance P, analogous to the activation of mechanisms that promote pain mediated by substance P associated with chronic opiate administration.74 In this regard, the mean serum concentration of substance P was significantly higher in patients with chronic liver disease and pruritus than in patients with chronic liver disease without pruritus, and in control subjects.76 These data suggest that substance P may mediate some manifestations of liver disease, including pruritus; accordingly, there is a rationale to study the effect of substance P antagonists on this symptom of liver disease, in studies that include behavioral methodology.
It was suggested in a recent publication that lipophosphatidic acid and the activity of the enzyme that generates it, autotaxin, play a critical role in the pruritus of cholestasis because the serum concentration of the former and the activity of the latter were reported to be higher in the serum of patients with cholestasis and pruritus, than in the serum of those with cholestasis without pruritus.77 Studies documenting the relevance between the serum concentration and/or activities of these compounds in the pathogenesis of the pruritus of cholestasis are awaited.78
The accumulation of substances, including bile acids79,80 and histamine,81 in tissues of patients with cholestasis, has been used as a rationale to propose their involvement in the pathogenesis of the pruritus associated with liver disease; however, in the 21st century, rigorously obtained scientific data must be provided in support of hypotheses that may lead to clinical trials of medications in patients who suffer from pruritus. In this regard, the use of animal models of scratching82 to test an anti-scratching effect of a drug, the inclusion of behavioral methodology in clinical trials of medications for the treatment of pruritus,25,83 the development of relevant quality of life measures that detect the impact of a therapeutic intervention in patients, and the incorporation of responses from patients reflecting improvement or lack of it in association with a treatment (i.e. yes or no) must be taken into consideration in pruritus research.
Bile acids have been advanced as the pruritogens in cholestasis for many years. They accumulate in the serum and tissues of patients with cholestasis;79,80 however, their role in the pruritus of cholestasis has not been demonstrated.2 Three lines of evidence do not support a role of bile acids in this type of pruritus: (a) in liver failure, when bile acids are maximally elevated, pruritus tends to cease,84 (b) an important number of patients with cholestasis and marked elevations of serum bile acids do not experience pruritus throughout the course of their disease, and (c) pruritus is intermittent, independently from changes in serum bile acid concentrations. A certain bile acid profile in the cholestatic liver milieu, or in the serum, and not total serum concentration of bile acids, may be relevant if these substances are involved in the pruritus of cholestasis
In the context of bile acids, results of a double blind, placebo controlled, dose response phase II study on the use of obeticholic acid for the treatment of patients with primary biliary cirrhosis who had not responded completely to treatment with ursodeoxycholic acid has been published in abstract form.85 Obeticholic acid is a synthetic derivative of chenodeoxycholic acid, that is an agonist at the farsenoid nuclear receptor (FXR) and which has choleretic properties.86,87 FXR is a bile acid sensor associated with a decrease in bile acid production.88 The report documented that the administration of obeticholic acid was associated with a decrease in the activity of serum alkaline phosphatase. Pruritus, however, was a significant side effect in association with the two highest doses (25 and 50 mgs) of obeticholic acid tested, than in those who received placebo. The intensity of pruritus required discontinuation of the drug in some patients. Fifty percent of patients who received the placebo compound reported to experience pruritus, which was similar to the 48% that reported pruritus at the lowest dose (10 mg) of obeticholic acid. In the latter group, the pruritus was severe in some patients; severe pruritus was not reported in patients who received placebo. Many questions arise from the results of this study: did normal volunteers experienced pruritus in association with obeticholic acid?, what is the basis of obeticholic acid-associated pruritus vis-à-vis the pruritus of cholestasis? . If the pruritus of cholestasis was worsened by this new drug, the mechanism by which this effect is mediated merits investigation, as this finding may shed light on some aspect of the pathogenesis of this type of pruritus.
