The dendritic spines are the main sites of excitatory synaptic contacts. Moreover, they present plastic responses to different stimuli of synaptic activity or damage, ranging from an increase or decrease in its total number, or a redistribution of progenitor dendritic spines, or variations in the size or shape. However, the spines can remain stable for a long time.
BackgroundThe use of experimental models has shown that different molecules of the F-actin binding and signalling pathways are closely related to the development, maintenance and plasticity of excitatory synapses, which could affect the number, size and shape of the dendritic spines; mechanisms that affect and depend on the reorganisation of the actin cytoskeleton.
DevelopmentIt is proposed that the filopodia are precursors of dendritic spines. Drebrin is an F-actin binding protein, and is responsible for focus F-actin and PSD-95 in filopodia that should lead to the formation of the new spine.
ConclusionThe specific mechanisms of actin regulation are an integral part in the formation, maturing process and plasticity of dendritic spines in association with the various actin cytoskeleton-binding proteins In addition, there are signalling pathways mediated by small GTPases, as well as the equilibrium between G-actin and F-actin.
Las espinas dendríticas representan los principales sitios de contactos sinápticos de tipo excitador. Además, presentan respuestas plásticas a diferentes estímulos propios de la actividad sináptica o daño, que van de un aumento o disminución de su número total a una redistribución a lo largo de las dendritas progenitoras o variaciones en su tamaño o forma. Sin embargo, las espinas pueden permanecer estables durante tiempos largos.
FuentesEl uso de modelos experimentales ha reportado que distintas moléculas de unión a los F-actina y vías de señalización están estrechamente relacionadas con el desarrollo, el mantenimiento y la plasticidad de las sinapsis de tipo excitador, lo que podría influir en el número, tamaño y la forma de las espinas dendríticas; mecanismos que afectan y depende el reordenamiento del citoesqueleto de actina.
DesarrolloSe ha propuesto que los filopodios son los precursores de espinas dendríticas. Drebrina es una proteína de unión a los F-actina y es la responsable de concentrar los F-actina y PSD-95 en los filopodios que guiarán la formación de la nueva espina.
ConclusionesLos mecanismos específicos de regulación de la actina son parte integral en la formación, maduración y plasticidad de espinas dendríticas en correlación con diversas proteínas de unión al citoesqueleto de actina. Además, de las vías de señalización mediadas por pequeñas GTPasas, así como la relación entre la G-actina y F-actina.
The connections and neural circuits formed by the brain are responsible for different behaviours, thoughts, emotions, and memories. The neuron's ability to function within neural circuits is mediated by contact sites called synapses. Chemical synapses regulate electrical communication within neural networks and transmit information from the presynaptic axon terminals to postsynaptic regions. Building neural circuits during brain development has to be controlled precisely so that the activity of the neural network is carried out correctly. Most excitatory synapses in mammal brains are situated on small dendritic projections called dendritic spines.1 Experiments have shown that synaptic plasticity may lead to morphological changes in dendritic spines.2 Furthermore, we know