Cervicogenic dizziness is a musculoskeletal disorder mainly characterised by dizziness and disequilibrium associated with neck pain. The pathophysiology is unclear and the neurophysiological basis remains to be ascertained. The aim of this study is to compare the vestibulo-ocular reflex and postural control between patients with cervicogenic dizziness and asymptomatic subjects, and to assess the association between debilitating dizziness and other psychosocial variables.
Materials and methodsA total of 20 patients and 22 asymptomatic subjects were selected. Vestibulo-ocular reflex was assessed by performing the head impulse test. Computerised dynamic posturography was used to evaluate the postural control by means of the sensory organisation test. In addition, subjects self-reported their degree of disability due to dizziness, cervical disability, kinesiophobia, and state of anxiety and depression.
ResultsThere were no differences in the vestibulo-ocular reflex (P>.05). However, we found differences with a medium-to-large effect size (d>0.60) in variables related to proprioception and visual information integration; the former variable set was related to disability due to dizziness. Disability due to dizziness presents strong-to-moderate associations with cervical disability, kinesiophobia, and anxiety.
ConclusionOur data rule out changes in the vestibular system in cervicogenic dizziness, but they do point to proprioceptive impairment. According to our results, the association between dizziness-related disability and other psychosocial factors in cervicogenic dizziness is very relevant for clinical medicine and for future research projects.
El mareo cervicogénico es una afección que se caracteriza por mareos y desequilibrio que se asocia a dolor de cuello. La fisiopatología no está clara, y es necesario conocer la base neurofisiológica del trastorno. El objetivo de estudio es comparar la actividad del reflejo vestíbulo-ocular y el control postural entre pacientes que presentan mareo cervicogénico y sujetos asintomáticos; además, se pretende evaluar la asociación entre la discapacidad por mareo con otras variables psicosociales.
Material y métodosSe seleccionaron un total de 20 pacientes y 22 sujetos asintomáticos, a los que se realizó una valoración del reflejo vestíbulo-ocular con el test del impulso cefálico y una valoración del control postural mediante posturografía dinámica y el test de organización sensorial, además se evaluaron mediante autoinforme la discapacidad por mareo, la discapacidad cervical, el miedo al movimiento y el estado de ansiedad y depresión.
ResultadosNo se encontraron diferencias en la actividad del reflejo vestíbulo-ocular (p>0,05); a nivel del control postural se encontraron diferencias con un tamaño del efecto mediano-grande (d>0,60) en variables relacionadas con la propiocepción e integración de la información visual, asociándose esta variable a la discapacidad por mareo. La discapacidad por mareo presentó asociaciones moderadas-fuertes con la discapacidad cervical, el miedo al movimiento y la ansiedad.
ConclusiónLos resultados obtenidos descartan una alteración del sistema vestibular en el mareo cervicogénico, aunque sí se comprueba la existencia de una alteración propioceptiva. La asociación de la discapacidad por mareo con otras variables psicosociales a la vista de nuestros resultados debe tomarse en cuenta en la clínica y en futuras investigaciones.
Cervicogenic dizziness is a disorder characterised by dizziness and imbalance associated with neck pain1 and rigidity. The condition is aggravated by movements or particular positions of the neck.1–3 Some authors suggest that cervicogenic dizziness is a common symptom in patients with degenerative cervical spine disorders, trauma (e.g. whiplash injury),1,4,5 or idiopathic neck pain.6 Cervicogenic dizziness has been observed in 25% to 50% of patients with whiplash injury.7
Basic research has shown that the upper cervical region contains large numbers of muscle spindles and more connections with the visual and vestibular systems, and contributes to reflex activity to a greater extent than other regions of the cervical spine.8,9 It has also been suggested that proprioceptive alterations in neck muscles may cause asymmetrical functioning of the vestibulo-ocular reflex (VOR), which may in part explain the pathogenesis of cervicogenic dizziness.10
Some studies of patients with traumatic neck pain have found hyperactive VOR and low head movement velocity in tasks requiring gaze stability and head-eye coordination.11,12 In a recently published study, L’Heureux-Lebeau et al.13 compared sensorimotor variables in patients with cervicogenic dizziness and patients diagnosed with benign paroxysmal positional vertigo using a range of clinical tests. According to their results, patients with cervicogenic dizziness were more likely to experience a subjective feeling of drunkenness and lightheadedness, alterations in cervical proprioception, and pain induced during the physical examination of the upper cervical vertebrae and paravertebral muscles.