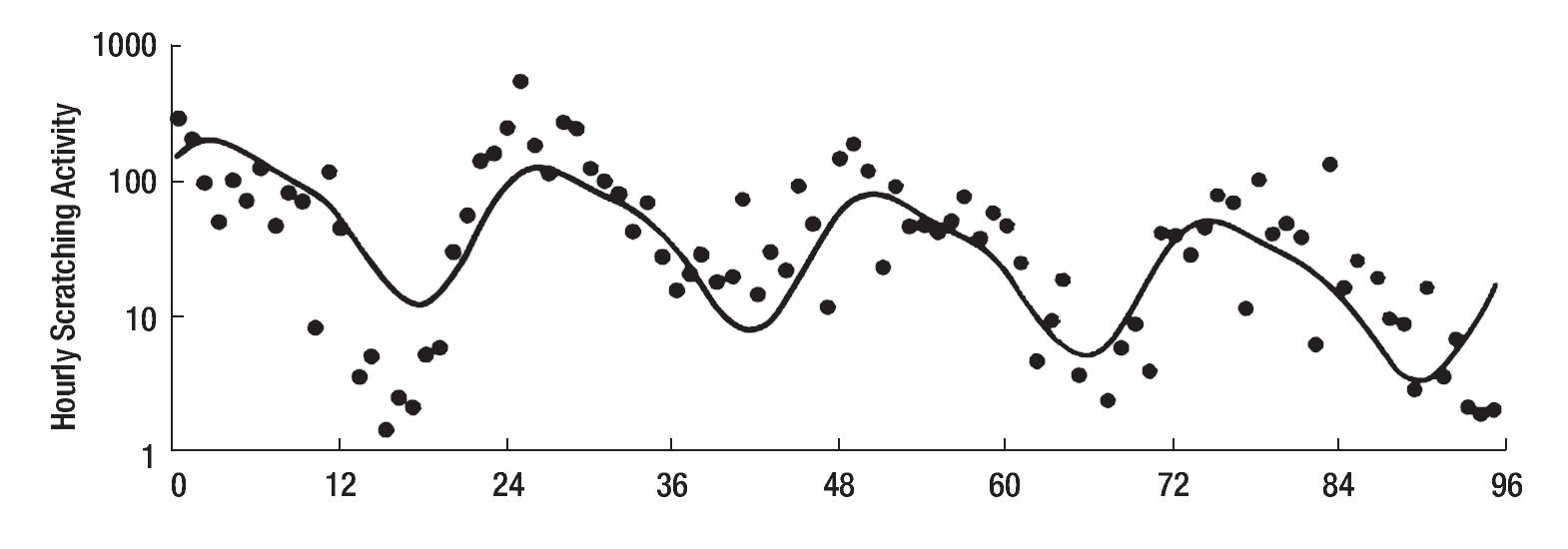
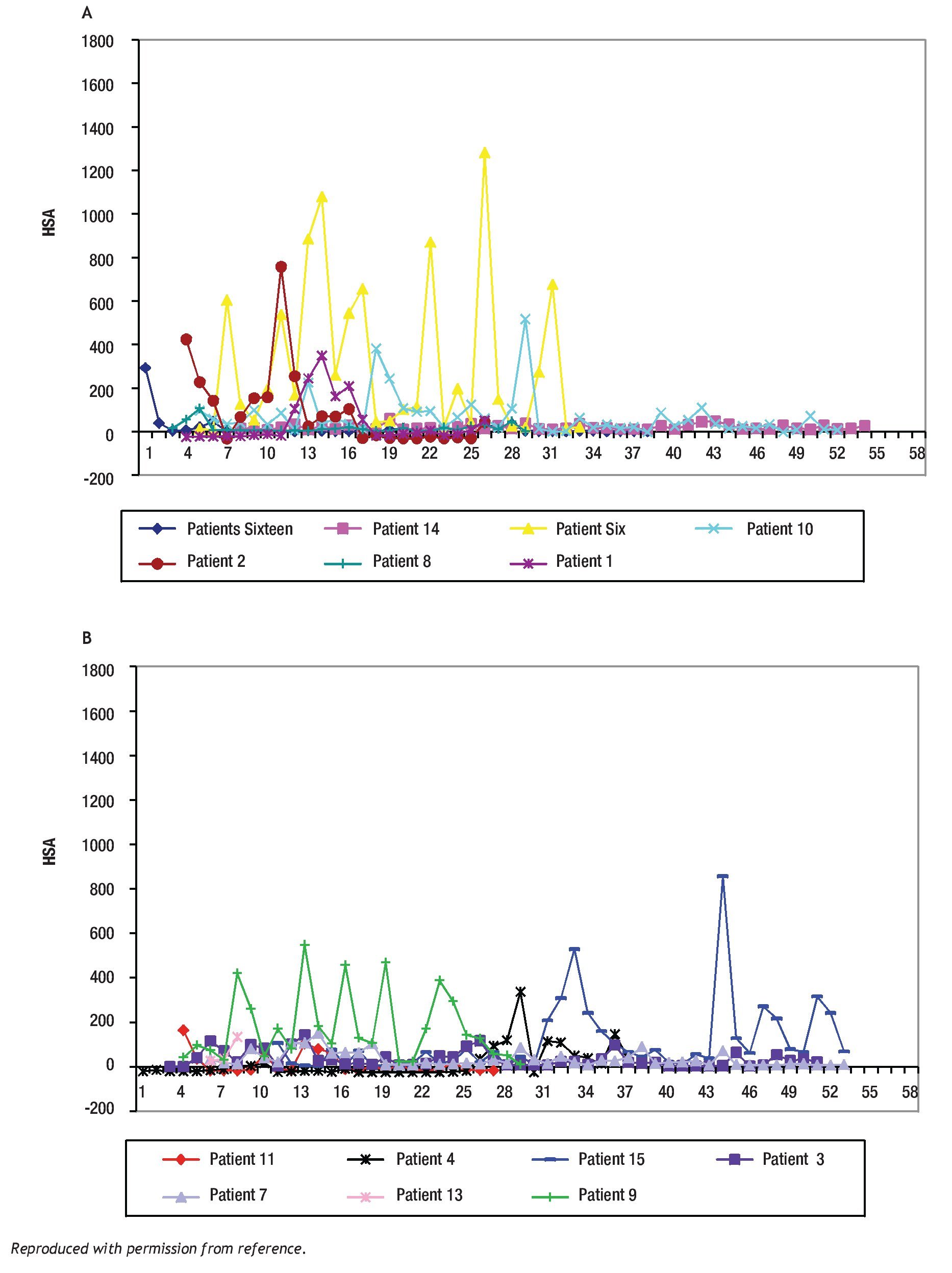
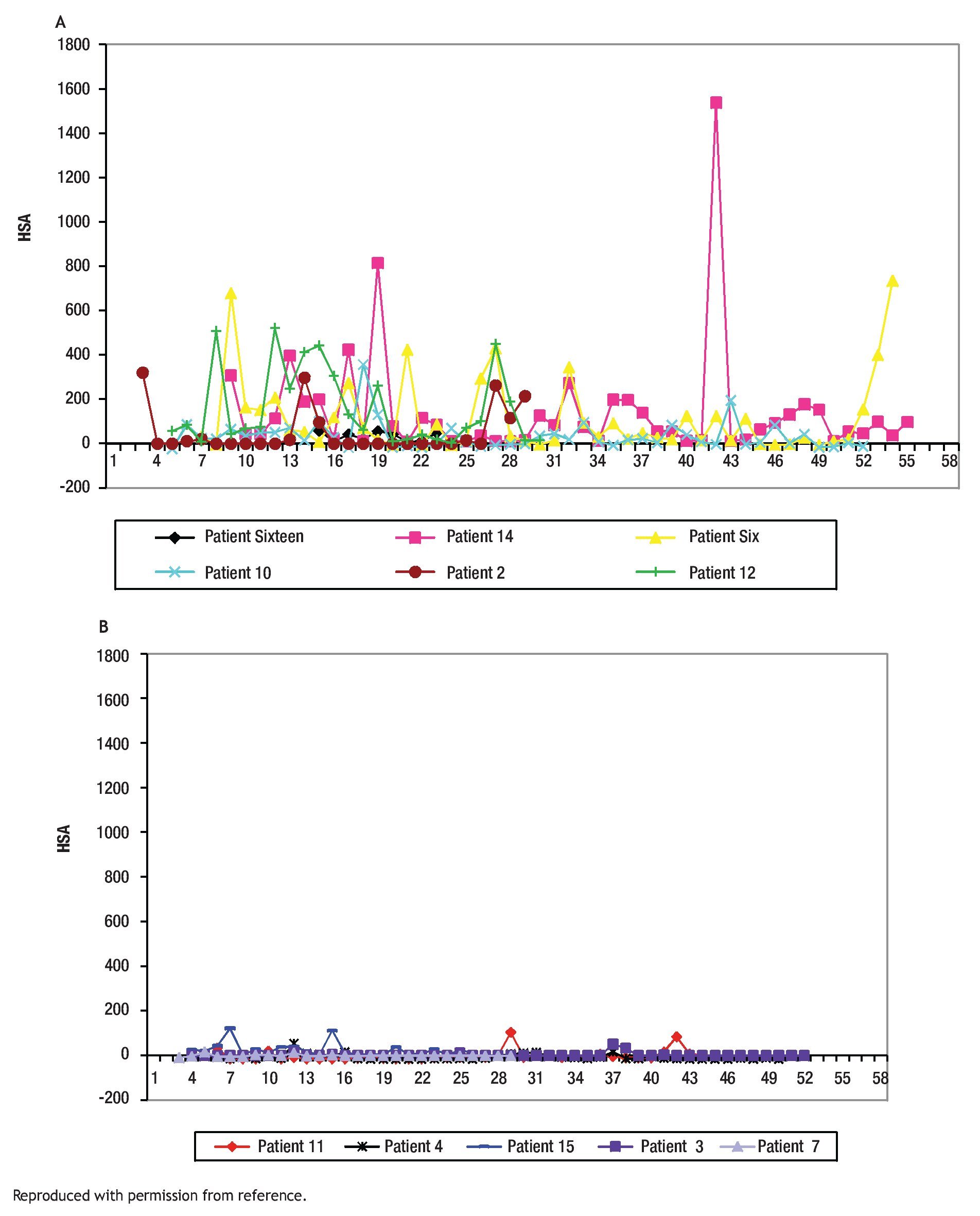
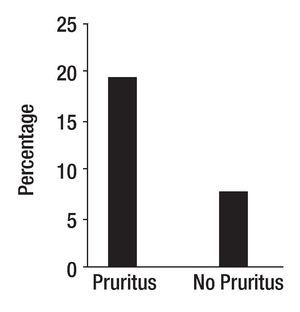
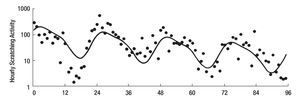
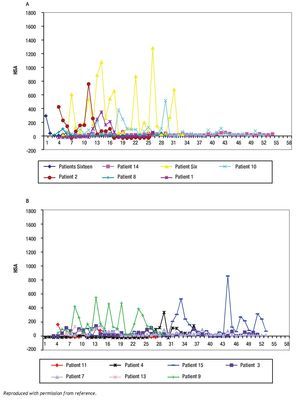
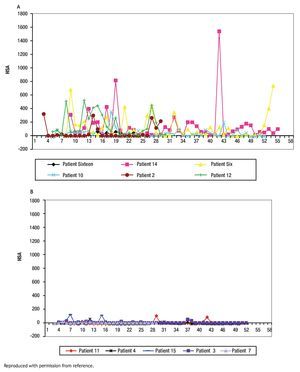
The study of symptoms is difficult because of their inherent subjective nature; however, pruritus is associated with scratching behavior, which can be measured by available methodology. In this context, the study of the pruritus of cholestasis has been advanced by the inclusion of behavioral methodology in clinical trials with well defined end points (i.e. decrease in scratching activity). In addition, behavioral methodology has revealed the complexity of scratching behavior: (a) in a double-blind randomized placebo controlled study of naloxone infusions for the pruritus of cholestasis, a 24 hour rhythm in scratching behavior that tended to peak from 1200 to 1600 hours, was detected in some patients46 (Figure 2); accordingly, the interpretation of data obtained from subjective methodology (i.e. visual analogue scales, diaries, questionnaires) may