that the brain's ability to store information depends on whether existing synapses are growing stronger or weaker, and on the appearance or elimination of dendritic spines. These functional and structural changes in spines and synapses are thought to be the processes behind learning and memory.3 Dendritic spines are tiny protoplasmic protuberances that cover the surface of many neurons4 and constitute the contact sites of excitatory synapses in neurons of the hippocampus, neocortex, and other regions of the brain. These structures were first described by Ramón y Cajal,5 who hypothesised that they served to connect axons and dendrites, and that they were structural components in the nervous system.5 Since then, a number of studies have shown that actin-binding proteins are fundamental to the formation, elimination, motility, stability, size, and shape of dendritic spines.6,7 Furthermore, in the synapses, the actin cytoskeleton contributes to the morphological structure of the neuron, in addition to participating in the organisation of proteins present in the post-synaptic density (PSD) and in anchoring different types of postsynaptic receptors needed for receiving messages and transmitting synaptic information.8
The purpose of this review is to summarise current evidence about the mechanisms regulating dendritic spine morphogenesis. We also describe the actin cytoskeleton's participation in the organisation and structuring of dendritic spines, and the signalling pathways that regulate spine formation through the formation of filopodia (microspikes).
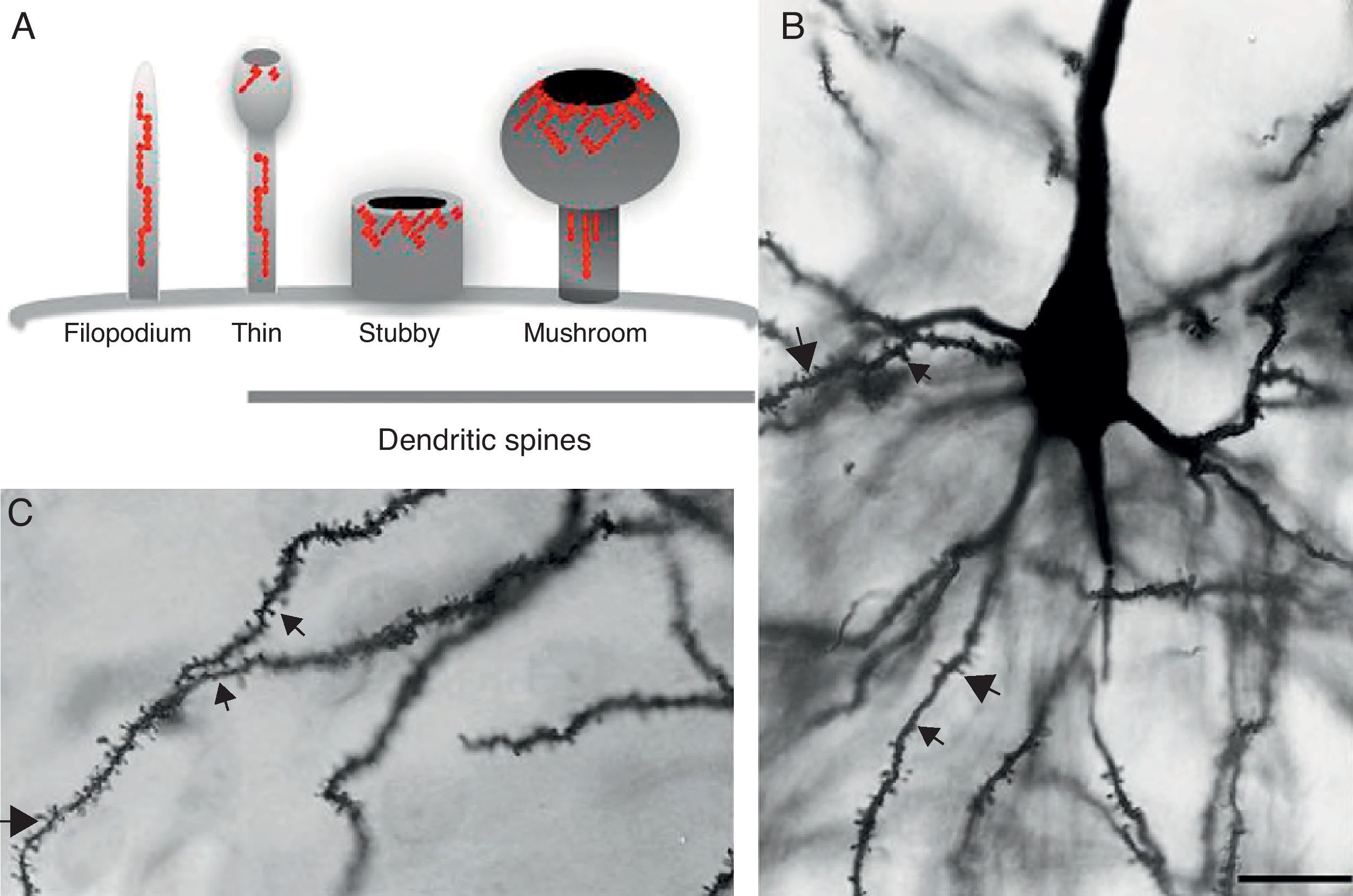
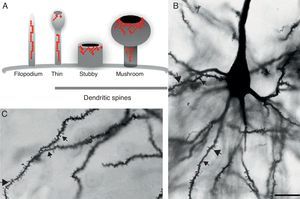
DevelopmentDendritic spines: structure and functionDendritic spines are tiny protoplasmic protuberances that cover the surface of many neurons and constitute the principal contact sites for excitatory synapses. Dendritic spine density ranges from 1 to 10 spines per micrometre along the dendrite. Some neurons, such as those in the hippocampus, contain thousands of spines along the dendritic branch structure.9 A spine contains 3 basic components: (a) a base joined to the dendrite, (b) a neck, and (c) a head which may connect with an axon. Spine shapes and sizes vary. They range in length from 0.2 to 2m, with a volume of 0.001 to 1m3. Based on their morphology, spines are classified as thin, stubby, or mushroom spines1 (Figs. 1A, B and 2). The interesting thing about these structures is that they are not static; their morphology undergoes constant change, including throughout adulthood, which reflects the nature of synaptic plasticity.10 In vitro and in vivo studies report that spine morphology may be modified by neuronal activity relating to experience.11 Activity patterns that induce long-term potentiation (LTP) constitute one of the main cellular mechanisms underlying learning and memory and inducing spine enlargement. It has been suggested that these changes are fundamental to the formation of memory pathways.12
(A) Diagram of a filopodium and dendritic spines (thin, stubby, and mushroom). Modified from Sekino et al.24 (B and C) Photomicrograph of a pyramidal cell from the third layer of the rat prefrontal cerebral cortex. The photo shows the different shapes of dendritic spines along the dendritic shaft: (
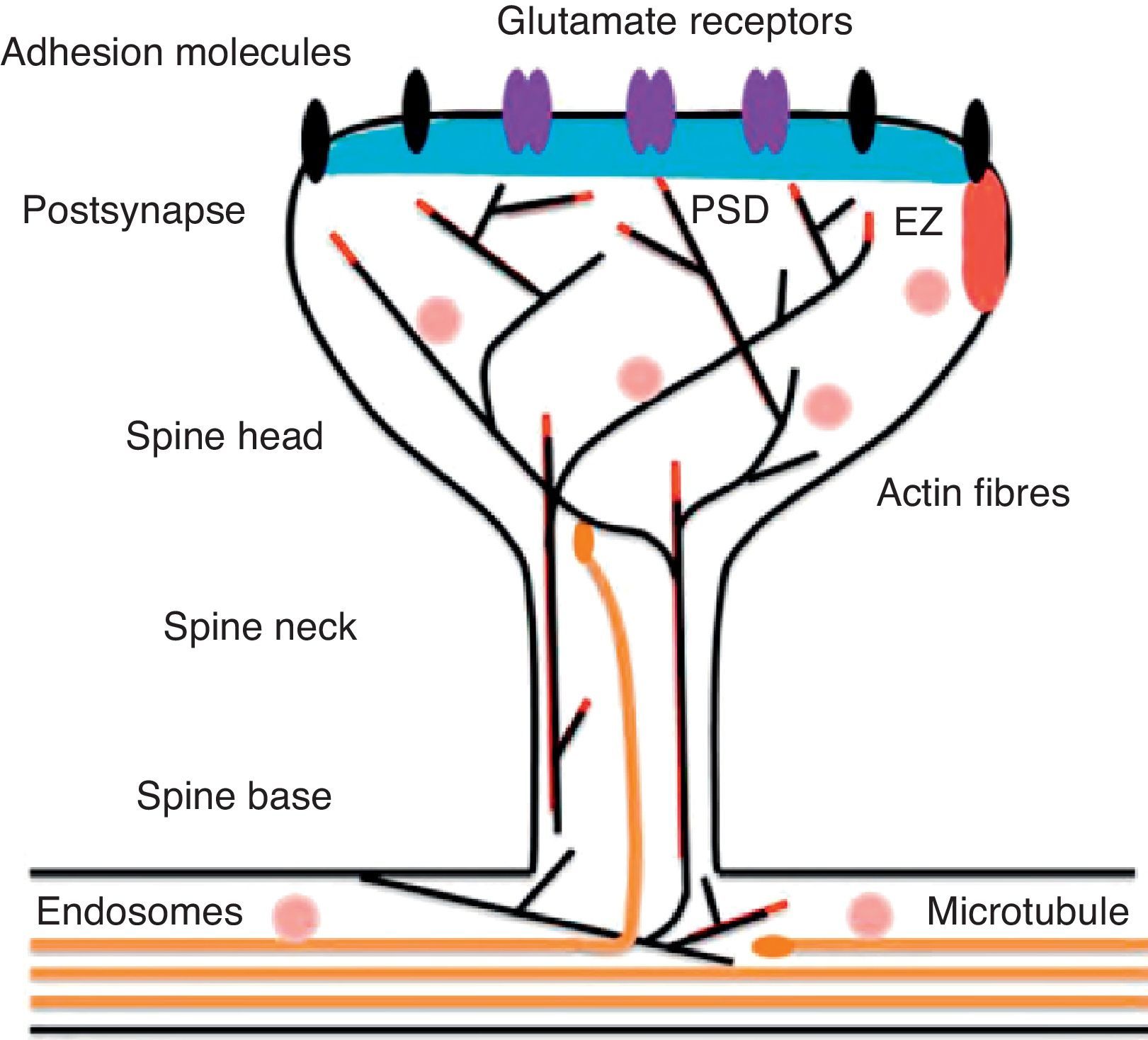
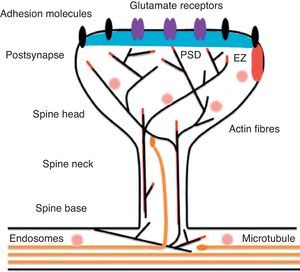
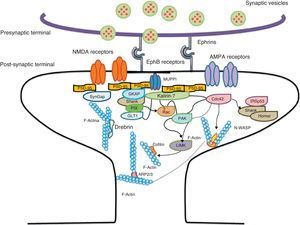
Diagram of a mushroom spine showing the postsynaptic structures necessary for the transmission of the nervous impulse.