Despite mounting evidence that cervicogenic dizziness is a distinct clinical entity, the diagnosis and definition of the condition are debated, with supporters and detractors in the clinical and research fields.14,15 It should be noted that some studies have found no significant neuro-otological differences between patients with cervicogenic dizziness and asymptomatic patients.16 Furthermore, there is no expert consensus on the most suitable test battery to determine cervical dysfunction in patients with dizziness.17 We therefore deem it necessary to gain deeper understanding of the role of the vestibular system (and more specifically the activity of the vestibulo-ocular system), as well as postural control and sensorimotor integration according to evaluation with computerised dynamic posturography.
The main purpose of this study is to compare VOR activity and postural control in patients with cervicogenic dizziness and asymptomatic individuals. As a secondary objective, we evaluated the association between disability due to subjective feelings of dizziness, neck disability, fear of movement, and anxiety and depression, and the association between these variables and those related to the VOR and postural control.
Material and methodsStudy designWe conducted a cross-sectional observational study including 2 groups of patients drawn by non-probability sampling. The first group included patients with cervicogenic dizziness and met the inclusion criteria, whereas the second group included asymptomatic individuals (controls).
Our study complies with the ethical standards of the Declaration of Helsinki and was approved by the ethics committee of the Centre for Advanced Studies at Universidad La Salle, in Madrid, Spain. The study was conducted at the La Salle Functional Rehabilitation Institute. All participants received detailed information about the study and signed informed consent forms prior to inclusion. The study methodology complies with the recommendations for observational studies established by the STROBE statement.18
Participant selectionParticipants were consecutively selected from the local population by non-probability sampling using leaflets, posters, and social media. Both groups were equivalent in terms of the main demographic data. Data were gathered between January 2015 and June 2015.
The patient group included individuals meeting the following inclusion criteria: (1) presence of neck pain according to the visual analogue scale for pain; (2) presence of neck pain associated with disability according to the Neck Disability Index (NDI); (3) presence of a subjective feeling of dizziness associated with pain, movement, rigidity, or certain positions of the neck; (4) presence of neck pain and dizziness for more than 3 months; and (5) age between 18 and 65 years. The exclusion criteria were presence of trauma or recent surgery in the head, face, neck, or chest; an otorhinolaryngological diagnosis of central or peripheral vertigo, and receiving physiotherapy during the study period.
Asymptomatic individuals were examined and included in the study if they met the following criteria: (1) experiencing no pain or dizziness over the past 6 months, and (2) being 18 to 65 years old. The following exclusion criteria were common to both groups: (1) presence of cognitive disabilities that may make it difficult for the patient to understand audiovisual material; (2) illiteracy; (3) difficulty understanding or communicating; and (4) insufficient knowledge of Spanish to follow instructions.
ProcedureAfter signing the informed consent forms, participants completed a sociodemographic questionnaire gathering data on sex, birth date, education level, and height. Participants subsequently used a series of self-reported measures to give a physical, functional, and emotional assessment of pain and neck disability, fear of movement, anxiety and depression, and the physical, functional and emotional aspects of a subjective feeling of dizziness. Lastly, all participants underwent a neurological examination using a battery of tests; VOR activity was evaluated using the head impulse test, and postural control and balance with dynamic posturography. These tests were used to detect any potential proprioceptive changes and to determine the role of the visual system in balance.
Primary variablesVOR activity was assessed using the head impulse test or the video head impulse test (vHIT). This test is used in clinical practice to evaluate vestibular function and more specifically dynamic function of the semicircular canals.19 This test makes use of a pair of video-oculography goggles (GN Otometrics, Denmark) containing a high-speed camera which records compensatory eye movements during head impulse. The instrument also includes 2 gyroscopes and an accelerometer.
