Superficial venous aneurysms of the lower extremities are considered rare and their clinical significance is poorly defined. The purpose of this article is to report a case of a 72-year-old woman with a thrombosed great saphenous vein aneurysm along with deep venous thrombosis and review its clinical presentation, diagnosis and treatment.
Venous aneurysms (VA) are a rare vascular pathology. First described by Sir William Osler in 1913,1 VAs can be located anywhere throughout the venous system and do not have a preference regarding sex or age.2
VAs are usually located in the lower extremities, and can be deep or superficial, depending on whether the affected vein is over or under the muscle fascia. Deep VAs are the most frequent, because the popliteal vein is the most commonly affected (between 60% and 70% of the cases), and the most studied because of their high thromboembolism risk.3
Superficial VAs are rare, with under 60 reported cases in medical literature4 and are taken less seriously than deep VAs, due to the fact that superficial VAs are considered to have a low risk of life-threatening complications.
The purpose of this article is to present a case of a patient affected by a thrombosed great saphenous vein aneurysm along with deep venous thrombosis. Additionally, the literature is reviewed with a discussion on clinical implications and the diagnostic and therapeutic approach of this lesser known vascular pathology.
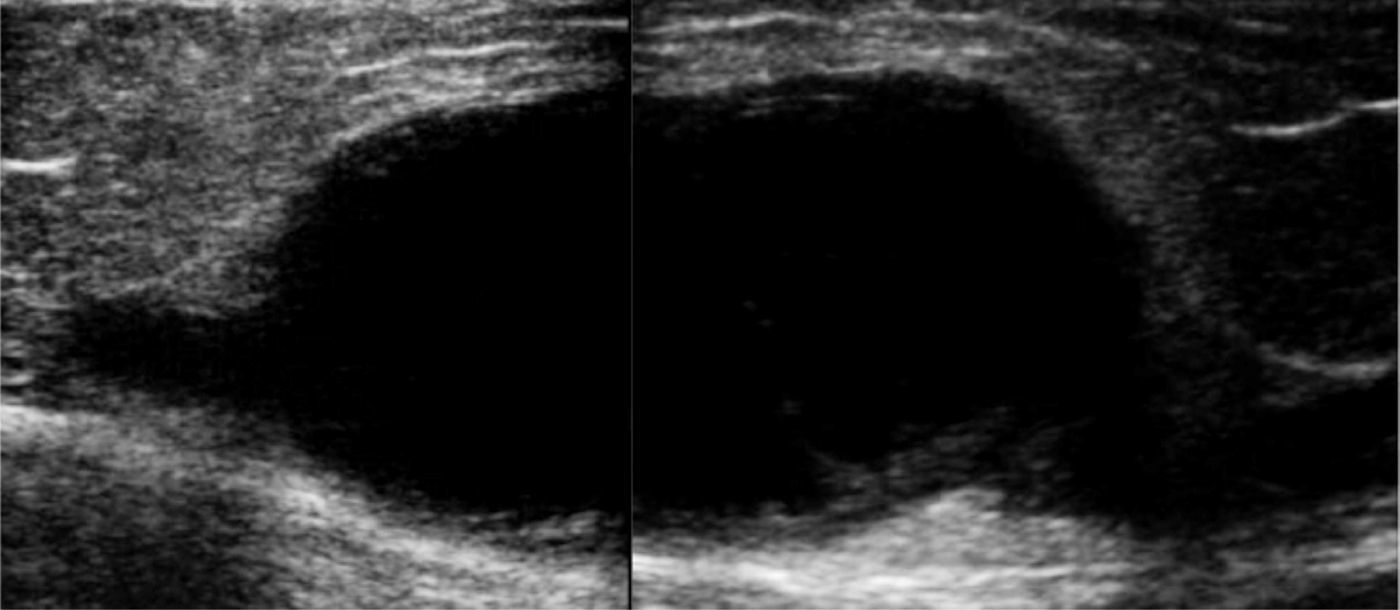
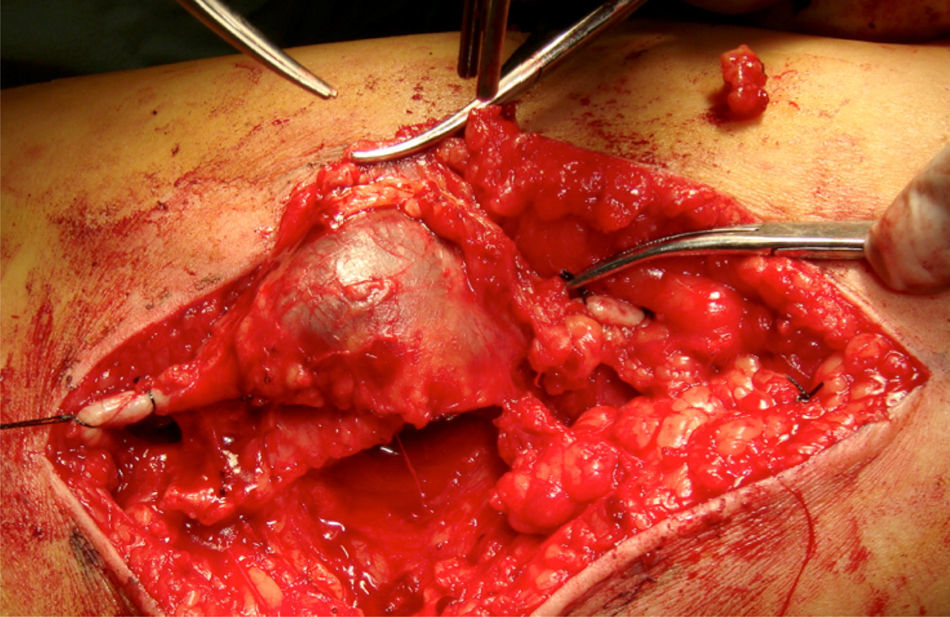
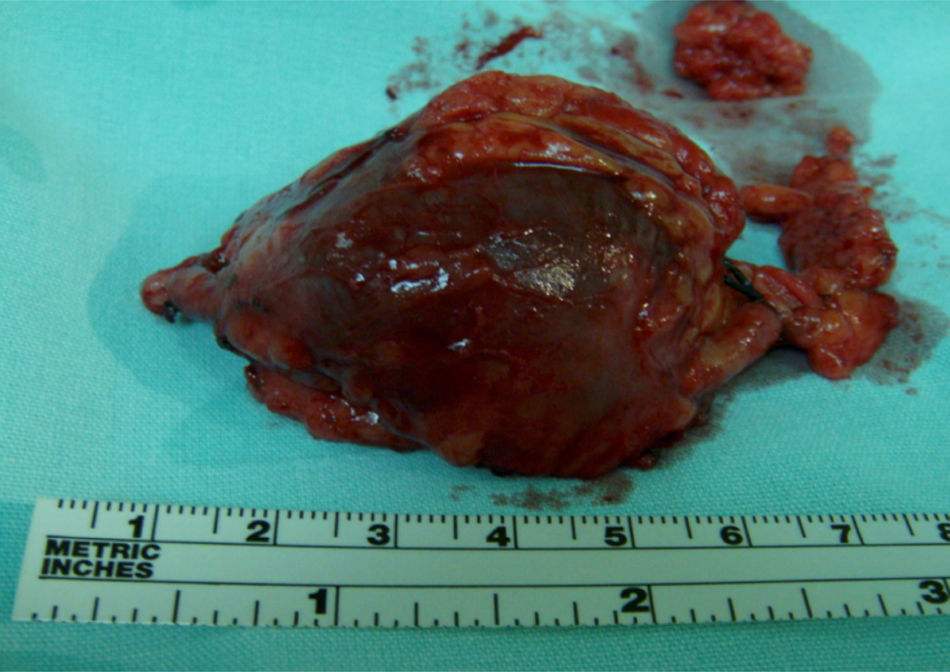
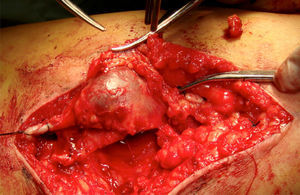
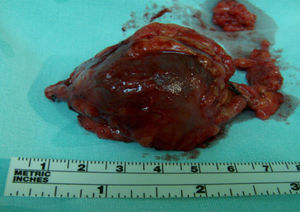