Dendritic spines contain the postsynaptic mechanisms needed in order to transmit nerve impulses. They include glutamate receptors, PSD proteins, and the actin cytoskeleton, along with a wide variety of organelles from the endomembrane system (smooth endoplasmic reticulum, mitochondria, and endosomes)13 (Fig. 2). The PSD is generally found on the posterior part of the head of the dendritic spine in apposition to the presynaptic active zone. The PSD works as an organisational structure for different groups of receptors, adhesion molecules, and ionic channels. It concentrates a large array of different signalling molecules in the postsynaptic membrane.14 Most intracellular signalling pathways seem to control the shape of the spines in addition to converging directly in the actin cytoskeleton.
Actin organisationG-actin is a monomeric protein that regulates cellular motility and structure. The strength of motility is regulated by the ATPase activity of myosin (an enzyme hydrolysing adenosine triphosphate (ATP)) or by the modulation of the polymerisation and depolymerisation processes of actin filaments (F-actin). F-actin is the main cytoskeleton protein found in dendritic spines.15 It forms short networks and long branches in the neck of the spines, and in the head just below the PSD (Fig. 2). The main function of post-synaptic proteins in mature spines is to stabilise and modulate the structure of the head. Mass spectrometry studies of PSD fractions have reported that calcium/calmodulin-dependent protein kinase IIβ (CaMKIIβ), cortactin, drebrin, and neurabin I are present in this zone.16 This study indicated that regulating the listed proteins reduces dendritic spine formation and maturation.17 It seems that these proteins are crucial for synaptic plasticity and for forming certain types of memory.18