be inaccurate, as the timing of collection will surely influence the results, and (b) in a double-blind, randomized placebo-controlled study of gabapentin for the pruritus of cholestasis, (Figures3A, 3B, 4A, and 4B) the placebo intervention was associated with a significant reduction of scratching activity (Figure 4B).89 This finding has confirmed the placebo effect considered to be inherent in all studies of pruritus and also, it identifies the inclusion of expectations at the time of enrollment of patients in clinical trials of pruritus because expectations can have an impact on the placebo response.90,91
Figure 2. Mean hourly scratching activity during the 96-hour study period of a patient with cholestasis and pruritus. The continuous line indicates the 24-hour rhythm that best fits the observations; this line has a significant downward linear trend (slope = -0.0081±0.0021) (P < 0.001), which is consistent with the sequence of treatments (placebo, placebo, naloxone, naloxone, the latter being associated with a decrease in hourly scratching activity). Reproduced with permission from reference 46 .
Figure 3A and B. Mean hourly scratching activity (HAS) from patients who participated in a randomized, double blind, placebo control study of gabapentin for the pruritus of cholestasis (89). All patients underwent baseline continuous recording of scratching activity for at least 24 hours after which, they were randomized to receive gabapentin or placebo. Figures 3A and 3B depict mean baseline hourly scratching activity (HSA) from the patients who were subsequently randomized to gabapentin (3A) and to placebo (3B). Reproduced with permission from reference 89.
Figures 4A and B. Mean hourly scratching activity (HAS) from patients who participated in a randomized, double blind, placebo control study of gabapentin for the pruritus of cholestasis (89). After the patients had been randomized to the study drug (gabapentin or its placebo) and had been on treatment for at least four weeks, scratching activity was recorded for a period of at least 24 hours. Gabapentin was not associated with an amelioration in hourly scratching activity, in fact, it was associated with an increase (Figure 4A); in contrast, the placebo tablet, was associated with a significant reduction of hourly scratching activity (Figure 4B). Reproduced with permission from reference 89.
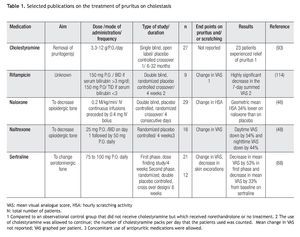
Selected therapies for the treatment of the pruritus of cholestasis (Table 1)
Differences in the pruritus experienced by patients with different types of liver diseases have not been reported. At present, therapies for the pruritus of cholestasis tend to be prescribed independently from the etiology of the liver disease. The treatment of the pruritus of cholestasis, however, can be aimed at two targets: (a) decreasing the degree of cholestasis; this relates to treating the specific cause of cholestasis such as relieving common bile duct obstruction, and in patients with primary biliary cirrhosis, administering ursodeoxycholic acid, a choleretic bile acid approved for the treatment of that disease, and (2) the specific treatment of pruritus; in this context, the only specific therapy for which a rationale is known is the administration of opiate antagonists. In this section, selected therapies for the pruritus of cholestasis will be listed, including the rationale that is given to support their use.