Clinical caseThe patient is a 72-year-old female, with no positive background of chronic degenerative diseases or of any other type, and a negative history of smoking. The patient presented an increase in volume of the right pelvic limb 10 days after undergoing surgery to correct a direct inguinal hernia using the Bassini–Shouldice technique. The subject pointed out that for over 40 years she presented a soft tumor of approximately 10cm, located in the middle third of the medial part of the right thigh 22cm from the inguinal fold. The tumor was soft, palpable and painless with a variation in volume depending on the position and did not cause any discomfort. Subsequent to the hernioplasty the patient began noticing that the tumor hardened and caused pain, along with redness and an increase in local temperature, consequently the physician diagnosed a cellulitis and prescribed antibiotic therapy. The symptomatology did not improve with the prescribed treatment and now presented an increase in volume of the entire pelvic member. She was therefore referred to our service. During the examination a difference of 5cm in volume between both pelvic members was observed, noticing a hard, non-mobile, painful lump of 9×6cm. The rest of the examination was normal. A vascular ultrasound was performed, showing a non-palpable, anechoic growth, connected to the great saphenous vein, of 9×5cm (Fig. 1) without the presence of venous reflex neither in the great saphenous nor in the saphenofemoral junction. Moreover, a posterior tibial vein thrombosis was detected. With a great saphenous vein aneurysm diagnosis, the patient was transferred to surgery where an aneurysmectomy and SFJ ligation were performed (Figs. 2 and 3).