It has been shown that reorganising actin has effects on the formation and/or loss of dendritic spines, as well as producing morphological changes (form and number of spines). Both the monomeric (G-actin) and polymeric (F-actin) forms of the protein are present in spines. The degree of actin polymerisation (and therefore, the G-actin/F-actin ratio) has an effect on dendritic spine morphology. It has been reported that LTP induction increases the F-actin in the G-actin/F-actin ratio and increases the volume of dendritic spines. At the same time, inducing long-term depression (LTD) increases G-actin in the G-actin/F-actin ratio by decreasing actin filaments, and also provokes contraction of dendritic spines.19,20
Dendritic spines and filopodiaImmature or developing dendrites are characterised by the presence of small cytoplasmic protuberances that are headless, fine, and hairlike, measuring more than 3μm in length. They are found in immature neurons called filopodia, which form synaptic contacts covering a large part of the dendritic extensions of developing neurons,21 and may come to be replaced by mature, stable spines.
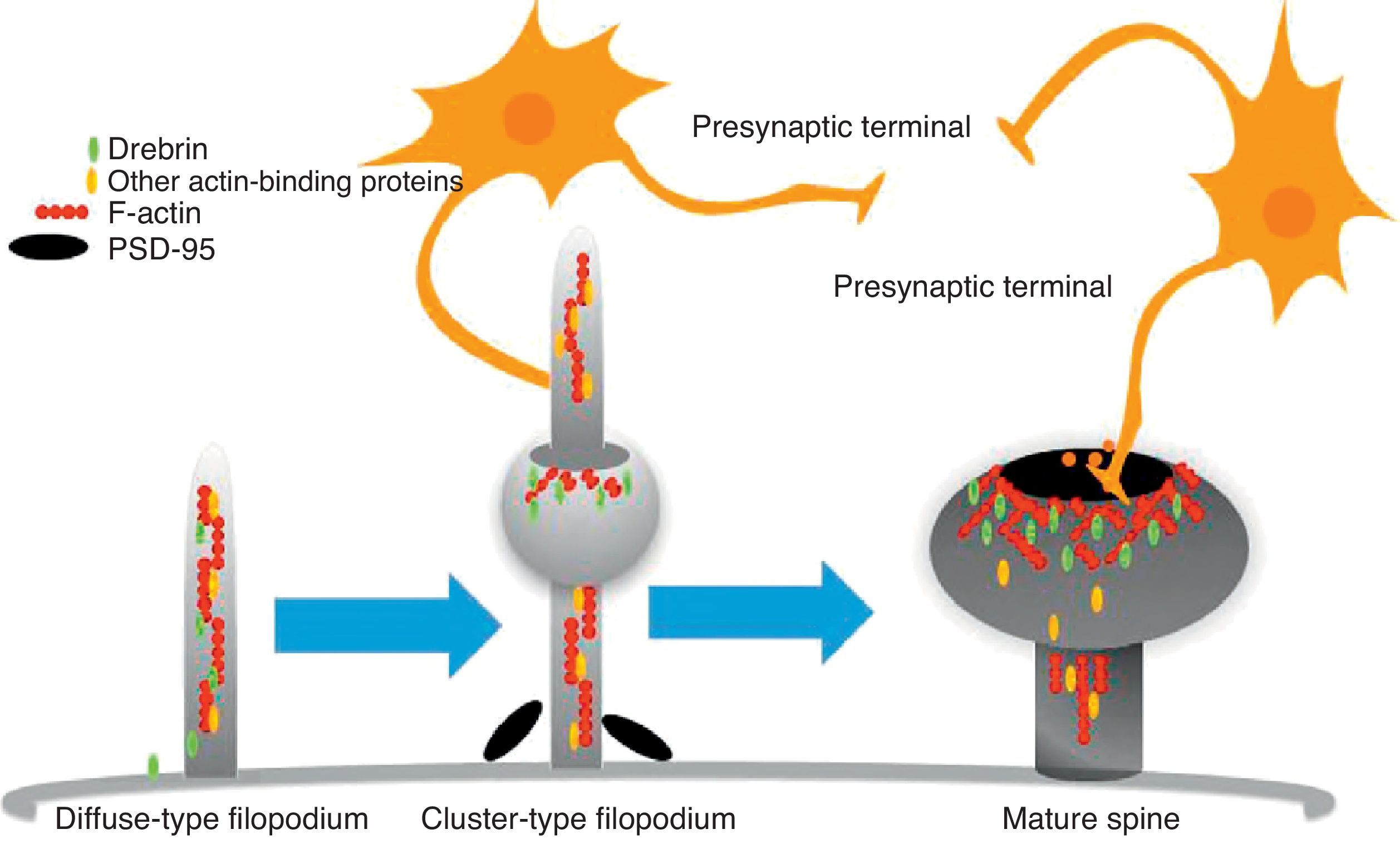
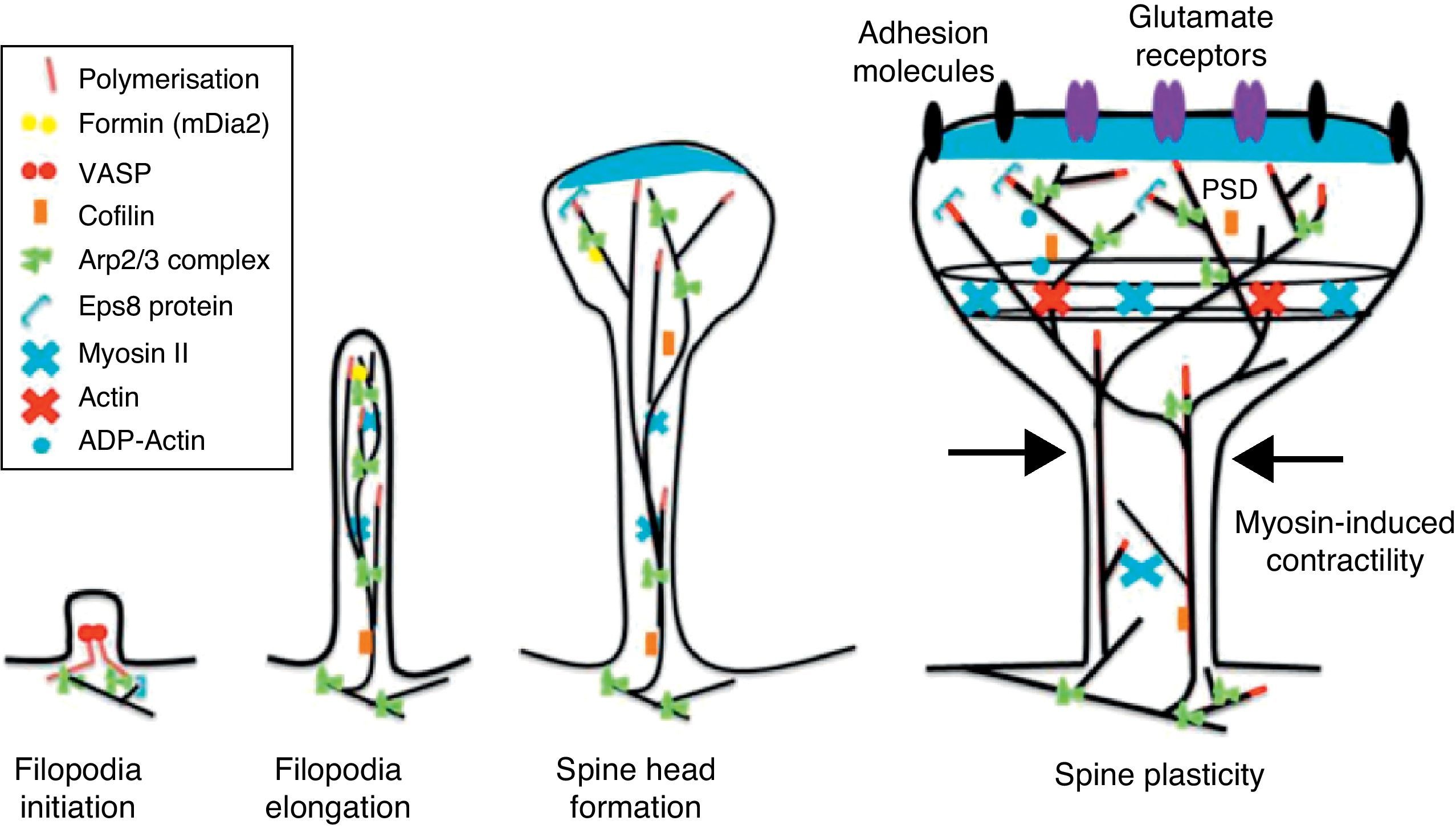
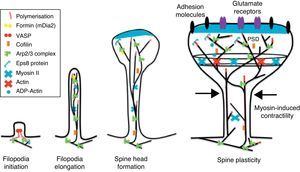
The motility of filopodia and spines depends on the presence of F-actin in the spine cytoskeleton. Based on the above, it has been suggested that the transition from a microspike to a mature spine would involve actin regulation.22 Although filopodia disappear as spines mature, researchers have not observed any type of proportional correlation between the numbers of filopodia retracted and the number of spines that form, and this lack of correlation has come to be widely accepted. Researchers have also reported that filopodia may grow or retract directly by means of synaptic contact at any point along the extension of the structure. It is hypothesised that filopodia may constitute anatomical guides used in spine construction23 (Fig. 3).
