Some vegetable foodstuffs contain toxic compounds that, when consumed, favour the development of certain diseases. Cassava (Manihot esculenta Crantz) is an important food source, but it contains cyanogenic glucosides (linamarin and lotaustralin) that have been associated with the development of tropical ataxic neuropathy and konzo. In rats, intraperitoneal administration of acetone cyanohydrin (a metabolite of linamarin) produces neurological disorders and neuronal damage in the hippocampus. However, it is unknown whether hippocampal area CA1 plays a role in neurological disorders associated with acetone cyanohydrin.
MethodA total of 32 male Wistar rats 3 months old were assigned to 4 groups (n=8 per group) as follows: vehicle (1μl physiological saline), and 3 groups with acetone cyanohydrin (1μl of 10, 15, and 20mM solution, respectively). The substances were microinjected intrahippocampally every 24hours for 7 consecutive days, and their effects on locomotor activity, rota-rod and swim tests were assessed daily. On the fifth day post-treatment, rats underwent further assessment with behavioural tests to identify or rule out permanent damage induced by acetone cyanohydrin.
ResultsMicroinjection of acetone cyanohydrin 20mM resulted in hyperactivity, motor impairment, and reduced exploration from the third day of treatment. All concentrations of acetone cyanohydrin produced rotational behaviour in the swim test from the first day of microinjection.
ConclusionThe hippocampal area CA1 is involved in motor alterations induced by microinjection of acetone cyanohydrin, as has been reported for other cassava compounds.
Algunos vegetales destinados a la alimentación contienen compuestos tóxicos que, al ser consumidos, predisponen al desarrollo de algunas enfermedades. La yuca (Manihot esculenta Crantz) es una fuente importante de alimento, pero contiene glucósidos cianogénicos (linamarina y lotaustralina) que han sido asociados con el desarrollo de la neuropatía atáxica tropical y el konzo. En la rata, la administración intraperitoneal de acetonacianohidrina (un metabolito de la linamarina) produce alteraciones neurológicas y daño neuronal en el hipocampo. No obstante, se desconoce si el área CA1 del hipocampo participa en las alteraciones neurológicas asociadas a la acetonacianohidrina.
MétodoTreinta y dos ratas macho Wistar de 3 meses de edad fueron destinadas a 4 grupos (n=8 cada grupo): vehículo (1 de solución salina fisiológica) y 3 grupos con acetonacianohidrina (1 de solución 10, 15 y 20mM). Las sustancias fueron microinyectadas intrahipocampalmente durante 7 días consecutivos (cada 24h); los efectos fueron evaluados diariamente en las pruebas de actividad locomotora, rota-rod y nado. Al quinto día postratamiento se evaluaron nuevamente en las pruebas conductuales para identificar o descartar la permanencia del daño inducido por la acetonacianohidrina.
ResultadosLa microinyección de acetonacianohidrina 20mM produjo hiperactividad, incoordinación motora y reducción de la exploración a partir del tercer día del tratamiento. En la prueba de nado, todas las concentraciones de acetonacianohidrina produjeron la conducta de giro desde el primer día de microinyección.
ConclusiónEl área CA1 del hipocampo participa en las alteraciones motoras inducidas por la microinyección de acetonacianohidrina, como ha sido reportado para otros compuestos de la yuca.
Various plant foods regarded as daily staples in some parts of the world contain compounds that can have a toxic effect under certain conditions. Under normal conditions, these foodstuffs are processed properly and are safe to consume; however, when they have not been prepared correctly, the toxic compounds that remain may cause illness, including different central nervous system diseases.1 One of these foodstuffs is manioc root or cassava (Manihot esculenta Crantz), which is a good source of energy. The root and the above-ground parts of the cassava plant contain cyanogenic compounds (linamarin and lotaustralin); hydrolisation of these compounds via the linamarase enzyme produces glucose and acetone cyanohydrin, which in turn decomposes into acetone and hydrogen cyanide, a neurotoxin.2
Earlier studies reported that rats treated with juice from cassava root (equivalent to 0.30mg/2mL of linamarin) developed hyperactivity and loss of motor coordination,3 as well as a reduction in the number of neurons in hippocampal area CA1.4 These studies point to the role of the hippocampus in the motor impairment caused by cassava consumption, and probably also in the aetiology of such neurological diseases as konzo and tropical ataxic neuropathy among cassava consumers.4 Linamarin microinjections to the hippocampus (CA1) elicit hyperactivity and loss of motor coordination in rats, manifesting as higher numbers of crossed squares in the open field test, shorter latency to fall on the rotarod test, and spinning behaviour on the swimming test.5 Aside from these findings, we do not yet know whether other compounds in cassava, such as acetone cyanohydrin, may also impair coordination and motor activity when delivered to the hippocampus by microinjection. We attempt to answer this question by studying how intrahippocampal microinjections of acetone cyanohydrin affect behaviour in Wistar rats, evaluated using locomotor function, rotarod, and swimming tests.
