Obesity has become a worldwide epidemic, and its prevalence has been projected to grow by 40% in the next decade. This increasing prevalence has implications for the risk of diabetes, cardiovascular disease and also for Chronic Kidney Disease. A high body mass index is one of the strongest risk factors for new-onset Chronic Kidney Disease. In individuals affected by obesity, a compensatory hyperfiltration occurs to meet the heightened metabolic demands of the increased body weight. The increase in intraglomerular pressure can damage the kidneys and raise the risk of developing Chronic Kidney Disease in the long-term. The incidence of obesity-related glomerulopathy has increased tenfold in recent years. Obesity has also been shown to be a risk factor for nephrolithiasis, and for a number of malignancies including kidney cancer. This year the World Kidney Day promotes education on the harmful consequences of obesity and its association with kidney disease, advocating healthy lifestyle and health policy measures that makes preventive behaviors an affordable option.
La obesidad se ha convertido en una epidemia mundial y se estima que su prevalencia crecerá en un 40% durante la próxima década. Este incremento de prevalencia tiene implicaciones de riesgo para el desarrollo de diabetes, enfermedad cardiovascular y enfermedad renal crónica. Un índice elevado de masa corporal es uno de los factores de riesgo más importantes para el inicio de la enfermedad renal crónica. En los individuos afectados por obesidad se produce una hiperfiltración compensatoria para cubrir el exceso de demanda metabólica que impone el incremento del peso corporal. El aumento de la presión intraglomerular puede dañar el riñón y aumentar el riesgo de desarrollar enfermedad renal crónica en el largo plazo. La incidencia de la glomerulopatía relacionada con la obesidad ha aumentado 10 veces en años recientes. La obesidad también constituye un factor de riesgo para la nefrolitiasis y para un número de enfermedades malignas, incluyendo al cáncer de riñón. Este año el Día Mundial del Riñón promueve la educación sobre las consecuencias perjudiciales de la obesidad y su asociación con la enfermedad renal, a la vez que aboga por la adopción estilos de vida saludables y las políticas de salud que permitan convertir a la prevención en una opción asequible de prevención.
In 2014, over 600 million adults worldwide, 18 years and older, were obese. Obesity is a potent risk factor for the development of kidney disease. It increases the risk of developing major risk factors for Chronic Kidney Disease (CKD), like diabetes and hypertension, and it has a direct impact on the development of CKD and end-stage renal disease (ESRD). In individuals affected by obesity, a (likely) compensatory mechanism of hyperfiltration occurs to meet the heightened metabolic demands of the increased body weight. The increase in intraglomerular pressure can damage the kidney structure and raise the risk of developing CKD in the long-term.
The good news is that obesity, as well as the related CKD, is largely preventable. Education and awareness of the risks of obesity and a healthy lifestyle, including proper nutrition and exercise, can dramatically help in preventing obesity and kidney disease. This article reviews the association of obesity with kidney disease on the occasion of the 2017 World Kidney Day.
Epidemiology of obesity in adults and childrenOver the last 3 decades, the prevalence of overweight and obese adults (BMI ≥25kg/m2) worldwide has increased substantially [1]. In the US, the age-adjusted prevalence of obesity in 2013–2014 was 35% among men and 40.4% among women [2]. The problem of obesity also affects children. In the US in 2011–2014, the prevalence of obesity was 17% and extreme obesity 5.8% among youth 2–19 years of age. The rise in obesity prevalence is also a worldwide concern [3,4], as it is projected to grow by 40% across the globe in the next decade. Low- and middle-income countries are now showing evidence of transitioning from normal weight to overweight and obesity as parts of Europe and the United States did decades ago [5]. This increasing prevalence of obesity has implications for cardiovascular disease (CVD) and also for CKD. A high body mass index (BMI) is one of the strongest risk factors for new-onset CKD [6,7].
Definitions of obesity are most often based on BMI (i.e. weight [kilograms] divided by the square of his or her height [meters]). A BMI between 18.5 and 25kg/m2 is considered by the World Health Organization (WHO) to be normal weight, a BMI between 25 and 30kg/m2 as overweight, and a BMI of >30kg/m2 as obese. Although BMI is easy to calculate, it is a poor estimate of fat mass distribution, as muscular individuals or those with more subcutaneous fat may have a BMI as high as individuals with larger intraabdominal (visceral) fat. The latter type of high BMI is associated with substantially higher risk of metabolic and cardiovascular disease. Alternative parameters to more accurately capture visceral fat include waist circumference (WC) and a waist–hip ratio (WHR) of >102cm and 0.9, respectively, for men and >88cm and >0.8, respectively, for women. WHR has been shown to be superior to BMI for the correct classification of obesity in CKD.