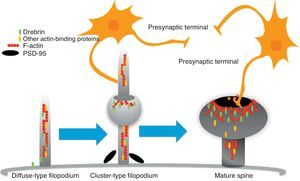
Diagram of the formation of a dendritic spine. Dendrites are bristled with many filopodia on which drebrin is distributed. Once an axon terminal establishes contact with a filopodium, a drebrin–actin complex forms in a postsynaptic contact site. The drebrin–actin complex is essential for the maturation of dendritic spines.
Since filopodia and spines are both small projections (0.5–8μm) along the shaft of the dendrite,24 confusion between the two structures can sometimes arise. The term ‘filopodia’ is therefore used to indicate small, fine, headless projections, while ‘dendritic spine’ refers to all other protuberances found along the dendritic shaft. It is generally believed that filopodia and dendritic spines are both intrinsically different from other types of projections, and that the role of dendritic filopodia is to capture axons and establish early synapses, rather than evolve into spines (Fig. 1).
Actin-binding proteins and dendritic spinesDendritic spines play host to various different identified actin-binding proteins, such as Arp2/3, cortactin, actin-depolymerising factor (ADF)/cofilin, profilin, gelsolin, drebrin, and neurabin.25 These proteins play an essential role in the regulating F-actin organisation. One of the proteins that regulate the length of F-actin by cutting filaments is calcium-dependent gelsolin. This protein is vital to F-actin assembly and disassembly. F-actin breakage is the first direct step in shortening actin filaments. This protein's activity is increased by activation of the NMDA receptor (N-methyl-d-aspartate) and the resulting influx of calcium ions (Ca2+).26
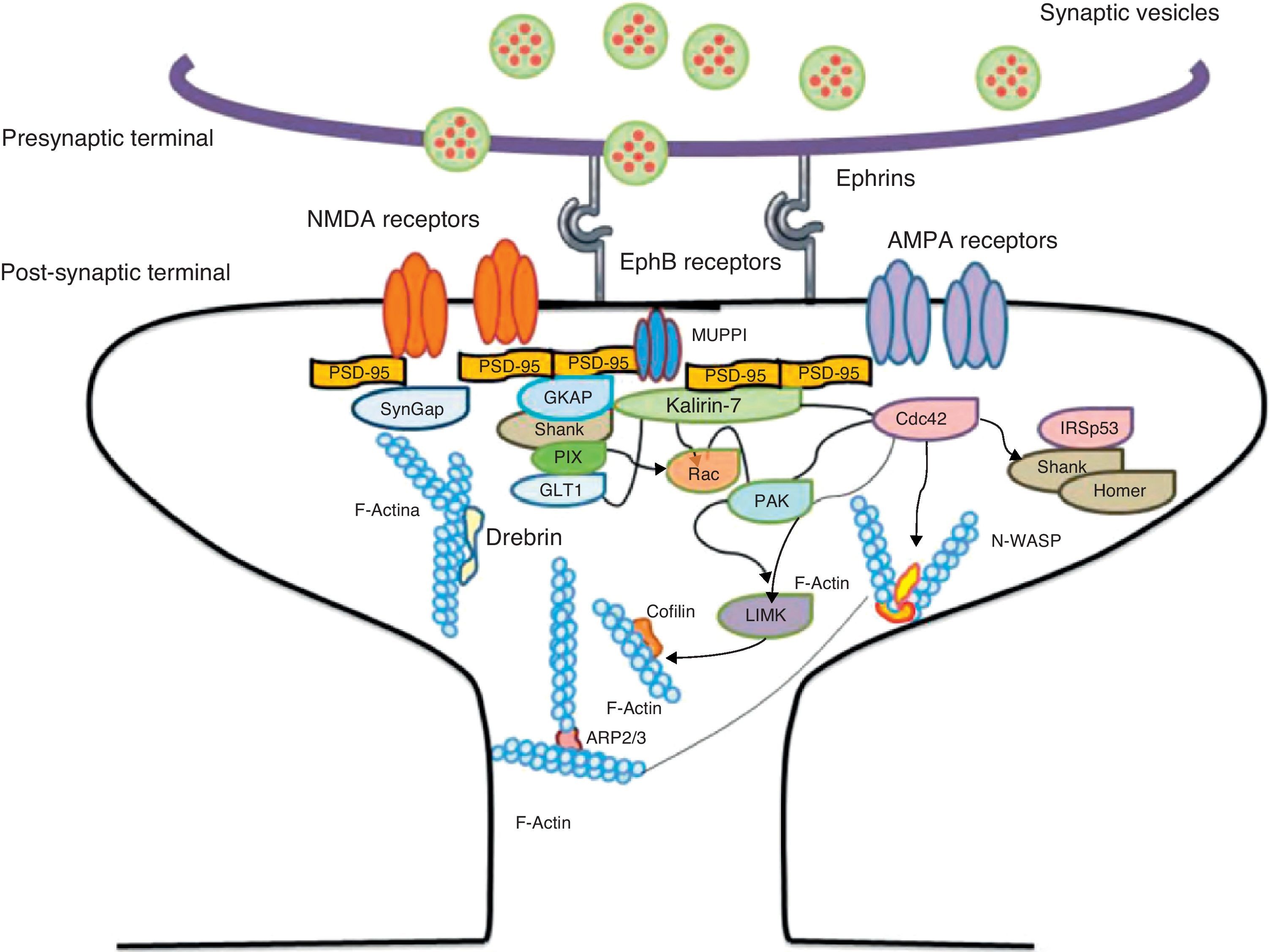
Another of the proteins that bind to F-actin is ADF/cofilin, which works with profilin and gelsolin to depolymerise F-actin and regulate its length. Cofilin is a member of the ADF/cofilin family and its amino acid sequence is 70% identical to that of ADF. This protein depolymerises the negative end of the microfilaments and inhibits reassembly, thereby creating more positive ends in the filament fragments. Cofilin/ADF performs the function of cutting actin filaments. The resulting monomers may be recycled by profilin, another protein associated with actin that facilitates the transformation of adenosine diphosphate (ADP) to ATP in actin monomers, thereby promoting polymerisation.27 Profilin is a multifunctional actin-binding protein involved in balancing the G-actin/F-actin ratio. In particular, it increases the exchange of ADP for actin-binding ATP, which promotes F-actin polymerisation.28 The profilin-controlled exchange of G-actin inhibits F-actin polymerisation. On the other hand, studies in cultured hippocampus neurons have shown that profilin II is responsible for stabilising the morphology of dendritic spines. Furthermore, profilin II may regulate the organisation of F-actin by binding small RhoA GTPases.29 In turn, the Arp2/3 complex induces F-actin branching and interlacing. Therefore, emerging strands of F-actin whose ends are covered by the Arp2/3 complex are involved in the formation of dendritic spines (Fig. 4).30
Diagram of the Rac and Cdc42 signalling cascade activated by EphB receptors via kalirin-7. Cdc42 may promote the polymerisation of actin by means of IRSp53 complex (found in spines and known as an actin cytoskeleton regulator). Activation of the pathways begins the assembly and grouping of F-actin that is needed for filopodia to form.