During the test, the examiner firmly holds the patient's head by the temporal and parietal regions and rotates it from right to left at least 20 times. VOR gain values and presence of refixation saccades are evaluated. According to previous studies, gain values below 0.6 are considered abnormal,20 whereas normal gain values are those approaching 1.19,21 The vHIT has been found to be valid compared to other vestibular tests and shows high reliability, sensitivity, and specificity.20,22
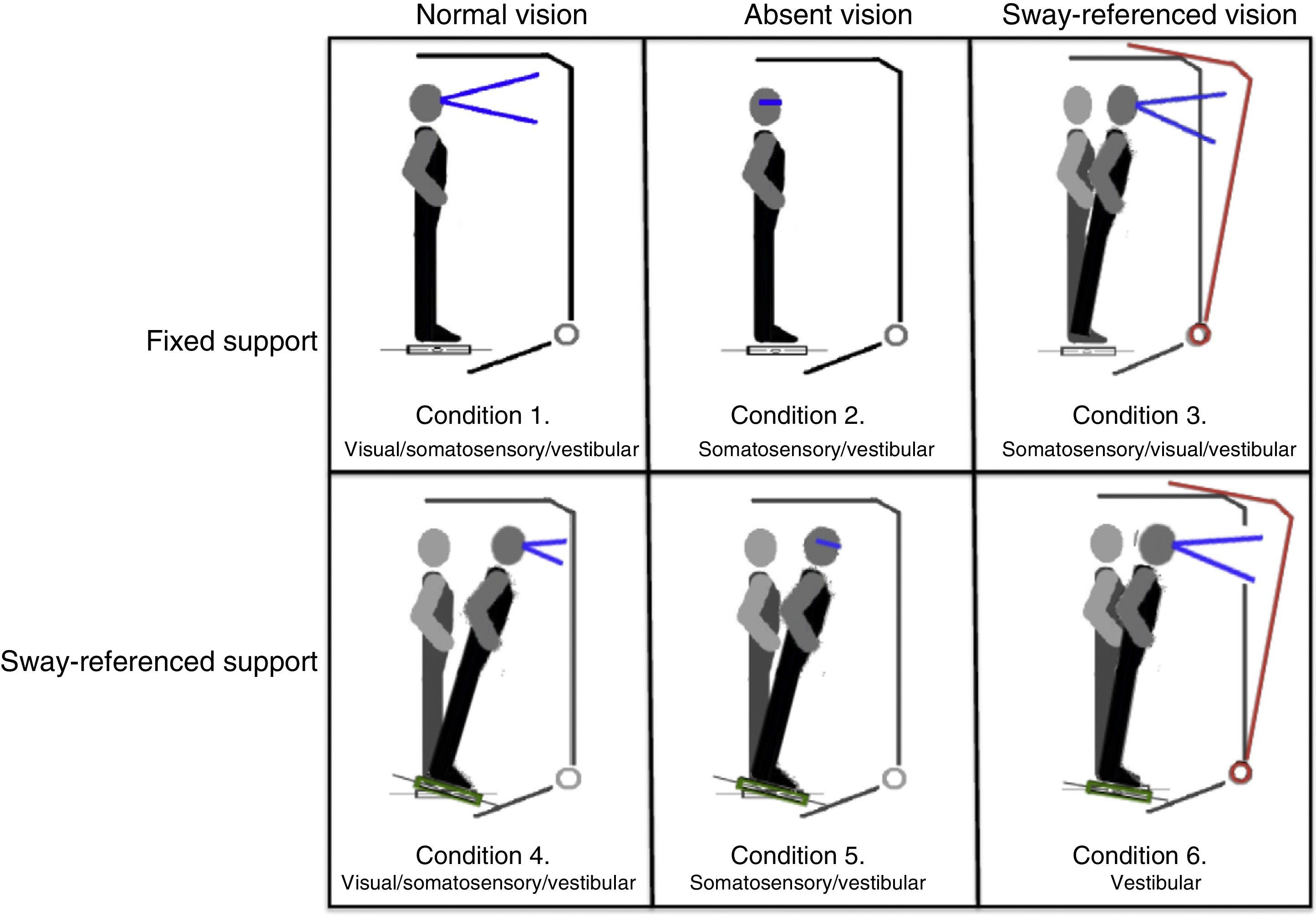
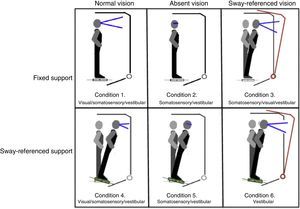
Postural control. Postural control was evaluated using computerised dynamic posturography, more specifically the Sensory Organisation Test (SOT). Tests were performed using the NeuroCom® SMART EquiTest® system, version 8.0 (NeuroCom International, Inc.; Clackamas, USA). The SOT assesses 6 conditions (Fig. 1); each condition is tested 3 times. The SOT result is calculated using the 6 conditions and the 4 ratios of sensory analysis (somatosensory, visual, vestibular, and visual preference) (Table 1).23 SOT has been shown to have adequate validity and reliability for assessing both patients with different conditions and asymptomatic individuals.24–27
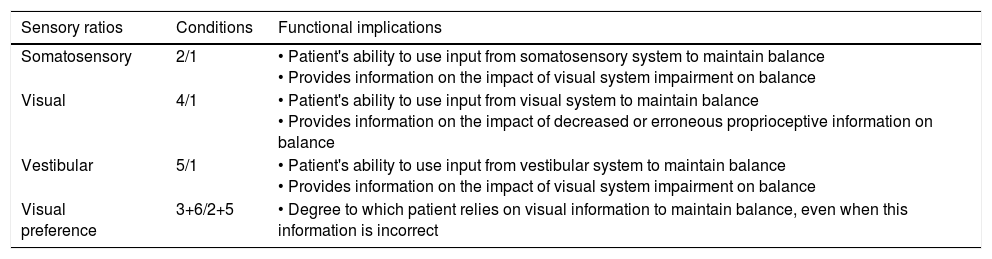
Description of sensory ratios of the Sensory Organisation Test.
| Sensory ratios | Conditions | Functional implications |
|---|---|---|
| Somatosensory | 2/1 | • Patient's ability to use input from somatosensory system to maintain balance • Provides information on the impact of visual system impairment on balance |
| Visual | 4/1 | • Patient's ability to use input from visual system to maintain balance • Provides information on the impact of decreased or erroneous proprioceptive information on balance |
| Vestibular | 5/1 | • Patient's ability to use input from vestibular system to maintain balance • Provides information on the impact of visual system impairment on balance |
| Visual preference | 3+6/2+5 | • Degree to which patient relies on visual information to maintain balance, even when this information is incorrect |