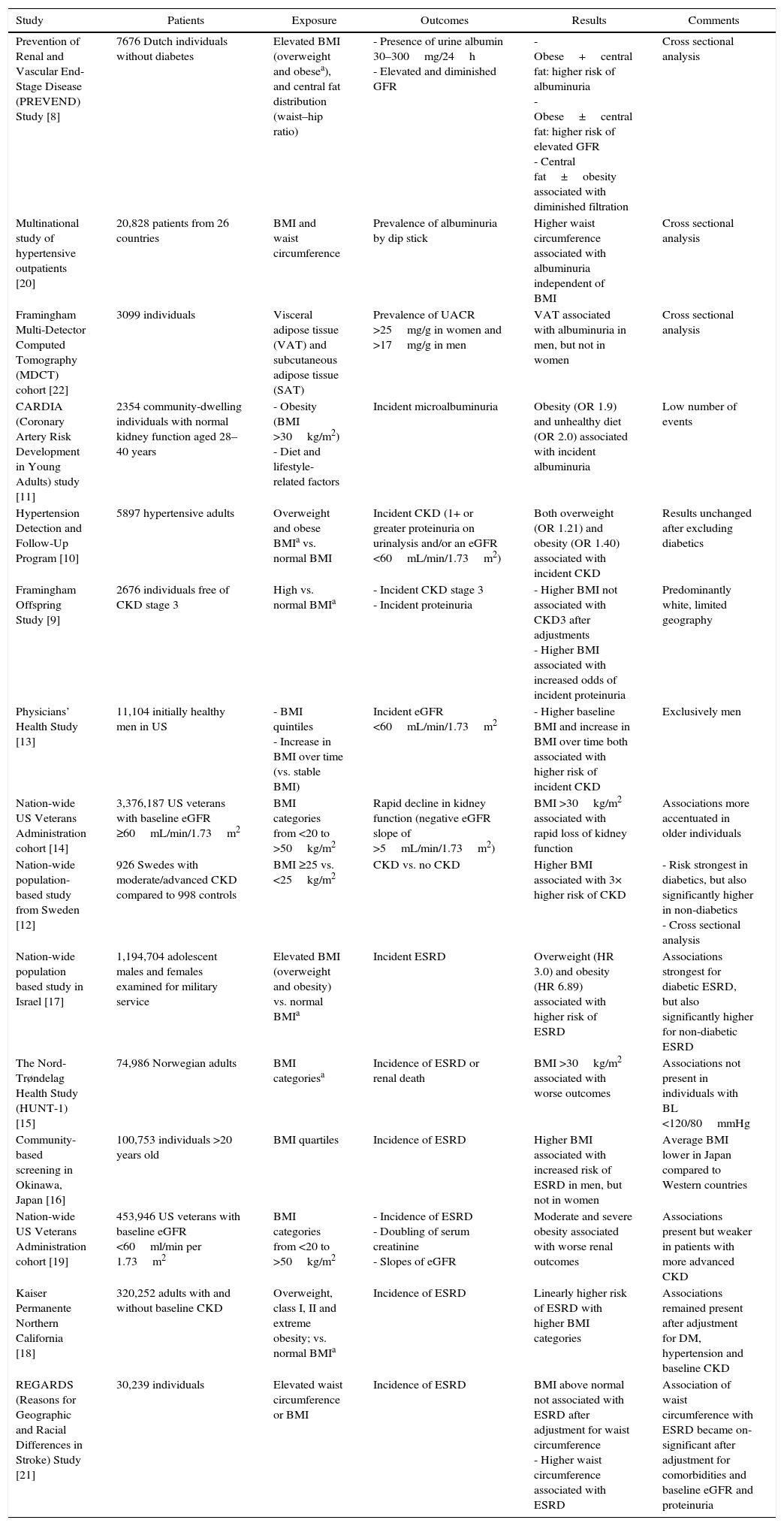
Association of obesity with CKD and other renal complicationsNumerous population based studies have shown an association between measures of obesity and both the development and the progression of CKD (Table 1). Higher BMI is associated with the presence [8] and development [9–11] of proteinuria in individuals without kidney disease. Furthermore, in numerous large population-based studies, higher BMI appears associated with the presence [8,12] and development of low estimated GFR [9,10,13], with more rapid loss of estimated GFR over time [14] and with the incidence of ESRD [15–18]. Elevated BMI levels, class II obesity and above, have been associated with more rapid progression of CKD in patients with pre-existing CKD [19]. A few studies examining the association of abdominal obesity using WHR or WC with CKD, describe an association between higher girth and albuminuria [20], decreased GFR [8] or incident ESRD [21] independent of BMI level.