MethodsSubjectsWe used 32 three-month-old male Wistar rats weighing 250 to 300g at the beginning of the study. Rats were housed in transparent acrylic cages in a vivarium kept at room temperature (25±2°C) with a 12:12 light-dark cycle (lights on at 7:00 am). Rats had ad libitum access to water and food. Rats were handled in accordance with the international ethical standards listed in the Guide for care and use of laboratory animals6 and official Mexican guidelines for the breeding, care, and use of laboratory animals.7
Stereotactic surgeryAs described in previous studies, animals were deeply anaesthetised during the unilateral implantation of a guide cannula.8 Researchers used a stereotactic apparatus (Stoelting, Wood Dale, IL, USA) to immobilise the rats’ heads before exposing the skull. A dental lab drill (Saeshin Dental Lab 35000 RPM, South Korea) was used to trepan the skull and implant a cannula in the dorsal hippocampal area CA1. The stereotactic coordinates from bregma9 were AP=−3.8mm; L=−2mm; H=−2mm. A stainless steel guide cannula (8mm long, 0.7mm in diameter) was subsequently implanted and secured to the skull with dental acrylic (Arias Distribuidora Dental; Tlalnepantla, Mexico). Four days after implantation of the cannula, microinjections were started.
Experimental groups and treatmentRats were randomly assigned to 4 groups (n=8 rats per group): one group received the vehicle (physiological saline) and the remaining 3 groups received acetone cyanohydrin dosed at different concentrations (10, 15, and 20mM) that were calculated based on earlier studies.10 Microinjections of either the vehicle or acetone cyanohydrin were delivered every 24hours for 7 consecutive days. Five minutes after the microinjection, rats were evaluated using the behavioural tests to determine the effect of treatment; on the fifth day post-treatment, the behavioural tests were repeated without treatment to detect or rule out any permanent damage caused by acetone cyanohydrin.
MicroinjectionResearchers used an injection cannula (10mm long, 0.7mm in diameter) consisting of a stainless steel needle measuring 0.7mm×32mm (22G×1 1/4″) attached to a 10L Hamilton syringe by means of a polyethylene tube. An automatic infusion pump (KD Scientific; Holliston, MA, USA) was used to microinject 1μL of liquid at a constant rate of 0.1μL/min for 10minutes. During this procedure rats were able to move freely. After microinjection, the injection cannula was left in place for 5 additional minutes to allow diffusion of the injected substance and prevent it from returning by capillarity. We used behavioural testing to evaluate each rat immediately after treatment on the 7 consecutive days of treatment, and on day 5 post-treatment.
Locomotor activity testEach rat was placed inside an opaque acrylic cage (44×33×20cm) whose base was divided into squares measuring 11×11cm. We assessed the following: (a) number of crossed squares (the rat was considered to have crossed a square when at least three-fourths of its body passed from one square to another); and (b) number of vertical behaviours (times standing on its hind legs). Crossed squares were an indicator of spontaneous motor activity and vertical behaviour was observed to detect any potential alterations in motor coordination.
Rotarod testDuring the 5 days prior to beginning microinjections, rats were trained on a rotarod (LE 8300, LSI Letica, Panlab Scientific Instruments, Barcelona, Spain) at a speed of 18rpm. After receiving microinjections, rats were placed on the rotarod for daily assessments of latency to fall, that is, the time it takes the rat to fall off the rod. This variable is used to identify any alterations in motor coordination and balance.