Disability associated with a subjective feeling of dizziness was measured with the Spanish-language version of the Dizziness Handicap Inventory (DHI). This tool quantifies the impact of dizziness on the patient's quality of life by evaluating a series of dimensions. This self-reported measure includes 25 items corresponding to 3 categories (emotional, functional, and physical). The Spanish-language version of the test has been shown to have good psychometric properties.28
Neck disability was assessed with the Spanish-language version of the NDI.29 This tool has a single-factor structure comprising 10 items which assess different activities of daily living on a 6-point Likert-type scale (from 0 to 5). Higher scores indicate more severe pain and greater disability. The Spanish-language version of the NDI has adequate psychometric properties and is suitable both for research purposes and for clinical practice.29
Neck pain intensity was evaluated with the visual analogue scale (VAS) for pain. This reliable tool consists of a 100-mm horizontal line between the extremes ‘no pain’ (left) and ‘worst imaginable pain’ (right).30
Fear of movement was quantified with the Spanish-language version of the Tampa Scale for Kinesiophobia (TSK-11). This instrument has been shown to be reliable and valid for assessing chronic pain.31 As a rule, the TSK-11 has similar psychometric properties to those of the original TSK plus the advantage that it is quick to administer. Total score ranges between 11 and 44 points, with higher scores indicating a higher degree of kinesiophobia.31
Anxiety and depression were evaluated with the Hospital Anxiety and Depression Scale (HADS). This self-administered questionnaire contains 14 items, rated on a 4-point Likert-type scale (from 0 to 3 points). The tool includes 2 subscales of 7 items that assess anxiety and depression. The Spanish-language version of the HADS has good psychometric properties.32