Studies examining the association of obesity with various measures of CKD.
| Study | Patients | Exposure | Outcomes | Results | Comments |
|---|---|---|---|---|---|
| Prevention of Renal and Vascular End-Stage Disease (PREVEND) Study [8] | 7676 Dutch individuals without diabetes | Elevated BMI (overweight and obesea), and central fat distribution (waist–hip ratio) | - Presence of urine albumin 30–300mg/24h - Elevated and diminished GFR | - Obese+central fat: higher risk of albuminuria - Obese±central fat: higher risk of elevated GFR - Central fat±obesity associated with diminished filtration | Cross sectional analysis |
| Multinational study of hypertensive outpatients [20] | 20,828 patients from 26 countries | BMI and waist circumference | Prevalence of albuminuria by dip stick | Higher waist circumference associated with albuminuria independent of BMI | Cross sectional analysis |
| Framingham Multi-Detector Computed Tomography (MDCT) cohort [22] | 3099 individuals | Visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) | Prevalence of UACR >25mg/g in women and >17mg/g in men | VAT associated with albuminuria in men, but not in women | Cross sectional analysis |
| CARDIA (Coronary Artery Risk Development in Young Adults) study [11] | 2354 community-dwelling individuals with normal kidney function aged 28–40 years | - Obesity (BMI >30kg/m2) - Diet and lifestyle-related factors | Incident microalbuminuria | Obesity (OR 1.9) and unhealthy diet (OR 2.0) associated with incident albuminuria | Low number of events |
| Hypertension Detection and Follow-Up Program [10] | 5897 hypertensive adults | Overweight and obese BMIa vs. normal BMI | Incident CKD (1+ or greater proteinuria on urinalysis and/or an eGFR <60mL/min/1.73m2) | Both overweight (OR 1.21) and obesity (OR 1.40) associated with incident CKD | Results unchanged after excluding diabetics |
| Framingham Offspring Study [9] | 2676 individuals free of CKD stage 3 | High vs. normal BMIa | - Incident CKD stage 3 - Incident proteinuria | - Higher BMI not associated with CKD3 after adjustments - Higher BMI associated with increased odds of incident proteinuria | Predominantly white, limited geography |
| Physicians’ Health Study [13] | 11,104 initially healthy men in US | - BMI quintiles - Increase in BMI over time (vs. stable BMI) | Incident eGFR <60mL/min/1.73m2 | - Higher baseline BMI and increase in BMI over time both associated with higher risk of incident CKD | Exclusively men |
| Nation-wide US Veterans Administration cohort [14] | 3,376,187 US veterans with baseline eGFR ≥60mL/min/1.73m2 | BMI categories from <20 to >50kg/m2 | Rapid decline in kidney function (negative eGFR slope of >5mL/min/1.73m2) | BMI >30kg/m2 associated with rapid loss of kidney function | Associations more accentuated in older individuals |
| Nation-wide population-based study from Sweden [12] | 926 Swedes with moderate/advanced CKD compared to 998 controls | BMI ≥25 vs. <25kg/m2 | CKD vs. no CKD | Higher BMI associated with 3× higher risk of CKD | - Risk strongest in diabetics, but also significantly higher in non-diabetics - Cross sectional analysis |
| Nation-wide population based study in Israel [17] | 1,194,704 adolescent males and females examined for military service | Elevated BMI (overweight and obesity) vs. normal BMIa | Incident ESRD | Overweight (HR 3.0) and obesity (HR 6.89) associated with higher risk of ESRD | Associations strongest for diabetic ESRD, but also significantly higher for non-diabetic ESRD |
| The Nord-Trøndelag Health Study (HUNT-1) [15] | 74,986 Norwegian adults | BMI categoriesa | Incidence of ESRD or renal death | BMI >30kg/m2 associated with worse outcomes | Associations not present in individuals with BL <120/80mmHg |