Drebrin is one of the most extensively studied proteins due to its marked expression in both spines and filopodia, its close relationship with F-actin, and its participation in dendritic spine morphogenesis.31 It is an actin-binding protein, and expressed by most neurons. A number of studies have shown that it is most commonly expressed in postsynaptic contact sites on dendrites. It was initially described as a brain regulator protein with a maximum expression occurring during embryogenesis and decreasing in adulthood.31 We distinguish between 2 isoforms: drebrin E during the embryonic phase, and drebrin A during the adult phase. The latter is abundantly expressed in the anterior cerebrum, cerebral cortex, and hippocampus. Expression is low in the cerebellar cortex, medulla oblongata and spinal cord. Expression can be detected at the fourth week after birth and, and a low level of expression remains during the first 10 weeks after birth.17 This suggests that drebrin isoforms convert rapidly during the formation of spines and synaptic contacts. Drebrin A is responsible for regulating the phases of maturity of proto-spines once their morphology has been established.31 In mature neurons, drebrin A is predominantly located in dendritic spines, the soma, and the dendritic shaft. It is never found in presynaptic terminals.17,31 One in vivo immunoelectron microscope study revealed that low levels of drebrin may be detected in approximately 70% of the spines. However, acute inhibition of NMDA receptors significantly increases the number of drebrin-positive spines, which leads us to suggest that drebrin A may be regulated by NMDA receptor activity.32 It has also been shown that drebrin A accumulates in the submembranous region of the axon-dendrite contact site. It therefore may be involved in the first step in drebrin binding to F-actin (DBF-actin) to form filopodia.
Signalling pathways and the actin cytoskeletonWe know that intracellular signalling pathways related to the morphology of dendritic spines are mainly regulated by the glutamate receptors NMDA and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid). NMDA receptors regulate signalling pathways in the actin cytoskeleton by the following mechanisms: (a) the regulation of the calcium ion flux towards post-synaptic neurons, modulating the activity of some actin-binding proteins, such as CaMKIIβ and gelsolin, and (b) by binding directly to actin or regulating proteins such as CaMKII, α-actinin.33 Additionally, several receptors of tyrosine kinase, such as members of the Trk family (BDNF receptor) and the EphB (ephrin B) family, as well as adhesion molecules, have shown themselves to be important in the actin regulation in dendritic spines.34
EphB receptorsEphB receptors are a family of intercellular adhesion molecules that control processes related to neural development, such as synapse formation and maturation, and structural and functional synaptic plasticity. EphB receptors are bound to protein kinases located in the post-synaptic terminal; receptors are activated by ephrin located in the axon.35 This group of molecules was initially described in the embryonic nervous system. Molecules are constituted by a group of Eph receptors and their ligands, called ephrins; both are bound to the extracellular membrane (Fig. 4). It is claimed that signalling mediated by these receptors is essential for the formation of spines in the hippocampus.36 Experiments in transgenic mice with a double or triple mutation for EphB1, EphB2, or EphB2 receptors have shown that when these 3 receptors are absent, spine formation fails in hippocampus cells (in a cell culture). Furthermore, in vivo experiments provide evidence that spine density decreases by nearly 25% under these conditions, but that dendritic branching is normal and spine head size decreases. Lastly, the studies state that polymerisation of F-actin was not modified in either in vivo or in vitro experiments.36
GTPases and Rho GTPasesGTPases (guanosine triphosphate) are a family of enzymes with more than 100 structurally related proteins. They regulate a wide range of biological functions. They are hydrolase-type enzymes capable of binding to and hydrolysing guanosine triphosphate molecules (GTP). All GTPases have a common mechanism that enables them to activate or deactivate signal transduction by means of a one-way change from active GTPase to the inactive form GDP (guanosine diphosphate). This change is brought about by GTP hydrolysis through the intrinsic activity of GTPase, which is what deactivates the signalling cascade. This reaction is initiated by specific proteins known as GTPase activator proteins (GAPs). GTPase deactivation can be cancelled by the action of GEFs (guanine nucleotide exchange factor), which facilitates replacement of GTP by GDP.37 Proteins such as Rac, Cdc42, Rnd1, and RhoA belong to the Rho GTPase family and are reported to be important actin cytoskeleton regulators. They are involved in spine morphogenesis and also have the function of promoting dendritic spine growth and stabilisation. When RhoA is expressed in high concentrations, it can inhibit or contract spines until they are completely eliminated.38 On the other hand, Cdc42 is involved in F-actin polymerisation. This process is necessary in order for a microspike to become long enough to form a mature spine. Furthermore, Cdc42 together with Rac causes the re-ordering of actin fibres in the heads of spines38 (Fig. 4).
Rnd1 may regulate dendritic spines in the neurons of the hippocampus. Its expression increases during the period of synaptogenesis and causes elongation of the spines since decreases in Rnd 1 expression have been correlated to a slight decrease in spine density.39 Different GEFs and the activation of GTPase-type proteins seem to regulate Rho GTPase function.