Forced swim testWe placed rats in a glass tank (base: 26×29cm; height: 50cm) filled with water at a temperature of 25±1°C. The water level was such that rats could touch the bottom of the tank with their hind feet and tails. This test was used to evaluate the number of spins, that is, periods during which the rat was moving in a circular direction rather than forward.11,12
We recorded video feed from all sessions of the locomotor activity and swimming tests. Two independent observers quantified open field test variables until reaching a concordance of at least 95%. In the forced swimming test, the variable ‘number of spins’ was quantified by analysing video feeds with a software tool (ANY-maze 4.73; Stoelting, Wood Dale, IL, USA).
Verification of the microinjection siteAfter completing the behavioural tests, rats were euthanised with pentobarbital (PiSA Agropecuaria, Guadalajara, Mexico) and transcardially perfused with 100mL of physiological saline (NaCl 0.9%) followed by 100mL of formaldehyde 30% (J.T. Baker, Ecatepec, Mexico). The microinjection site was marked with Evans blue. Brains were removed and cut into thick slices to analyse the site of microinjection under a light microscope; Paxinos and Watson's rat brain atlas was used as a reference.9 The statistical analysis included data from only those rats in which cannulas were confirmed to have been implanted correctly in hippocampal area CA1.
Statistical analysisData were analysed with the 2-way repeated measures ANOVA; the 2 factors were treatment and days of treatment. Given P-values≤.05, we applied the post hoc Student–Newman–Keuls test. Results are expressed as means±standard deviation.
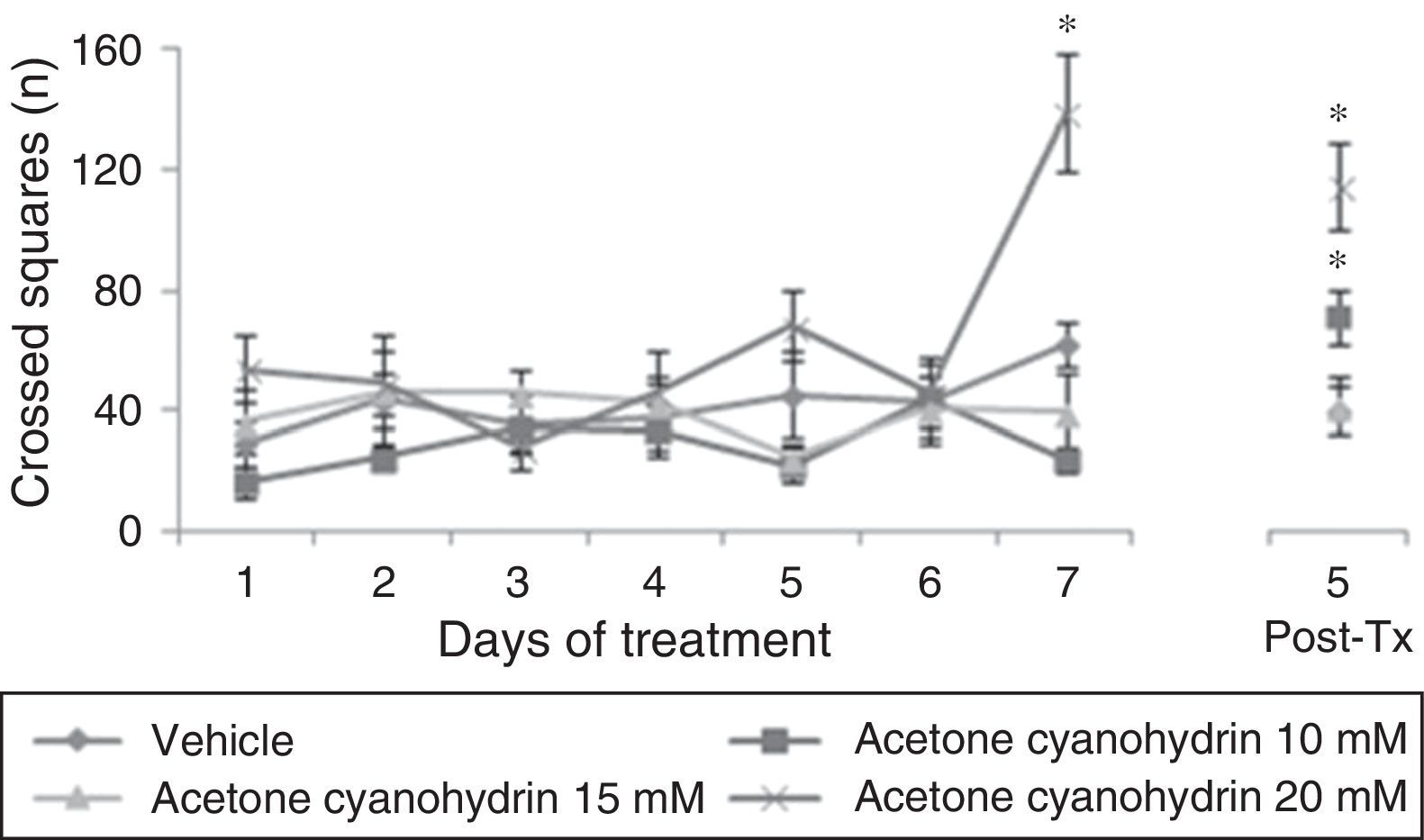
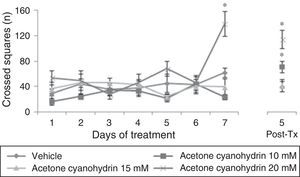