| Community-based screening in Okinawa, Japan [16] | 100,753 individuals >20 years old | BMI quartiles | Incidence of ESRD | Higher BMI associated with increased risk of ESRD in men, but not in women | Average BMI lower in Japan compared to Western countries |
| Nation-wide US Veterans Administration cohort [19] | 453,946 US veterans with baseline eGFR <60ml/min per 1.73m2 | BMI categories from <20 to >50kg/m2 | - Incidence of ESRD - Doubling of serum creatinine - Slopes of eGFR | Moderate and severe obesity associated with worse renal outcomes | Associations present but weaker in patients with more advanced CKD |
| Kaiser Permanente Northern California [18] | 320,252 adults with and without baseline CKD | Overweight, class I, II and extreme obesity; vs. normal BMIa | Incidence of ESRD | Linearly higher risk of ESRD with higher BMI categories | Associations remained present after adjustment for DM, hypertension and baseline CKD |
| REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study [21] | 30,239 individuals | Elevated waist circumference or BMI | Incidence of ESRD | BMI above normal not associated with ESRD after adjustment for waist circumference - Higher waist circumference associated with ESRD | Association of waist circumference with ESRD became on-significant after adjustment for comorbidities and baseline eGFR and proteinuria |
Normal weight: BMI 18.5–24.9kg/m2; overweight: BMI 25.0–29.9kg/m2; class I obesity: BMI 30.0–34.9kg/m2; class II obesity: BMI 35.0–39.9kg/m2; class III obesity: BMI ≥40kg/m2.
BMI, body mass index; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; HR, hazard ratio; OR, odds ratio; UACR, urine albumin–reatinine ratio.
Higher visceral adipose tissue measured by computed tomography has been associated with a higher prevalence of albuminuria in men [22]. The observation of a BMI-independent association between abdominal obesity and poorer renal outcomes is also described in relationship with mortality in patients with ESRD [23] and kidney transplant [24], and suggests a direct role of visceral adiposity. In general, the associations between obesity and poorer renal outcomes persist even after adjustments for possible mediators of obesity's cardiovascular and metabolic effects, such as high blood pressure and diabetes mellitus, suggesting that obesity may affect kidney function through mechanisms in part unrelated to these complications (vide infra).
The deleterious effect of obesity on the kidneys extends to other complications such as nephrolithiasis and kidney malignancies. Higher BMI is associated with an increased prevalence [25] and incidence [26,27] of nephrolithiasis. Furthermore, weight gain over time, and higher baseline WC were also associated with higher incidence of nephrolithiasis [27]. Obesity is associated with various types of malignancies, particularly cancers of the kidneys. In a population-based study of 5.24 million individuals from the UK, a 5kg/m2 higher BMI was associated with a 25% higher risk of kidney cancers, with 10% of all kidney cancers attributable to excess weight [28]. Another large analysis examining the global burden of obesity on malignancies estimated that 17% and 26% of all kidney cancers in men and women, respectively, were attributable to excess weight [29]. The association between obesity and kidney cancers was consistent in both men and women, and across populations from different parts of the world in a meta-analysis that included data from 221 studies (of which 17 examined kidney cancers) [30]. Among the cancers examined in this meta-analysis, kidney cancers had the third highest risk associated with obesity (relative risk per 5kg/m2 higher BMI: 1.24, 95%CI 1.20–1.28, p<0.0001) [30].