Activation of RhoA in dendritic spines has been shown to be necessary if LTP is to be expressed through cofilin inactivation.40 In fibroblasts, Cdc42 induces filopodia formation; in dendritic spines, it induces growth of the head of the spine.38 RhoA is important for inhibiting cofilin activity, which results in stabilisation of actin filaments and the dendritic spine. Rac and Cdc4d regulate formation of the head, primarily by activating the Arp2/3 complex and inhibiting actin depolymerisation via cofilin. We should note that the Rho GTPase family Rif (Rho in filopodia) is an important regulator of filopodia formation.41
Kalirin-7Kalirin-7 is a protein belonging to the GEF family of Rho GTPases, and the most common in adult brains. It regulates the morphogenesis of pyramidal neurons and participates in structural plasticity, which is crucial for learning and memory.42 It works as a regulation centre for multiple signalling pathways that control numerous processes having to do with plasticity in the spines. It is considered a GEF for Rac, Cdc42, and Rnd1. It is expressed in dendritic spines and causes them to enlarge and take on a wide variety of shapes. Furthermore, it can perform the single function of propagating signals from the receptors to the synaptic membrane, since each molecule of kalirin-7 activates several Rac1 molecules, and this is an important step in the signalling cascade. It also regulates spine structure and the synaptic expression of AMPA receptors.42 Additionally, we know that kalirin-7 is rapidly phosphorylated by CaMKII proteins in response to NMDA receptor stimulation, which is an essential mechanism for elongating and maintaining spines.43 Kalirin may also work through the cytoskeleton to regulate the anchoring of AMPA receptors to the actin cytoskeleton. This is possible since kalirin-7 signalling affects the target of those receptors through interactions with transmembrane AMPA receptor regulatory proteins (TARP), which are essential for anchoring AMPA receptors. All of these factors indicate that kalirin-7 may play a crucial role in transmitting signals from NMDA receptors to the actin cytoskeleton, and also in AMPA transport, which is the fundamental basis for synaptic plasticity on both the structural and functional levels44 (Fig. 4). It has been reported that EphB activation catalyses phosphorylation of tyrosine kinases of kalirin-7. That GEF comes into contact with Rac1 GDP, and the latter catalyses the transformation of GDP to GTP when Rac1 is activated. Kalirin-7 is localised to the PSD, where it may interact with different proteins, including PSD-95.45 Specifically, researchers have reported that Rac1 activation by kalirin-7 is mediated by the activation of EphB receptors, which is necessary for spine formation in cultured hippocampus neurons. They also reported that the overexpression of kalirin-7 blocks the effects of EphB ligands, thereby inhibiting spine formation.42
Kalirin-7 may be associated with N-cadherin proteins through interactions with the PDZ domain of AF-6/afadin. AF-6 is a binding protein regulated by GTPase-Rap. Binding by these proteins induces spine growth, which involves Rac1 and PAK and grants the molecules the ability to regulate post-synaptic actin reorganisation. This channel can therefore serve as the basis for rapid coordination of the synapse during synaptic maturation and plasticity. It can also guarantee spine stability in mature neurons.46
Kalirin-7 interacts with various PSD proteins, such as PSD-95, SAP101, and SAP97, which either transport or assemble glutamate receptors.45 Studies have reported that kalirin-7 may be regulated by Arf6 GTPase, and that it works in conjunction with the EFA6 activator (Arf6 change factor involved in membrane recycling and cytoskeleton remodelling) to regulate the transition from filopodia to mature, stable spines.47
Another GEF involved in the morphogenesis of dendritic spines is PIX. Through another channel, PIX is also able to activate Rac and Cdc42.48 Cdc42 is one of the main signalling channels promoting branching by dendritic spine heads through the activation of N-WASP (neuronal Wiskott-Aldrich syndrome protein). Actin polymerisation is mediated by the Arp2/3 complex. Studies describe 2 families of proteins that activate N-WASP. The activation of Arp2/3 polymerises actin and causes extension of dendritic spine heads49 (Fig. 4). Ras is another protein in the GTPase family that may also activate certain Rho and Ras GTPase activators (GEF) and inhibitors (GAP), which are proven to be fundamental for neuron morphology and growth.50 An important postsynaptic inhibitor of Ras signalling is synaptic Ras GAP (SynGAP), located in the PSD. The elimination of SynGAP in mice has been shown to increase the number of mushroom heads on spines in hippocampus neurons.51 Various mutations in the family of Rho and Ras GEFs have been linked to mental retardation.52
Rap GTPases are closely linked to Ras, but their role seems to be in opposition to synaptic plasticity. Ras and Rap act as antagonists with respect to postsynaptic action to regulate spine morphology.53
Initiation and elongation of dendritic filopodiaThe molecular mechanisms involved in initiating dendritic filopodia are not well known. Filopodia begin forming along the dendritic stalk, often from small collections of branched actin or lamelipodia.20 Researchers have suggested that filopodia begin forming at random locations in a process induced by a signal. Studies show that glutamate released by the presynaptic terminal has an effect on filopodia formation and elongation.54 Certain mechanisms potentially involved in the formation and elongation process have been described. (a) The Ena/VASP protein binds filaments generated by the Arp2/3 complex, which may initiate the filament lengthening process. (b) A protein called formin DRF3/mDia2 is important for proper filopodia formation. Other proteins in the formin family may also participate in the polymerisation of actin filaments and the initiation and elongation of filopodia. (c) One hypothesis is that myosin may initiate filopodia formation and that lateral movement of the myosin in the ends of the actin filaments creates the base of the filopodium. (d) F-actin is often localised in microtubules in the dendritic stalk. In this way, groups of F-actin associated with microtubules initiate the filopodium. (e) Microtubules and F-actin bound to septin-7 polymerise heterotrimetric filaments and form small ring-shaped structures. They can therefore initiate filopodia formation and act as a diffusion barrier during the maturation of dendritic spines. (f) Lastly, filopodia may begin to form as a result of deformation of both the membrane and protein domains known as I-BAR (inverse-bin Amphiphysin/Rvs). Electrostatic interactions between the positively charged ends of the I-BAR domains and negatively charged hydrophobic phosphatidyl-ionositol-4-biphosphate [PI (4,5) P2] groups cause curvature of the membrane due to the convex shape of the lipid-binding interface. In cultured neurons, suppression of I-BAR proteins has been shown to affect dendritic spine morphogenesis21 (Fig. 5).
Actin regulatory mechanisms during dendritic spine development.