ResultsLocomotor activity testWe found significant differences in the number of crossed squares for treatment type (F[3,177]=15.906, P<.001), days of treatment (F[7,177]=5.878, P<.001), and the interaction between factors (F[21,177]=3.578, P<.001). The post hoc test indicated that the group treated with acetone cyanohydrin 20mM crossed a higher number of squares on day 7 of treatment, and that this difference remained on day 5 post-treatment, with respect to the vehicle and acetone cyanohydrin 10mM and 15mM groups measured on the same day (Fig. 1).
Number of squares crossed in the locomotor activity test. This number was higher in rats treated with 20mM acetone cyanohydrin on day 7 of treatment compared to results from other groups on the same day.
Post-Tx: 5 days post-treatment.
*P<.001 vs all groups on day 7 treatment and day 5 post-treatment.
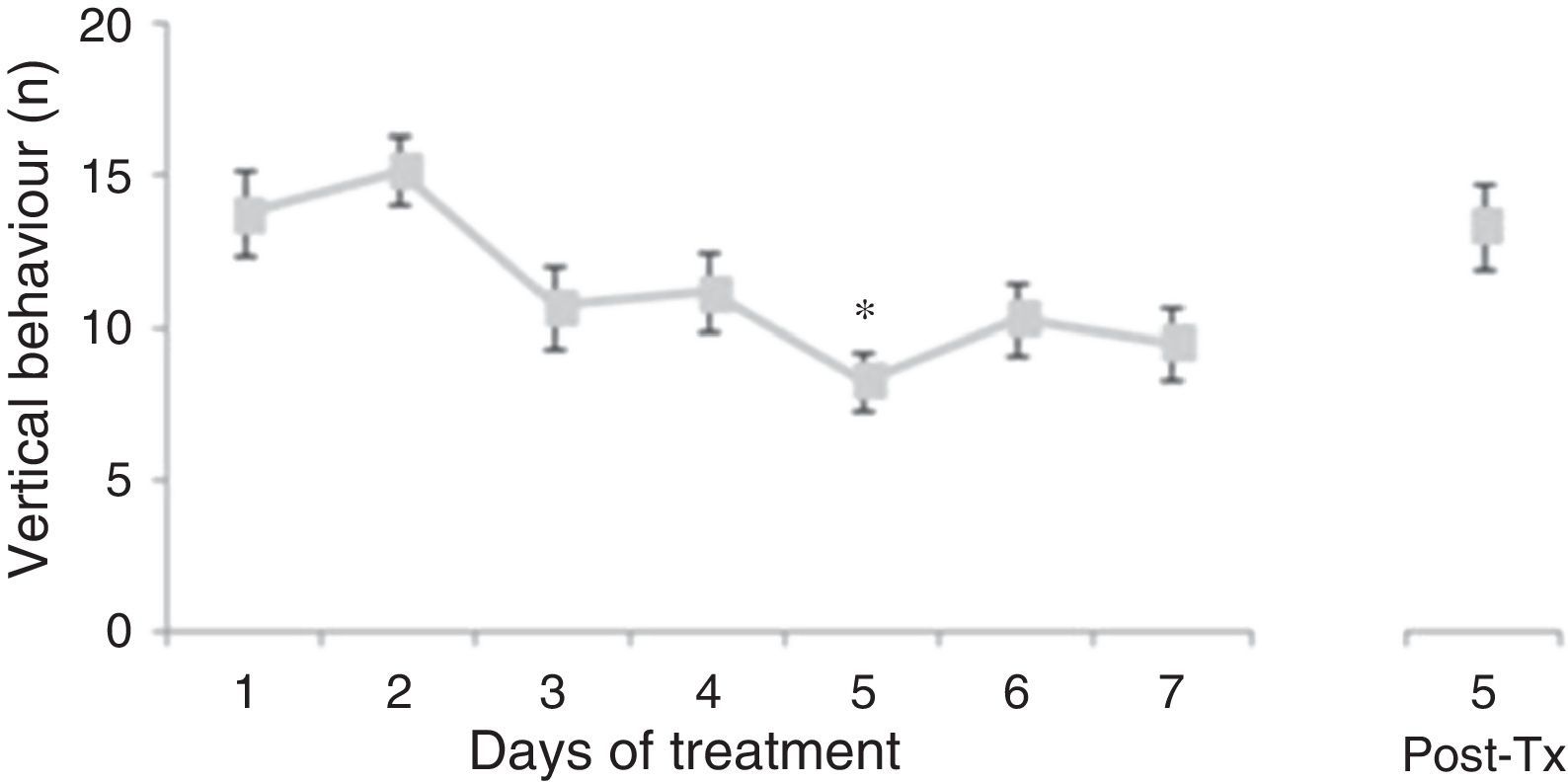
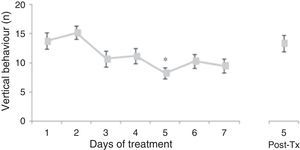
Analysis of the number of vertical behaviours did not reveal significant differences linked to treatment type (F[3,177]=0.645, P=.578) or to interaction between factors (F[21,177]=0.796, P=.723). Nevertheless, the analysis revealed significant differences with respect to the day of treatment (F[7,177]=3.437, P=.002). The post hoc test showed that the number of vertical behaviours decreased beginning on day 3 of treatment; the difference was significant with respect to days 1 and 2, and it was still present at 5 days post-treatment (Fig. 2).
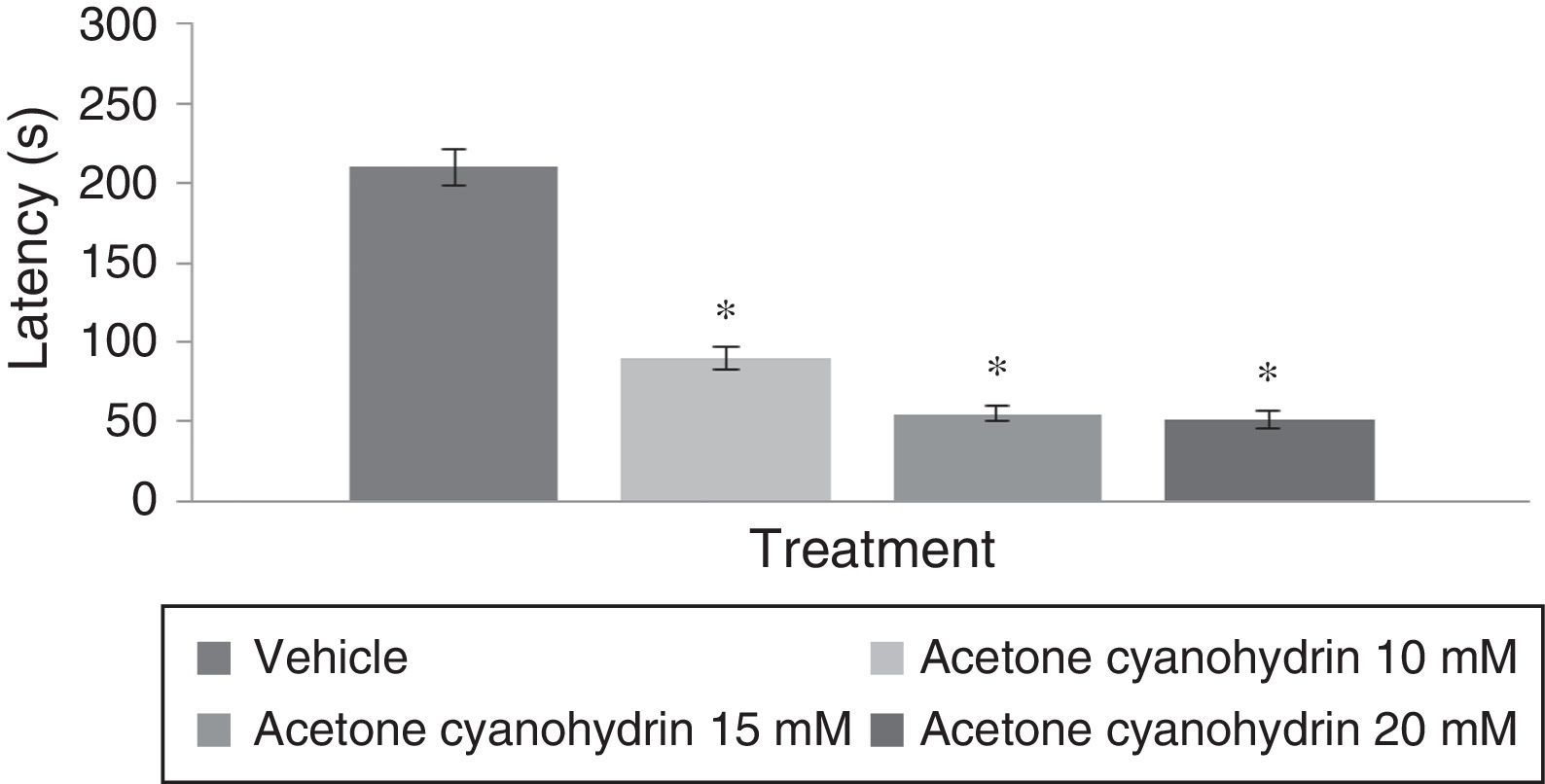
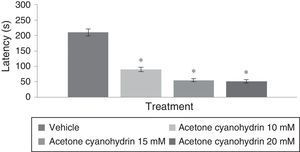
Rotarod testAnalysis of time to fall on the rotarod test found significant differences among treatment groups (F[3,192]=6.382, P<.001); the post hoc test showed that groups treated with 10mM, 15mM, and 20mM of the substance had shorter times to fall from the rotarod compared to the vehicle group. The group treated with 20mM displayed the shortest latencies on the rotarod test (Fig. 3). Analyses by day of treatment (F[7,192]=1.307, P=.249) or by interaction between factors (F[21,192]=0.342, P=.997) did not reveal significant differences.