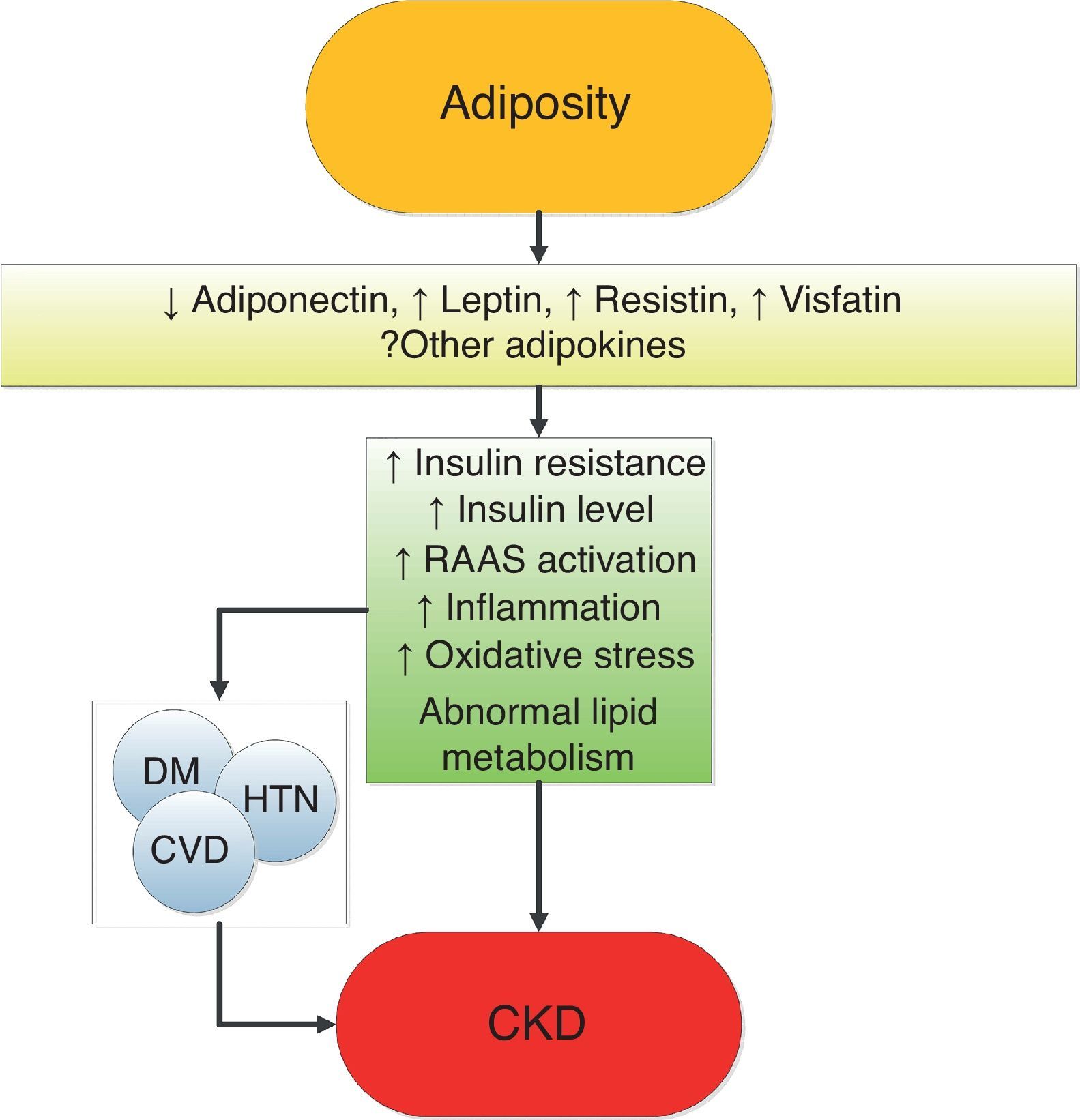
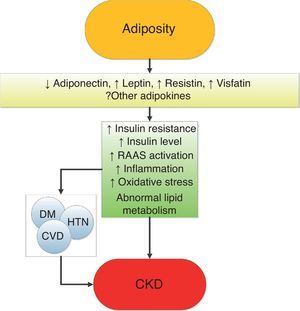
Mechanisms of action underlying the renal effects of obesityObesity results in complex metabolic abnormalities which have wide-ranging effects on diseases affecting the kidneys. The exact mechanisms whereby obesity may worsen or cause CKD remain unclear. The fact that most obese individuals never develop CKD, and the distinction of up to as many as 25% of obese individuals as “metabolically healthy” suggests that increased weight alone is not sufficient to induce kidney damage [31]. Some of the deleterious renal consequences of obesity may be mediated by downstream comorbid conditions such as diabetes mellitus or hypertension, but there are also effects of adiposity which could impact the kidneys directly, induced by the endocrine activity of the adipose tissue via production of (among others) adiponectin [32], leptin [33] and resistin [34] (Fig. 1). These include the development of inflammation [35], oxidative stress [36], abnormal lipid metabolism [37], activation of the renin–angiotensin–aldosterone system [38], and increased production of insulin and insulin resistance [39,40].