Sample sizeThe sample size was calculated to detect inter-group differences in the results for the primary variables (VOR activity and postural control) with a statistical power of 80% (1−β) for an α error probability of 0.05. We used the t test for independent samples and an effect size of 0.80. We determined to include a total of 42 participants (20 patients with cervicogenic dizziness and 22 asymptomatic individuals). The sample size was calculated using G*Power statistical software version 3.1.7 for Windows (G*Power®; Universität Düsseldorf, Germany).33
Statistical analysisDescriptive statistics were used to analyse continuous variables; these are expressed as means±SD, 95% confidence intervals (CI), and relative frequencies (percentages). The chi-square test was used to compare differences between categorical (nominal) variables. The Shapiro–Wilk test was used to test for normality. All but 2 variables followed a normal distribution; data was therefore not transformed, but rather analysed with parametric tests.
The t test for independent samples was used to compare continuous variables between the 2 groups (primary and secondary variables).
The effect size (Cohen's d) was calculated for the main variables. According to Cohen's method, effect sizes 0.20 to 0.49 were considered small, 0.50 to 0.79 medium, and 0.8 large.34
The association between primary and secondary variables was determined using the Pearson correlation coefficient. Correlation coefficients >0.60 indicate a strong correlation, 0.30 to 0.60 a moderate correlation, and <0.30 a weak correlation.35 The SPSS version 21 (SPSS Inc.; Chicago, IL, USA) software package was used for statistical analysis. Statistical significance was set at P<.05 for all tests.
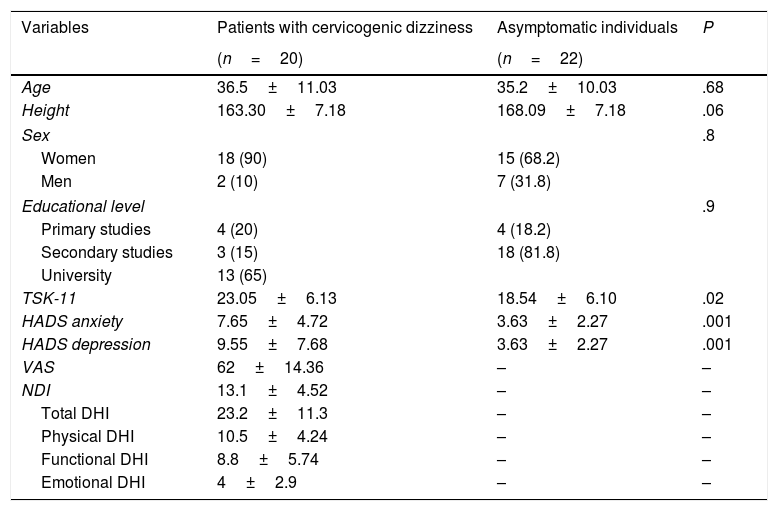
ResultsThe final sample included 42 participants (20 with cervicogenic dizziness and 22 asymptomatic individuals) who met the inclusion criteria. No statistically significant differences were found in sociodemographic variables between groups (P>.05). We did, however, find significant inter-group differences in some clinical characteristics, such as anxiety and depression and fear of movement (P<.05). Descriptive statistics of sociodemographic and clinical characteristics are shown in Table 2. None of the participants reported prescribed use of analgesics or psychoactive drugs.