Forced swim testSince the vehicle group did not display spinning behaviour, only the groups treated with acetone cyanohydrin (10, 15, and 20mM) were included in the statistical analysis.
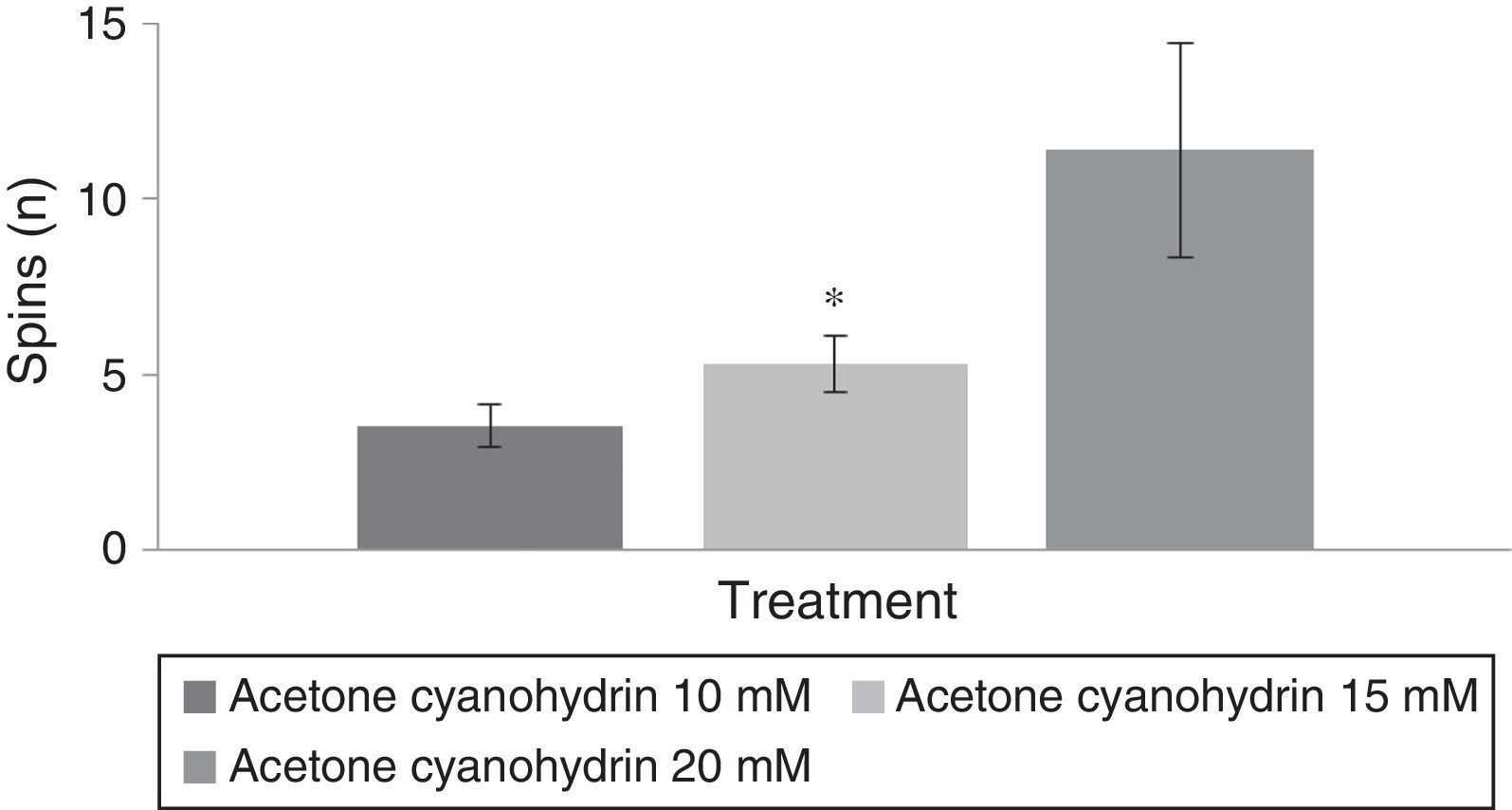
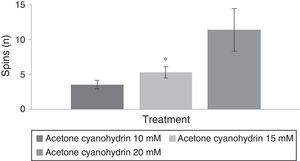
Spinning behaviourAn analysis of the number of spins per treatment group revealed statistically significant differences (F[3,177]=8.840; P<.001). The post hoc test indicated that the group treated with 20mM acetone cyanohydrin displayed more spins than did the 10mM and 15mM treatment groups (Fig. 4). The analysis did not detect any significant differences regarding days of treatment (F[7,177]=0.736; P=.642). An analysis of the interaction of factors did not reveal any significant differences (F[21,177]=0.560, P=.940).
DiscussionIn this study, interhippocampal delivery of acetone cyanohydrin in rats resulted in decreased coordination and motor activity. These findings indicate that the metabolite, like other compounds present in cassava, may form a part of the neurotoxic substrate associated with the development of neuropathies in humans who consume cassava and its derivatives under certain conditions. Locomotor activity testing allows us to identify changes in the rats’ motricity (hypoactivity or hyperactivity) and exploratory behaviour, which in turn may point to potential neurological damage (peripheral neuropathies) caused when certain drugs or toxic substances affect the central nervous system.13,14 The locomotor activity test employed in our study helps us identify changes in rats’ motor function by analysing their exploratory patterns and the coordination of their movements, as other authors have observed.15 Hippocampal lesions in adult rats elicit behavioural changes related to the dopaminergic system; these include increased locomotor activity in new environments, as well as learning and memory deficiencies.16,17 This study reports that the group receiving 