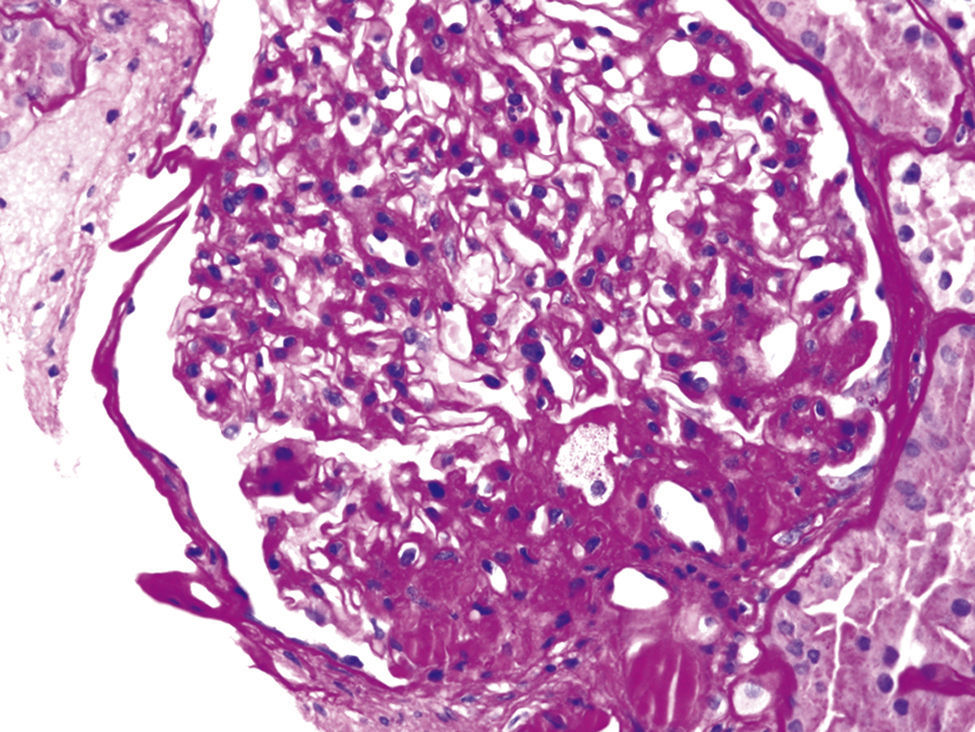
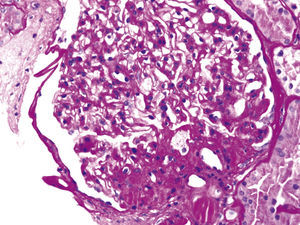
These various effects result in specific pathologic changes in the kidneys [41] which could underlie the higher risk of CKD seen in observational studies. These include ectopic lipid accumulation [42] and increased deposition of renal sinus fat [43,44], the development of glomerular hypertension and increased glomerular permeability caused by hyperfiltration-related glomerular filtration barrier injury [45], and ultimately the development of glomerulomegaly [46], and focal or segmental glomerulosclerosis [41] (Fig. 2). The incidence of the so-called obesity-related glomerulopathy (ORG) has increased tenfold between 1986 and 2000 [41]. Importantly, ORG often presents along with pathophysiologic processes related to other conditions or advanced age, conspiring to result in more accentuated kidney damage in patients with high blood pressure [47] or in the elderly [14,39].
Obesity is associated with a number of risk factors contributing to the higher incidence and prevalence of nephrolithiasis. Higher body weight is associated with lower urine pH [48] and increased urinary oxalate [49], uric acid, sodium and phosphate excretion [50]. Diets richer in protein and sodium may lead to a more acidic urine and decrease in urinary citrate, also contributing to kidney stone risk. The insulin resistance characteristic of obesity may also predispose to nephrolithiasis [51] through its impact on tubular Na–H exchanger [52] and ammoniagenesis [53], and the promotion of an acidic milieu [54]. Complicating the picture is the fact that some weight loss therapies result in a worsening, rather than an improvement in the risk for kidney stone formation; e.g. gastric surgery can lead to a substantial increase in enteral oxalate absorption and enhanced risk of nephrolithiasis [55].