Procedures aimed at the removal of pruritogens from the body
Non-absorbable resins: The administration of non-absorbable resins (e.g. cholestyramine, cholesevalam), which bind anions in the small intestine92 have been reported to have antipruritic effect in some patients with cholestasis,93,94 and with polycythaemia vera,95 which is not associated with cholestasis and with an effect no different from placebo, in a controlled study that applied subjective methodology.96 The use of cholestyramine approved to treat hypercholesterolemia is incorporated in the practice guidelines on the management of pruritus in patients with primary biliary cirrhosis.97 The drug can be administered at doses of 4 g immediately before and after breakfast, as the idea that suggests its use is the increase in the fecal excretion of the pruritogens, which are stored in the gallbladder during the overnight fast, and that are poured into the duodenum at the first morning meal. The addition of 4 g of cholestyramine at lunch and/or dinner time can be considered but more than 16 g per day is not recommended in the use of this drug.
Extraction of pruritogens from plasma: The intervention to remove pruritogens from plasma on which there are several published articles is the extracorporeal liver support systems (Molecular Adsorbent Recirculating System (MARSTM)98-101 and PrometheusTM.102 The placebo effect of these interventions may be substantial and the relief temporary. The most recent series published reported that no major adverse effects had been associated with the procedure; thus, this intervention can be considered in selected patients with cholestasis and pruritus difficult to manage.101
Procedures associated with removal of bile the gastrointestinal tract have been in use for some time. The use of nasobiliary drainage in patients with pruritus secondary to primary biliary cirrhosis has been reported.103 Procedures to divert bile flow usually performed in children with some congenital cholestatic liver diseases have been reported to be associated with relief of pruritus, including partial external diversion of bile.104,105 A recently published retrospective review documented that the long term postoperative outcome in seven patients with progressive familial intrahepatic cholestasis who underwent partial biliary diversion over a four year period.105 It was reported that six patients experienced short-term resolution of jaundice and pruritus and three, qualified as symptom-free, had not required liver transplantation after partial biliary diversion from a post-procedure period duration of 59 to 78 months. Serious complications were also reported. The authors concluded that partial biliary diversion for progressive familial intrahepatic cholestasis is effective as a bridge to liver transplantation in improving jaundice and pruritus but that it may be associated with a high incidence of complications related to the stoma. This review highlights the difficulty that treating pruritus in patients with cholestasis, including children, poses and also, emphasizes the need to consult experts in the matter prior to this type of intervention.105
Changes in neurotransmission
Opiate antagonists: The rationale that support the use of opiate antagonists to treat the pruritus of cholestasis2 and the results of several clinical studies,27-29,45-51,106 including those that used behavioral methodology28,29,45,46 support the administration of opiate antagonists to treat the pruritus of cholestasis (see above). The opiate withdrawal like reaction that some patients experience is generally characterized by a constellation of symptoms including tachycardia, goose-bumps, abdominal pain, and unpleasant dreams.27-29 Although the duration of this reaction is limited and in most cases, the degree of severity mild, the risk for its occurrence can discourage the prescription of this type of drug. The treatment with oral opiate antagonists (i.e. naltrexone) can be started at the lowest possible dose to decrease the probability and intensity of the potential opiate withdrawal like reaction.107-109 In this regard, the only opiate antagonist with oral bioavailability available commercially is naltrexone. This drug, which is approved for the treatment of alcoholism,110 is produced in 50 mg tablets; thus, one alternative to its use is to break the tablet in four to yield approximately 12.5 mg per dose, which can be the starting dose, to be increased gradually to 50 mg per day. Another alternative is to admit patients to the hospital for intravenous infusions of naloxone, another opiate antagonist, and to convert to oral medication (i.e. naltrexone) after one or two days of intravenous medications. The initial dose of naloxone is 0.4 mg bolus intravenous administration, followed by its continuous infusion of naloxone at a dose of 0.2 micrograms for kilogram of body weight for minute.46 Metabolites of naltrexone can accumulate in patients with decompensated liver disease;84 thus, the use of lower than approved doses has been recommended in those patients. Pruritus, however, tends to cease in patients with hepatic synthetic dysfunction;111 thus, treatment of pruritus with naltrexone is usually not necessary in patients with decompensated liver disease.