20mM acetone cyanohydrin displayed increased spontaneous motor activity on the open field test. In this case, hyperactivity may have been associated with the neurotoxic effects of acetone cyanohydrin on the hippocampus, which would prevent the animal from forming memories. This was observed with linamarin in an earlier study.5 Under natural conditions, a rat undergoing repeated locomotor activity evaluations would demonstrate a reduction in spontaneous motor activity (crossed squares, vertical behaviours, and grooming); this decrease indicates familiarity with the experimental setting.18 In contrast, an animal subjected to neural damage in the hippocampus and then evaluated repeatedly on the open field test would maintain or even increase the level of motor activity. This behaviour may indicate decreased consolidation of memory resulting in continued exploration of what is viewed as a novel setting.18 Humans with neuropathies associated with cassava consumption develop motor coordination problems.19,20 This study found that treatment with acetone cyanohydrin (10, 15, or 20mM) promotes loss of motor coordination and balance on the rotarod test, in line with findings for other neurotoxic compounds.21
Additionally, intrahippocampal microinjection of acetone cyanohydrin led to the appearance of spinning motions during the forced swim test, but this behaviour decreased as the experiment progressed. It is important to point out that spinning motions have been linked to alterations in limb motor coordination in rats. These changes may be similar to behaviours observed in patients with tropical ataxic neuropathy and konzo, specifically, the development and presence of slow, progressive paralysis of the upper and lower limbs.22 We emphasise that certain compounds present in cassava can cause demyelination of the spinal cord, resulting in ataxia with decreased limb coordination.20 Acetone cyanohydrin may also contribute to the motor impairment associated with long-term consumption of cassava, including the alterations occurring in tropical ataxic neuropathy and konzo.3