The mechanisms behind the increased risk of kidney cancers observed in obese individuals are less well characterized. Insulin resistance, and the consequent chronic hyperinsulinemia and increased production of insulin-like growth factor 1 and numerous complex secondary humoral effects may exert stimulating effects on the growth of various types of tumor cells [56]. More recently, the endocrine functions of adipose tissue [57], its effects on immunity [58], and the generation of an inflammatory milieu with complex effects on cancers [59,60] have emerged as additional explanations.
Obesity in patients with advanced kidney disease: the need for a nuanced approachConsidering the above evidence about the overwhelmingly deleterious effects of obesity on various disease processes, it is seemingly counterintuitive that obesity has been consistently associated with lower mortality rates in patients with advanced CKD [19,61] and ESRD [62,63]. Similar “paradoxical” associations have also been described in other populations, such as in patients with congestive heart failure [64], chronic obstructive pulmonary disease [65], rheumatoid arthritis [66], and even in old individuals [67]. It is possible that the seemingly protective effect of a high BMI is the result of the imperfection of BMI as a measure of obesity, as it does not differentiate the effects of adiposity from those of higher non-adipose tissue. Indeed, studies that separated the effects of a higher waist circumference from those of higher BMI showed a reversal of the inverse association with mortality [23,24]. Higher muscle mass has also been shown to explain at least some of the positive effects attributed to elevated BMI [63,68]. However, there is also evidence to suggest that higher adiposity, especially subcutaneous (non-visceral) fat, may also be associated with better outcomes in ESRD patients [62]. Such benefits may indeed be present in patients who have very low short term life expectancy, such as most ESRD patients [69]. Indeed, some studies that examined the association of BMI with time-dependent survival in ESRD have shown a marked contrast between protective short term effects vs. deleterious longer term effects of higher BMI [70]. There are several putative short term benefits that higher body mass could portend, especially to sicker individuals. These include a benefit from the better nutritional status typically seen in obese individuals, and which provides better protein and energy reserves in the face of acute illness, and a higher muscle mass with enhanced antioxidant capacity [63] and lower circulating actin and higher plasma gelsolin levels [71], which are associated with better outcomes. Other hypothetically beneficial characteristics of obesity include a more stable hemodynamic status with mitigation of stress responses and heightened sympathetic and renin–angiotensin activity [72]; increased production of adiponectines [73] and soluble tumor necrosis factor alfa receptors [74] by adipose tissue neutralizing the adverse effects of tumor necrosis factor alfa; enhanced binding of circulating endotoxins [75] by the characteristically higher cholesterol levels seen in obesity; and sequestration of uremic toxins by adipose tissue [76].
Potential interventions for management of obesityObesity engenders kidney injury via direct mechanisms through deranged synthesis of various adipose tissue cytokines with nephrotoxic potential, as well as indirectly by triggering diabetes and hypertension, i.e. two conditions that rank among the strongest risk factors for CKD. Perhaps due to the survival advantage of obesity in CKD, the prevalence of end stage kidney disease is on the rise both in the USA [77] and in Europe [78]. Strategies for controlling the obesity related CKD epidemic at population level and for countering the evolution of CKD toward kidney failure in obese patients represent the most tantalizing task that today's health planners, health managers and nephrologists face.
Countering CKD at population levelCalls for public health interventions in the community to prevent and treat CKD at an early stage have been made by major renal associations, including the International Society of Nephrology (ISN), International Federation of the Kidney Foundation (IFKF), the European renal association (ERA-EDTA) and various national societies. In the USA, Healthy People 2020, a program that sets 10-year health targets for health promotion and prevention goals, focuses both on CKD and obesity. Surveys to detect obese patients, particularly those with a high risk of CKD (e.g. hypertensive and/or diabetic obese people) and those receiving suboptimal care to inform these patients of the potential risk for CKD they are exposed to, are the first step toward developing public health interventions. Acquiring evidence that current interventions to reduce CKD risk in the obese are efficacious and deployable, is an urgent priority to set goals and means for risk modification. Appropriate documentation of existing knowledge distilling the risk and the benefits of primary and secondary prevention interventions in obese people, and new trials in this population to fill knowledge gaps (see below) are needed. Finally, surveillance programs that monitor progress on the detection of at-risk individuals and the effectiveness of prevention programs being deployed [79] constitute the third, fundamental element for establishing efficacious CKD prevention plans at population level.