Opiate-induced scratching behavior can be prevented by the stimulation of kappa opioid receptors in primates.39 Nalfurafine is a drug with kappa agonist activity that was reported to be associated with a decrease in pruritus in patients with kidney disease on hemodialysis.112 A study on the use of this drug in patients with primary biliary cirrhosis is on going in Japan (www.clinicaltrials.gov).
Serotonin re-uptake inhibitors: The original report stating that sertraline was associated with a decrease in pruritus in patients with primary biliary cirrhosis came from a retrospective review of data recorded by patients who were participants in a clinical trial that compared the effect of ursodeoxycholic acid and placebo to that of ursodeoxycholic acid and methotrexate.67 The finding was further explored in a randomized placebo controlled study the report of which documented that sertraline at doses of 75 to 100 mg was associated with relief of pruritus, as assessed by a visual analogue scale, and healing of excoriations, as evaluated on physical examination.68
Antiobiotics: Rifampicin is a ligand of the pregnane X receptor, the stimulation of which induces drug-metabolizing enzymes and transporters.113 Rifampicin has been studied in clinical trials114 and it is listed as an alternative to the management of the pruritus in patients with primary biliary cirrhosis in the clinical guidelines published by the American Association for the Study of Liver Disease.97 There is concern on the use of rifampicin for the treatment of this type of pruritus because of the risk of hepatotoxicity;115-117 thus, when this drug is prescribed, a rigorous monitoring of the patient to detect any evidence of liver toxicity, for which the drug should be stopped is necessary.
Antihistamines: Antihistamines, including hydroxyzine, tend to be prescribed for the treatment of pruritus in patients with liver disease, however, there is no evidence to support that histamine plays a role in this type of pruritus as the findings consistent with histamine mediated pruritus (i.e. edema and erythema) are absent from the skin of patients with cholestasis and pruritus; in contrast, excoriations, fresh and old, and prurigo nodularis, are the hallmark of chronic scratching. The relief of pruritus in association with this class of drug, as reported by 27 of the 164 (16.5%) patients with primary biliary cirrhosis who participated in an internet survey21 may be due to the sedative properties of antihistamines.118
Miscellaneous types of drugs that have been reported to relief pruritus in cholestasis: Phototherapy to the skin to treat the pruritus of cholestasis is used by some hepatologists, gastroenterologists and dermatologists,119-121 although the rationale for that type of therapy is not evident. Circadian rhythms are regulated by light.122 The 24 hour rhythm in scratching behavior displayed by some patients with cholestasis (Figure 2) suggested a circadian nature,46 and thus, provided a rationale to test bright light phototherapy indirectly directed towards the eyes, in a pilot study in which this intervention was associated with a decrease in scratching activity in the participants.123
Anesthetics including propofol124-126 and lidocaine127 have been reported to decrease pruritus in patients with cholestasis. The reported antipruritic effect of lidocaine may suggest a potential role of the transient receptor potential vanilloid receptor-1 (TRPV1) in the transmission of pruritogenic stimuli in cholestasis.128-130
The central processing of the sensation of itch poses a challenge in clinical studies because of methodological limitations to the study of brain function and to the study symptoms in general; however, continuous technological process will open avenues for research into this area. The reconciliation of consequences of cholestasis (e.g. accumulation in tissues of substances that are normally excreted in bile) with the neurophysiology and neuropathophysiology of the itch sensation in human beings is a challenge and a priority in clinical research.
Correspondence: Nora V. Bergasa, M.D.
Chief Department of Medicine Metropolitan Hospital Center 1901 First Avenue New York, New York. 10029
Received: January 2011.
Accepted: January 2011