Regarding the post-treatment effect, that is, results gathered 5 days after the last intrahippocampal microinjection, rats receiving 20mM acetone cyanohydrin continued to show motor hyperactivity in the open field test (increased number of crossed squares and vertical behaviours). In this case, the rat's hyperactive exploratory behaviour might be linked to the neurotoxic effect of acetone cyanohydrin microinjections to hippocampal area CA1, and it might indicate a permanent effect on exploratory behaviour. Studies by other teams working with young rodents have shown that damage to pyramidal neurons in hippocampal area CA1 elicits changes in learning and spatial memory,23 and in locomotor ability and exploration and searching strategies.24,25 Other studies indicate that these alterations may correspond to functional and morphological changes in the hippocampus,26 and they link them to decreased neurogenesis in this region.25 This mechanism may explain how acetone cyanohydrin would prevent the consolidation of memory in rats. Furthermore, the rotarod test completed 5 days after the last intrahippocampal microinjection demonstrated that the lesion caused by acetone cyanohydrin (10, 15, and 20mM) had caused lasting impairment to the rats’ coordination and motor equilibrium27; results on day 5 post-treatment indicated a significant decrease in time to fall. Lastly, animals in the 15mM and 20mM treatment groups evaluated with the forced swim test on day 5 post-treatment, with no new microinjections, continued to display spinning motions. This behaviour indicates persistent motor deterioration and loss of coordination and balance responding to the neural damage caused by acetone cyanohydrin microinjections to the hippocampus.3,4,11 Lastly, although this study did not