There were no complications during the procedure, the evolution was good and the patient was discharged on the second postoperative day with a compressive and anticoagulant DVT treatment for a period of 3 months.
DiscussionThe terminology used to describe venous dilatations can cause confusion. The terms phlebectasia, varicose vein and/or venous aneurysm are considered synonyms in the medical community; however, they mean different things. Phlebectasiais defined as a fusiform and diffusely dilated vein. The association of dilated and tortuous veins is known as varicose veins.5 There is no precise criteria regarding size and when a venous dilation is considered an aneurysm; however, Mateo6 and McDevitt7 established that whenever the vein's diameter is twice as large as the normal diameter, then it is considered to be an aneurysm (the saphenous vein's normal size at the saphenofemoral joint is 3–5mm, 2–4mm at the thigh and 1–3mm at the ankle).8. Nevertheless, in order to consider it a primary venous aneurysm size is not the only factor considered. It must also be a localized dilation, conformed by three histological layers which constitute the normal venous wall. This could be saccular or fusiform (an important distinction because of its hemodynamic implications that influence the course of treatment) and should only communicate to the corresponding vein in a proximal and distal manner and neither be secondary to an arteriovenous fistula,9 nor be related to a varicose vein.10 Hilscher coined the term primary venous aneurysm, making a comparison to arterial aneurysms. Abbott was the one who integrated the criteria to define VAs.11 Our patient met the required criteria to classify her venous pathology as a primary venous aneurysm.
Primary VAs occur in the head and neck, thoracic and visceral veins as well as veins of the extremities. Aneurysms of the deep venous system are characteristic on the extremities, where a greater incidence of thromboembolic complications is reported.
The incidence of superficial venous aneurysms is approximately 0.1%,12 with a prevalence of 1.5%,13 equally in both sexes and it may occur at any age.
The pathogenesis of venous aneurysms is unknown; several mechanisms have been implicated in its formation, such as hemodynamic changes, arteriovenous fistulae, inflammatory processes, infections, trauma, congenital weakness of the venous wall and degenerative changes.5,14 The most accepted theory states that it is a result of the loss of connective tissue components of the venous wall, which can be caused by a congenital defect or secondary to a degenerative process due to aging.15,16 In a recent study, tissue from the wall of venous aneurysms was examined and it was reported that structural changes of the aneurysmatic wall can be related to the increased expression of metalloproteinase,17 which can be translated to a reduction of the muscle layer, fragmented elastic tissue, an increase in the fibrous tissue and infiltration of inflammatory cells. Schatz and Fine,18 believed that edophlebohypertophy was a major factor in the formation of VA, which begins with an increase in venous flow leading to a hypertrophy of the venous wall, then dilation and sclerosis. Pascarella et al.4 informed that superficial VAs on lower limbs were generally located distal to an incompetent venous valve, which causes venous reflex, hitting the venous wall and producing a turbulent flow, causing structural changes on the wall of the spleen, while those in deep veins are secondary to intrinsic changes of the venous wall.
The difference among findings in the reports suggests that the etiology of VAs is diverse and depends on their location and natural history.16,19–22 In our case, the etiology may come as a result of a congenital defect in the connective tissue structure, which generated a weak venous wall susceptible to dilation, hence contributing to the onset of the inguinal hernia.
According to the etiopathogeny, VAs can be divided into primary (congenital) or acquired. Based on the Hamburg classification for congenital vascular malformations,23 VAs correspond to a venous malformation of the troncular type, which can be presented as aplasia, hyperplasia, stenosis (i.e. the left iliac vein in May-Turner syndrome), dilations or aneurysms (the most common being the popliteal vein).24
Congenital VAs are less common but can occur in any vein of the body. In a study, Guillespie et al.,2 reported that 77% of VAs were located in the lower extremities (57% in the deep venous system), 10% in the upper extremities and 13% in the neck.
The clinical presentation of superficial VAs in lower extremities is that of a palpablesoft lump. It can change its size and location with the body's position or the Valsalva maneuver; they can be completely asymptomatic or painful with edema, and have signs and symptoms similar to those described in our case.