Descriptive statistics of sociodemographic data and self-reported measures.
| Variables | Patients with cervicogenic dizziness | Asymptomatic individuals | P |
|---|---|---|---|
| (n=20) | (n=22) | ||
| Age | 36.5±11.03 | 35.2±10.03 | .68 |
| Height | 163.30±7.18 | 168.09±7.18 | .06 |
| Sex | .8 | ||
| Women | 18 (90) | 15 (68.2) | |
| Men | 2 (10) | 7 (31.8) | |
| Educational level | .9 | ||
| Primary studies | 4 (20) | 4 (18.2) | |
| Secondary studies | 3 (15) | 18 (81.8) | |
| University | 13 (65) | ||
| TSK-11 | 23.05±6.13 | 18.54±6.10 | .02 |
| HADS anxiety | 7.65±4.72 | 3.63±2.27 | .001 |
| HADS depression | 9.55±7.68 | 3.63±2.27 | .001 |
| VAS | 62±14.36 | – | – |
| NDI | 13.1±4.52 | – | – |
| Total DHI | 23.2±11.3 | – | – |
| Physical DHI | 10.5±4.24 | – | – |
| Functional DHI | 8.8±5.74 | – | – |
| Emotional DHI | 4±2.9 | – | – |
VAS: visual analogue scale; HADS: Hospital Anxiety and Depression Scale; NDI: Neck Disability Index; DHI: Dizziness Handicap Inventory; TSK-11: Tampa Scale for Kinesiophobia.
Values are expressed as means±SD and numbers (%).
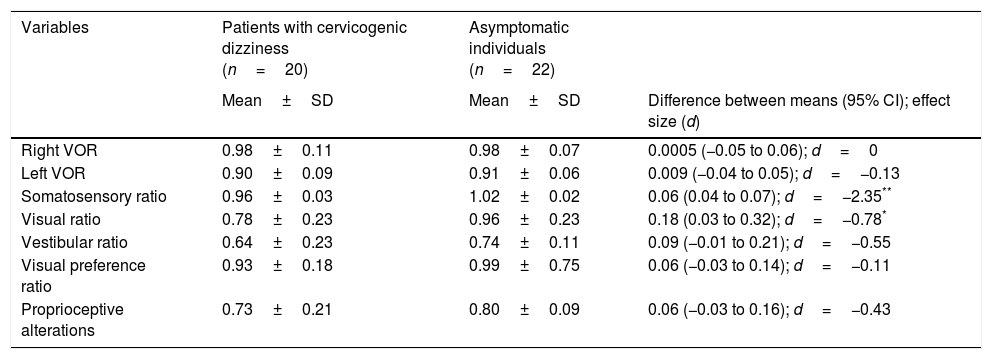
No statistically significant inter-group differences were found in the variables associated with the VOR and with postural control (Table 3), with the exception of the visual (P=.03) and somatosensory (P<.05) ratios, in which a medium-to-large effect was observed (d=0.78 and d=2.35, respectively).
Comparative analysis of the variables associated with the VOR and postural control.
| Variables | Patients with cervicogenic dizziness (n=20) | Asymptomatic individuals (n=22) | |
|---|---|---|---|
| Mean±SD | Mean±SD | Difference between means (95% CI); effect size (d) | |
| Right VOR | 0.98±0.11 | 0.98±0.07 | 0.0005 (−0.05 to 0.06); d=0 |
| Left VOR | 0.90±0.09 | 0.91±0.06 | 0.009 (−0.04 to 0.05); d=−0.13 |
| Somatosensory ratio | 0.96±0.03 | 1.02±0.02 | 0.06 (0.04 to 0.07); d=−2.35** |
| Visual ratio | 0.78±0.23 | 0.96±0.23 | 0.18 (0.03 to 0.32); d=−0.78* |
| Vestibular ratio | 0.64±0.23 | 0.74±0.11 | 0.09 (−0.01 to 0.21); d=−0.55 |
| Visual preference ratio | 0.93±0.18 | 0.99±0.75 | 0.06 (−0.03 to 0.14); d=−0.11 |
| Proprioceptive alterations | 0.73±0.21 | 0.80±0.09 | 0.06 (−0.03 to 0.16); d=−0.43 |
VOR: vestibulo-ocular reflex.