identify the mechanism underlying the motor impairment observed following acetone cyanohydrin microinjections to area CA1, we are able to offer a plausible explanation. A variety of neurological diseases share pathological features indicative of gradual and selective neuron loss, and many of these diseases are related to hippocampal alterations. By forming part of the limbic system and providing part of the glutamatergic excitatory pathway reaching the nucleus accumbens, the hippocampus has a direct role in motivating the execution of motor responses associated with emotionally arousing events. Based on the above, we can suppose that the sideways and spinning motions occurring in the forced swim test result from overactivation of glutamatergic receptors in the hippocampus subjected to chemical lesion with acetone cyanohydrin, while recognising that other brain structures may also have been damaged. Loss of neuronal membrane integrity results in loss of glutamate, which in turn contributes to prolonged activation of glutamatergic receptors in neighbouring cells.28 Furthermore, an influx of calcium will also provoke glutamate release by exocytosis, and this process establishes a positive feedback system that gives rise to prolonged neuronal overexcitation, followed by neuronal apoptosis.29 This mechanism provides an explanation for the motor alterations that may be caused by delivery of acetone cyanohydrin, a cassava derivative, to the hippocampus.
FundingThis study was partially supported by the study group for the biology, chemistry, and molecular functionality of vegetable metabolites (UV-GC-368) at Universidad Veracruzana.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rivadeneyra-Domínguez E, Vázquez-Luna A, Díaz-Sobac R, Briones-Céspedes EE, Rodríguez-Landa JF. Participación del área CA1 del hipocampo en la incoordinación motora inducida por acetonacianohidrina en la rata. Neurología. 2017;32:230–235.