The diagnosis can be made by the clinical history and physical examination in most cases, but it is usually confirmed by image studies. Within image studies, vascular ultrasound is the method of choice for the study of VAs.25 In the ultrasound, VAs are presented as an anechoic cystic structure, with well-defined walls, which can be secular or fusiform and with a low flow volume; also, they provide us with information on vascular connections, the existence of thrombosis, or if there is an associated arteriovenous fistula or any other pathologies,26 in addition to guiding the therapeutic approach. The CAT scan, the MRI and the phlebography are studies which can be performed in case of diagnostic doubt or when more exact information is required (size, extension, associated lesions and vascular origin).27 Clinical history and examination are the basis for reaching any diagnosis, but in the case of venous problems, vascular ultrasound evaluation is fundamental to reaching a correct diagnosis, and thus choosing the proper treatment. It is only in case of doubt, where further imaging studies are required. In the differential diagnosis, we must consider varicose veins, soft tissue tumors, hygromas, hemangiomas and, depending on location, inguinal hernias. Vascular aneurysm complications are: thrombophlebitis, deep venous thrombosis, pulmonary embolism and bleeding caused by rupture.
Coagulation disorders associated with VAs are characterized by blood stasis in the dilated vessels and with a low blood flow, which activates the coagulation cascade with the subsequent production of thrombin and the conversion of fibrinogen into fibrin.28,29 Then the fibrinolysis process begins which is reflected in the rise in fibrinogen derived products, including D-dimer. This is the simplified description of located intravascular coagulopathy which characterizes coagulopathy associated with venous malformations.30,31 Newly formed microthrombus attach to calcium and phleboliths are formed.32,33 These can be detected during physical examination and verified by imaging studies. The presence of phleboliths in VAs can indicate treatment with anticoagulants, especially in large and extensive VAs.34 Localized intravascular coagulopathy is of utmost importance because it is linked to the presence of local pain, thrombophlebitis, deep venous thrombosis and pulmonary embolism.
Even though deep venous aneurysms are more susceptible to presenting pulmonary embolism and their frequency is more common in this type of VA, superficial VAs are not free from presenting this complication. Due to this, they must be treated with anticoagulant therapy, just as the deep ones. Recapitulating Virchow's triad for venous thrombosis, which consists of venous stasis, hypercoagulability and endothelial lesion, in our case it presented venous stasis because of the hemodynamic changes brought on by the dimensions of the venous aneurysm, the existing coagulation disorders of this pathology associated with bed rest subsequent to hernioplasty, and the existing risk of thrombosis in any type of surgery, and constitute risk factors which contributed to the presence of superficial and deep venous thrombosis described in our report.
Regarding the risk of VA rupture, it represents something theoretical since there are no reported cases of this complication.
Superficial VA treatment can be conservative, endovascular or surgical. If the VA is not too large and does not cause symptoms it may be treated conservatively through compressive therapy and prophylaxis in order to avoid thrombophlebitis or deep venous thrombosis. The indications for surgical treatment in superficial VAs are: the presence of symptoms, the risk of thrombosis, compression of nearby structures and, more commonly, esthetic problems.35 Surgical treatment36 consists of ligation and total excision of the aneurysm. However, in some cases, endovascular methods can be used, like foam, laser or radiofrequency sclerotherapy.37 In the case of patients who present superficial venous thrombosis above the knee (as in our case) or deep venous thrombosis at any location, it is important to treat with anticoagulants for 3–6 months in patients with normal thrombophilic profiles, otherwise the anticoagulant is prescribed indefinitely. In the presented case, there is no doubt that the chosen surgical treatment is adequate and the treatment with anticoagulants due to the presence of deep thrombosis for a period of three months is acceptable.
The results of the treatment of these types of pathologies are excellent as long as they are not associated with other vascular malformations or pathologies; there are no reports of mortality in superficial VA surgical intervention in the lower extremities and the morbidity is the same as any venous surgical procedure.36
In our case, the primary care physician diagnosed the increase in volume as a soft tissue tumor (first differential diagnosis which should be made) and since it was asymptomatic, they did not give it much importance despite the fact that the patient reported that the tumor was growing progressively. As a result of the inguinal hernioplasty the patient was on bed rest for two weeks. Surgical trauma and prolonged bed rest are risk factors for thrombosis. The patient did not receive prophylaxis for thrombosis.
At first, and because the tumor looked red and was causing severe pain, it was treated as if it was cellulitis (thrombophlebitis, which is commonly confused with soft tissue infections). It was not until the size of the patient's leg began to increase that there was a suspicion of venous thrombosis and the case was referred to our service.
Thus we conclude that it is important to: (1) perform the clinical history and a thorough physical examination in all of our patients, (2) we must keep in mind all possible differential diagnoses before giving a definite one, (3) one must never forget prophylaxis for deep vein thrombosis in surgical patients, (4) not all skin redness equals infection, (5) venous thrombosis must be ruled out in every patient who presents a sudden increase in leg volume, (6) vascular ultrasound is an essential tool for diagnosis and (7) venous aneurysms do exist and they are not just varicose veins.
Conflict of interestThe authors have no conflicts of interest to declare.
FundingNo financial support was provided.