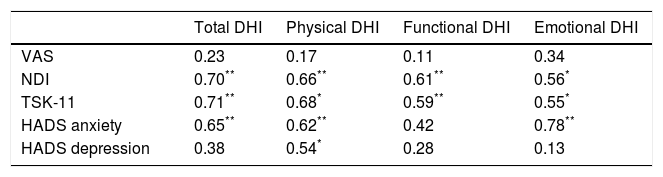
The correlation analysis performed with the Pearson correlation coefficient is shown in Table 4. No statistically significant inter-group differences were found in pain intensity or in the disability-related variables associated with a subjective feeling of dizziness (P<.05). However, a moderate-to-strong association was found between neck disability and fear of movement, and the different factors of disability associated with a subjective feeling of dizziness (Table 4); the strongest associations with these variables were found in total DHI scores (r=0.70, P<.001 and r=0.71, P<.001, respectively) and between emotional DHI scores and anxiety (r=0.78, P<.001).
Study of Pearson correlations between disability due to dizziness and psychosocial variables.
| Total DHI | Physical DHI | Functional DHI | Emotional DHI | |
|---|---|---|---|---|
| VAS | 0.23 | 0.17 | 0.11 | 0.34 |
| NDI | 0.70** | 0.66** | 0.61** | 0.56* |
| TSK-11 | 0.71** | 0.68* | 0.59** | 0.55* |
| HADS anxiety | 0.65** | 0.62** | 0.42 | 0.78** |
| HADS depression | 0.38 | 0.54* | 0.28 | 0.13 |
VAS: visual analogue scale; HADS: Hospital Anxiety and Depression Scale; NDI: Neck Disability Index; DHI: Dizziness Handicap Inventory; TSK-11: Tampa Scale for Kinesiophobia.
In the analysis of correlations between the variables associated with disability and the variables associated with VOR activity and postural control, only 2 moderate negative correlations were found: between physical DHI and the somatosensory ratio (r=−0.47, P<.05) and between emotional DHI and the visual preference ratio (r=−0.54, P<.05).
DiscussionThe purpose of our study was to evaluate the role of the vestibular system and postural control in cervicogenic dizziness and to analyse the association between these variables and perceived disability and fear of movement. Our results do not support the hypothesis that the vestibular system is directly involved in patients’ subjective feeling of dizziness. However, other variables associated with postural control do seem to play a role in cervicogenic dizziness. Furthermore, our results support the correlation between disability associated with a subjective feeling of dizziness, on the one hand, and neck disability, anxiety, and fear of movement, on the other.
Vestibulo-ocular reflex activityTo our knowledge, this is the first study to evaluate VOR activity in patients with cervicogenic dizziness unrelated to head or neck trauma (for example, whiplash injury). Our data reveal no significant differences in VOR activity between asymptomatic individuals and patients with cervicogenic dizziness in the vHIT. It should be noted that this test is highly sensitive in evaluating semicircular canal function and detecting peripheral vestibular dysfunction,36,37 with very similar results to those of the caloric stimulation test.37 In line with our findings, some studies have reported that vestibular dysfunction was absent in patients with cervicogenic dizziness.16 Other studies, in contrast, have reported conflicting results: in one recent study, L’Heureux-Lebeau et al.13 observed cervical sensorimotor alterations and vestibular dysfunction in patients with cervicogenic dizziness using videonystagmography. Likewise, some studies have also reported vestibular alterations in patients with cervicogenic dizziness secondary to whiplash injury.38,39 Nonetheless, it should be noted that a considerable proportion of patients with a history of head or neck trauma meet the diagnostic criteria for benign paroxysmal positional vertigo.40,41 It has also been suggested recently that the pathophysiological mechanism of vertigo secondary to whiplash injury may be associated with cerebrospinal fluid hypovolaemia42 or brainstem alterations.43 From a clinical and research viewpoint, these data suggest that cervicogenic dizziness secondary to whiplash injury is not comparable to that associated with non-specific neck pain, as in our study. Based on our findings, we feel that the term ‘cervicogenic dizziness’ should not be used for patients with non-specific neck pain.