Once the dendritic filopodium has been formed and axonal contact has been made, motility gradually decreases and the structure of the dendritic spine becomes stable.35 Recently formed spines are usually thin and long, with small heads. Arp2/3 complex is necessary for spine head growth. Alternatively, microtubules in dendritic spines may cause activation of the Arp2/3 complex. Microtubules have been shown to be associated with temporary morphological changes related to spine head formation and growth of the dendritic spine.55 Microtubules may spark a signalling cascade affecting the actin dynamic through the EB3 protein.55 A proteomics study for EB binding patterns in hippocampus neurons showed that p140Cap is a protein that interacts with SNAP-25 (PSD protein abundant in spines)55 and an important regulator of Src tyrosine kinases. The factor known as p140Cap is also able to interact with a protein named cortactin. At a given time, this protein may activate the Arp2/3 complex and stabilise and promote mushroom-type spine heads.55 It is likely that the association between EB3 molecules linked to the ends of microtubules and p140Cap is what controls Src kinase activity and regulates the function of cortactin, which may trigger activation of the Arp2/3 complex and growth of the head of the spine. Here, microtubules in mature neurons may work as a local signal in the reorganisation of the actin cytoskeleton and regulate the size of the head of the spine.
In turn, the action of myosin II modifies the size and shape of spines, which is an important process in dendritic spine morphogenesis. On the other hand, it has been shown that actin, together with proteins such as CaMKIIβ, neurabin, and drebrin A, participates in modifying and stabilising the heads of dendritic spines.17 Cofilin activity is important for spine morphology and stabilisation.41 In addition, studies state that synaptic plasticity is associated with rapid, persistent reorganisation of the actin cytoskeleton within the spine.
Dendritic spines and changes in brain functionDendritic spines may respond morphologically to a large variety of physiological stimuli. Responses to different conditions of stimulation or damage range from increases or decreases in the total number of spines, and spine redistribution along progenitor dendrites, to variations in the spine size or shape. Studies report that when a neural pathway is partially denervated, branches of remaining axons may undergo multiplication. This phenomenon is called axonal regrowth.56 We therefore observe that dendritic length and degree of branching, plus the density, shape, and distribution of dendritic spines, seem to be influenced by a wide variety of environmental factors such as malnutrition, sensory deprivation, stress, denervation, learning processes, and others.57
Researchers have demonstrated that inducing a pharmacological lesion in the rat dorsal raphe nucleus (DRN) with 5,7-dihydroxytryptamine (5,7-DHT) decreases serotonergic fibre content in the prefrontal cerebral cortex58 and modifies the cytoarchitecture of pyramidal cells in that region. Such lesions cause correlative changes in the completion of tasks requiring retrieval of short-term memory, plus increases in the number and shape of dendritic spines,59 as well as an increase in the number of 5-HT2A serotonin receptors and increased expression of drebrin.58,60 Based on the above, it is hypothesised that the activation of different receptors could induce mobility of cytoskeleton-binding proteins such as drebrin and modulate the morphological changes mentioned above as part of the plastic response initiated by the CNS when faced with a DRN lesion. However, we must not rule out participation by other cytoskeleton-binding proteins or other intracellular mechanisms.
On the other hand, some reports in the literature link morphological changes in dendritic spines with certain psychiatric and neurological disorders.61 For example, we know that mutations in the Shank3 synaptic protein and in adhesion molecules such as neuroligin3 and neuroligin4 are related to autism.62 Another study suggests that numerous memory disorders are directly related to deficiencies in actin cytoskeleton regulation.53 Meanwhile, decreases in PAK3 levels have been linked to alterations in synaptic transmission in Alzheimer disease. Experiments in mice have shown that PAK3 inhibition provokes memory loss. This has been correlated with PAK being a probable and potential participant in memory loss in Alzheimer disease.63 Further genetic studies will identify new mutations that may affect the reordering of the actin cytoskeleton of dendritic spines. In addition, cell-level study of the biological mechanisms that underlie dendritic spine development will deliver a better understanding of synaptic plasticity, brain function, and neurological diseases.
ConclusionsNumerous molecules and signalling pathways are involved in dendritic spine morphogenesis, possibly because dendritic spines are the main sites for excitatory synapses. Researchers have described several mechanisms affecting the development, maintenance, and plasticity of excitatory synapses, which in turn may affect the number, size, and shape of dendritic spines. On the other hand, the use of in vivo and in vitro experimental models designed to determine the function of the cytoskeleton and its relationship with dendritic spines has delivered important information about the molecular mechanisms underlying plastic changes linked to dendritic spine morphogenesis. Given the relationship between brain dysfunction and dendritic spine abnormalities, it is believed that spine shape is important to our understanding of synaptic function and, by extension, the higher cerebral functions. We know that changes in spine morphology are instant and caused by the dynamics of the dendritic spine's actin cytoskeleton. Furthermore, studies have shown that actin-binding proteins, such as drebrin A, are responsible for F-actin and PSD-95 formation in filopodia, which leads to the formation of dendritic spines. Since drebrin is one of the first actin-binding proteins, it controls the grouping of other F-actin binding proteins. We therefore find studies that identify actin-binding proteins as the agents responsible for regulating morphological changes in dendritic spines. We should stress that the precise function of some actin-regulating proteins is unclear at present. A detailed study of actin-binding proteins and the interaction of signalling pathways in dendritic spines will deliver information crucial to our understanding of the mechanism regulating F-actin organisation and its association with cognitive functions (learning and memory). On the other hand, use of molecular techniques in both in vivo and in vitro cerebral plasticity models will let us identify factors regulating dendritic spine growth and density in the future. Such techniques will also enable us to observe plastic phenomena used by the brain for the formation of new neural connections under both normal and pathological conditions. This is particularly important considering that abnormalities in dendritic spine morphology have been associated with a number of neurodegenerative diseases and developmental delay syndromes.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study was elaborated thanks to a grant for project PS-2009-610 awarded by Consejo Estatal de Ciencia y Tecnología de Estado de Jalisco (Science and Technology Board of the State of Jalisco, Mexico) to the University of Guadalajara (COECyTJal-UdG) and the lead author.
Please cite this article as: Soria Fregozo C, Pérez Vega MI. Participación de las proteínas de unión a la actina y vías de señalización asociadas a la formación y mantenimiento de las espinas dendríticas. Neurología. 2012;27:421–31.