A successful surveillance system for CKD has already been implemented in some places such as the United Kingdom (UK) [80]. A campaign to disseminate and apply K-DOQI CKD guidelines in primary care within the UK National Health Service was launched. This progressively increased the adoption of K-DOQI guidelines and, also thanks to specific incentives for UK general physicians to detect CKD, led to an impressive improvement in the detection and care of CKD, i.e. better control of hypertension and increased use of angiotensin-converting enzyme and angiotensin receptor blockers [80]. This system may serve as a platform to improve the prevention of obesity-related CKD. Campaigns aiming at reducing the obesity burden are now at center stage worldwide and are strongly recommended by the WHO and it is expected that these campaigns will reduce the incidence of obesity-related complications, including CKD. However obesity-related goals in obese CKD patients remain vaguely formulated, largely because of the paucity of high-level evidence intervention studies to modify obesity in CKD patients [81].
Prevention of CKD progression in obese people with CKDObservational studies in metabolically healthy obese subjects show that the obese phenotype unassociated with metabolic abnormalities per se predicts a higher risk for incident CKD [82] suggesting that obesity per se may engender renal dysfunction and kidney damage even without diabetes or hypertension (vide supra). In overweight or obese diabetic patients, a lifestyle intervention including caloric restriction and increased physical activity compared with a standard follow up based on education and support to sustain diabetes treatment reduced the risk for incident CKD by 30%, although it did not affect the incidence of cardiovascular events [83]. Such a protective effect was partly due to reductions in body weight, HbA1c, and systolic BP. No safety concerns regarding kidney related adverse events were seen [83]. In a recent meta-analysis collating experimental studies in obese CKD patients, interventions aimed at reducing body weight showed coherent reductions in blood pressure, glomerular hyper-filtration and proteinuria [81]. A thorough post hoc analysis of the REIN study showed that the nephron-protective effect of ACE inhibition in proteinuric CKD patients was maximal in obese CKD patients, but minimal in CKD patients with normal or low BMI [84]. Of note, bariatric surgical intervention has been suggested for selected CKD and ESRD patients including dialysis patients who are waitlisted for kidney transplantation [85–87].
Globally, these experimental findings provide a proof of concept for the usefulness of weight reduction and ACE inhibition interventions in the treatment of CKD in the obese. Studies showing a survival benefit of increased BMI in CKD patients, however, remain to be explained [88]. These findings limit our ability to make strong recommendations about the usefulness and the safety of weight reduction among individuals with more advanced stages of CKD. Lifestyle recommendations to reduce body weight in obese people at risk for CKD and in those with early CKD appear justified, particularly recommendations for the control of diabetes and hypertension. As the independent effect of obesity control on the incidence and progression of CKD is difficult to disentangle from the effects of hypertension and type 2 diabetes, recommendation of weight loss in the minority of metabolically healthy, non-hypertensive obese patients remains unwarranted. These considerations suggest that a therapeutic approach to overweight and obesity in patients with advanced CKD or other significant comorbid conditions has to be pursued carefully, with proper considerations of the expected benefits and potential complications of weight loss over the life span of the individual patient.
ConclusionsThe worldwide epidemic of obesity affects the Earth's population in many ways. Diseases of the kidneys, including CKD, nephrolithiasis and kidney cancers are among the more insidious effects of obesity, but which nonetheless have wide ranging deleterious consequences, ultimately leading to significant excess morbidity and mortality and excess costs to individuals and the entire society. Population-wide interventions to control obesity could have beneficial effects in preventing the development, or delaying the progression of CKD. It is incumbent upon the entire healthcare community to devise long-ranging strategies toward improving the understanding of the links between obesity and kidney diseases, and to determine optimal strategies to stem the tide. The 2017 World Kidney Day is an important opportunity to increase education and awareness to that end.
Note from the Editor: This Editorial note has been requested within the framework of the World Kidney Day campaign. This publication has been sent simultaneously to a series of Scientific Journals. Thus, it is as a duplicate publication that Nefrología Latinoamericana presents as Editorial note given the importance that it has to Public Health in Nephrology.
Members of the World Kidney Day Steering Committee are: Philip Kam Tao Li, Guillermo Garcia-Garcia, Mohammed Benghanem-Gharbi, Rik Bollaert, Sophie Dupuis, Timur Erk, Kamyar Kalantar-Zadeh, Csaba Kovesdy, Charlotte Osafo, Miguel C. Riella, Elena Zakharova.