Postural controlWe found statistically significant differences in postural control between patients and controls, with considerable changes in effect size (d>0.60) for the somatosensory and visual ratios. Both variables showed alterations in the patient group. A recent study by Yahia et al.44 reported similar results to our own.
According to the data for the somatosensory and visual ratios, patients with cervicogenic dizziness in our study showed features compatible with alterations in proprioception and visual integration, which are necessary to maintain balance. These findings may allow us to attribute these patients’ subjective feelings of dizziness partly to a non-generalised postural control disorder. Furthermore, we found that the factors identifying disability associated with physical and emotional aspects of dizziness were negatively correlated with the somatosensory and visual preference ratios. In addition, dynamic posturography tests assessing the role of the vestibular system (vestibular and visual preference ratios) found no statistically significant differences between patients and asymptomatic individuals.
Considering the limited evidence currently available and the need for further studies on the topic, our findings on proprioceptive deficits are relevant and possibly specific to cervicogenic dizziness; proprioceptive alterations associated with general postural control have not been found in patients with non-traumatic neck pain and no dizziness.44,45
Correlation between disability associated with dizziness and other variablesThe mean level of disability associated with dizziness recorded in our sample (23.2±11.3) corresponds to mild disability. The correlation analysis found moderate-to-strong associations with neck disability, anxiety, and fear of movement. These findings suggest that a number of biopsychosocial factors may be involved in the persistence and worsening of dizziness. Future research should aim to identify which of these factors are the main predictors of cervicogenic dizziness. Regarding the factor of neck disability, Humphreys and Peterson46 report that patients with dizziness associated with chronic cervical pain, with or without a history of trauma, had more severe disability and more intense pain than healthy individuals or patients with chronic cervical pain without dizziness.
Fear of moving and anxiety have been studied in the context of alterations in vestibular function and postural control. Both emotional factors have been included in a theoretical model according to which they may have an intrinsic or extrinsic impact, playing specific roles in disorders of movement, stability, or chronic subjective dizziness.47 Our findings support this model and point to the need for further research on this topic and for the introduction of self-assessment questionnaires to evaluate these factors.
LimitationsOur study has a number of limitations regarding the interpretation of results. Firstly, due to its cross-sectional design, the results are useful for proposing new hypotheses but not for establishing causal associations. Future studies should be longitudinal in design in order to analyse the behaviour of the variables included.
Second, our study did not include any specific measures of proprioception and neck movement; assessing these alterations would have been interesting, given how frequently they affect patients with non-specific neck pain. Furthermore, these data may be used to analyse the correlation with variables of postural control and VOR. When assessing postural control with the SOT in patients with cervicogenic dizziness, each condition should be measured with different head positions; there is evidence suggesting that this variable has an impact on general postural control.16,48,49
Lastly, future studies should also analyse developmental factors in order to identify the presence of such alterations as vitamin B12 or B9 deficiency and to determine their association with dizziness and imbalance.
ConclusionsOur study shows that VOR activity is similar in patients with cervicogenic dizziness and in asymptomatic individuals, ruling out the vestibular system as part of the pathogenesis of this condition. Postural control, in contrast, does seem to be impaired in these patients in terms of proprioception and visual integration; these variables are associated with disability due to dizziness. Such psychosocial factors as neck disability, fear of movement, and anxiety are associated with disability due to dizziness and should be considered in future studies aiming to determine the pathophysiological mechanisms or predictors of cervicogenic dizziness.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors are grateful for the support offered by the Centre for Advanced Studies at Universidad La Salle-Universidad Autónoma de Madrid. We would also like to thank Optomic and Natus for their support for the chair for research into sensorimotor rehabilitation and posturographic analysis.
Please cite this article as: Grande-Alonso M, Moral Saiz B, Mínguez Zuazo A, Lerma Lara S, La Touche R. Análisis bioconductual del sistema vestibular y el control postural en pacientes con mareo cervicogénico. Estudio observacional transversal. Neurología. 2018;33:98–106.